Abstract
This article aimed to collectively present the demographic, clinical, radiographic and histopathological features as well as the treatment performed along with its outcome for all the cases of adenoid ameloblastoma with dentinoid (AAD) reported in scientific literature till date. Ameloblastoma and adenomatoid odontogenic tumours are the most common odontogenic neoplasms. However, AAD, a hybrid variant of the two lesions, is found to be extremely rare. The lesion comprises of characteristic histopathological features of ameloblastoma and adenomatoid odontogenic tumour and shares certain clinical characteristics with either of the entities. AAD may be considered to be present at the more aggressive end of spectrum of benign odontogenic neoplasms. Owing to the frequent tendency of the lesions to be underdiagnosed, careful histopathological screening of submitted biopsies is warranted. With the increase in number of reported cases in the recent years, it is likely to be included as a separate entity in the upcoming World Health Organization classification.
Keywords: Odontogenic Tumors, Adenomatoid Odontogenic Tumor, Adenoameloblastoma, Immunohistochemistry
The process of odontogenesis involves complex molecular interactions. Disruptions in these interactions may result in a distinct spectrum of neoplasms, unique to the jaws, that are collectively termed odontogenic tumours (OTs).1 The World Health Organization (WHO) has defined OTs as lesions derived from epithelial, ectomesenchymal and/or mesenchymal elements that are or have been a part of the tooth-forming apparatus.2,3 Owing to their development from various components of the tooth-forming apparatus, OTs may present a considerable histopathological diversity.
Two or more distinct tumour entities may co-exist within the same lesion and are reported as hybrid tumours. Ide et al. defined these as lesions showing the combined histopathological characteristics of two or more previously recognised tumours and/or cysts of different categories.4 These tumours have not been included in the 2017 WHO classification system of odontogenic neoplasms due to the inadequate number of reported cases.5
One such variant of the hybrid odontogenic tumour may exhibit the histological features of both ameloblastoma (AM) and adenomatoid odontogenic tumour (AOT). This hybrid odontogenic tumour was first reported by Slabbert et al. in 1992 as dentinoameloblastoma.6 The diagnostic term adenoid ameloblastoma with dentinoid (AAD) was first reported by the Armed Forces Institute of Pathology in 1994 by Brannon.7 Over time, various terminologies have been associated with the lesions, such as atypical plexiform ameloblastoma with dentinoid, ameloblastoma with features of dentinoid, hybrid ameloblastoma and AOT originating within an unicystic ameloblastoma and atypical adenoid ameloblastoma.8–13 As a result of the paucity of reported cases of the lesions, the data available regarding their pathogenesis, clinical behaviour, diagnosis and prognosis are limited.
The present systematic review aimed to collectively present the demographic details, clinical features, histopathological patterns, molecular markers, treatment performed and the outcomes of the cases of AAD found in the literature in English. Another objective was to improve the understanding of the lesions with respect to their clinical characteristics, varied histopathological morphology and prognosis.
This systematic review has been registered in the International prospective register of systematic reviews – PROSPERO (Record ID: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=207062).
Methods
Case reports and case series of AAD were retrieved by a systematic search of scientific databases, including Ovid (Walter Kluwer, New York, USA), Medline (National Library of Medicine, Maryland, USA), PubMed Central (National Library of Medicine), Web of Science Citation Index Expanded (Clarivate Analytics, London, UK) and Google Scholar (Google, Mountain View, USA) with the keywords ‘Adenoid’ OR ‘Adenomatoid’ AND ‘Ameloblastoma’ AND ‘Dentinoid’. An additional search was performed using keywords ‘Hybrid’ AND ‘Odontogenic’ AND ‘Tumours’ and the retrieved literature was scanned to identify any cases reported with a name differing from AAD. Additionally, case reports and case series of AAD were also scanned from cross-references.
CRITERIA FOR SELECTION AND EXCLUSION
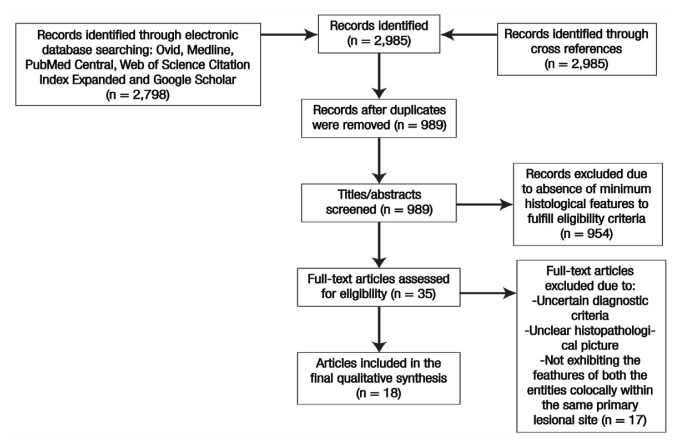
The case reports and case series of the lesions co-localised within the same primary lesion site, exhibiting the histopathological characteristics of both AM and AOT and available in English language were included in this review. However, lesions with uncertain diagnostic criteria, unclear histopathological characteristics and the absence of features of both the entities found co-locally were excluded from the review [Figure 1].
Figure 1.
PRISMA flow chart indicating selection process of articles for final qualitative synthesis in the present systematic review.
In order to be classified as AAD, a lesion should present characteristic histopathological features of both AM and AOT in association with extracellular dentinoid material. Therefore, the reported cases were included in this study if their histopathological pictures comprised a combination of at least one of the features of AM and AOT, respectively, along with dentinoid material [Table 1]. With respect to the AM component, any of the histopathological variants of solid/conventional AM as well as unicystic ameloblastoma (UAM), peripheral AM and metastatic AM were considered to be eligible for inclusion.
Table 1.
Coded labels provided to various histopathological features in the present systematic review
| Ameloblastoma component | |
|---|---|
| AM-F | Follicles of odontogenic epithelium with peripheral tall columnar ameloblast-like cells with reversal of polarity and central stellate reticulum-like cells in the form of follicles within a mature connective tissue stroma |
| AM-P | Odontogenic epithelium infiltrating into a mature connective tissue stroma in the form of interlacing strands or plexuses |
| UAM | Cystic lesion having lumen lined by tall columnar cells with hyperchromatic nuclei exhibiting nuclear palisading with reversal of polarity and cytoplasmic vacuolisation (Gorlin-Vickers Criteria)14 |
| Adenomatoid odontogenic tumour component | |
| AOT-S | Sheets/islands/cords/whorling of spindle to ovoid shaped odontogenic epithelial cells |
| AOT-D | Duct-like structures lined by epithelial cells with eosinophilic material/cystic space in the lumen |
| AOT-R | Rosette-like structures consisting of two layers of low to tall columnar epithelial cells with eosinophilic material/cystic space centrally in the lumen |
| Dentinoid material | |
| DM | Extracellular homogenous eosinophilic material (dentinoid) |
| Other features | |
| CC | Presence of clear cells within the tumor islands in AOT component of the lesion. |
| GC | Presence of ghost cells within odontogenic epithelial nests/islands in the AOT component or within ameloblastomatous epithelium |
AM = ameloblastoma; UAM = unicystic ameloblastoma; AOT = adenomatoid odontogenic tumour; DM = dentinoid material; CC = clear cells; GC = ghost cells.
Overall, in terms of the labels used in the present systematic review for the respective histological features, the following definite diagnostic formula denoting the minimum criteria for a lesion to be considered as AAD was proposed:
In addition to these minimum requisite features, lesions presenting other peculiar features, such as the presence of clear cells within the tumour islands in the AOT component of the lesion and ghost cells within the odontogenic epithelial nests/islands in the AOT component or within ameloblastomatous epithelium were also included and discussed in this review. Moreover, the desmoplastic changes, which may occur in the stromal component of AM, are now regarded as a histopathological variant of AM rather than a distinct entity.5 However, these additional features are not considered as definitive criteria for the lesion, but rather represent varying stages of odontogenic cell differentiation in the lesion.
DATA EXTRACTION
Data on the demographic, clinical, radiographic and histopathological features and molecular markers were assessed. The treatment performed and their outcomes in all the reported cases were elicited. In order to minimise the risk of bias in quality assessment, the authors were divided into two groups that independently screened the cases and extracted the data from the included articles. The data was entered into meta-analysis sheets (MS Office Excel, 2016; Microsoft Redmond Campus, Redmond, WA, USA).
Results and Discussion
A total of 29 reported cases of AAD were extracted from 18 publications available in English. The comprehensive findings following a detailed review of all the cases are collectively summarised [Tables 2 and 3].
Table 2.
Summarised parameters of cases of adenoid ameloblastoma with dentinoid
| Author and year of publication | Age in years | Gender | Duration | Arch | Side | Symptom | Radiographic feature | Histopathological feature | Special stains/IHC | Final diagnosis | Treatment (with number of recurrences) | Follow-up with NED |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slabbert et al.6 (1992) | 24 | Male | N/P | Mn | Left | Swelling | UL RL | AM-F, AM-P, AOT-S, DM | Van Gieson + Masson’s trichrome + Congo red − Alcian blue − |
Dentinoame-loblastoma | WE | N/P |
| Matsumoto et al.8 (2001) | 19 | Male | 1 mo | Mn | Right | Swelling | WD UL RL | AM-P, AOT-S, AOT-D, DM | Mucicarmine + Alcian blue + |
Atypical plexiform AM with dentinoid | Marsupialisation + enucleation; (1 rec after 2 yr); WE | 2.5 yr |
| Evans et al.9 (2004) | 39 | Male | N/P | Mn | CM | Swelling | WD UL RL | AM-P, AOT-S, AOT-D, DM, CC | N/P | AM with features of AOT | WE; WE + curettage enucleation + curettage; (3 recs over a 16-yr period); SR | 18 mo |
| Zhang et al.10 (2006) | 64 | Female | 16 mo | Mn | CM | Swelling + paresthesia | WD UL RL with RO clusters | AM-F, AOT-S, AOT-R, DM, GC | N/P | Hybrid odontogenic tumor characteristic of CCOT, solid multicystic AM and AOT | SR | 3 yr |
| Jivan et al.12 (2007) | 40 | Male | 7 mo | Mn | CM | Swelling | WD UL RL | UAM, AOT-S, AOT-D, AOT-R, DM | Calretinin ++ in cystic lining | AOT originating within a unicystic AM | N/P | N/P |
| Ghasemi-Moridani et al.15 (2008) | 19 | Female | 2 mo | Mx | Right | Swelling | WD UL RL | AM-P, AOT-S, AOT-D, DM | N/P | AAD | Excision | N/P |
| Ide et al.16 (2009) | 44 | Male | N/P | Mx | CM | N/P | WD UL RL | AM-P, AOT-S, AOT-D, AOT4, DM | Calretinin + | AAD | En; Excision; (3 recs over a 11-year period) Partial maxillectomy | 8 yr |
| Sonone et al.17 (2011) | 35 | Female | 1 year | Mn | Right | Swelling + numbness | WD UL RL with RO foci | AM-P, AOT-S, AOT-D, AOT-R, DM, GC Congo red − |
Van Gieson + | AAD | SR | 6 mo |
| Saxena et al.18 (2012) | 45 | Male | 2 wk | Mx | Left | Swelling | Diffuse RL | AM-P, AM4 AOT-D, AOT-S, DM | N/P | AAD | WE (3 recs) SR | N/P |
| Yamazaki et al.11 (2014) | 31 | Female | N/P | Mn | Right | None | WD UL RL with sclerotic area in distal portion | AM-F, AM-P, AOT-S, AOT-D, DM, CC CK17 + Calretinin + Ki-67 + Congo red + |
CK19 ++ | Hybrid AM and AOT | SR | 36 mo |
| Loyola et al.13 (2015) | 55 | Male | 5 mo | Mn | Left | Swelling | N/P | AM-F, AM-P, AOT-S, AOT-D, DM | CK19: ++ Ki-67 ++ CK8/18 − |
AAD | 1 rec; SR | 108 mo |
| 24 | Female | 6 mo | Mx | Left | Swelling | WD UL RL | None | Adenoid AM (hybrid/ mixed odontogenic tumour) | WE | 6 mo | ||
| Loyola et al.13 (2015) | 31 | Male | 1 mo | Mn | Right | Swelling | WD RL | None | Adenoid granular cell AM with dentinoid | Hemimandibulectomy | 18 mo | |
| 40 | Male | N/P | Mn | CM | Swelling, numbness, parasthesia | WD UL RL | AM-F, AM-P, AOT-S, AOT-D, DM | Calretinin ++ p53 ++ |
Atypical adenoid AM | Hemimandibulectomy | 14 mo | |
| 16 | Female | 2 mo | Mn | Right | Swelling | UL RL | None | AAD | WE | 12 mo | ||
| Kumar et al.19 (2013) | 55 | Male | 3 mo | Mn | Right | Swelling, pain, paresthesia | WD UL RL | AM-P, AOT-S, AOT-D, AOT-R, DM, GC | CK19 + | AAD | SR | 36 mo |
| Salehinejad et al.20 (2016) | 34 | Female | 43 mo | Mx | Right | Swelling | PD RL | AM-F, AM-P, AOT-D, DM | CK19 ++ Ki-67 ++ CK8/18 + |
AAD | 9 recs; SR | 19 mo |
| Khalele et al.21 (2016) | 33 | Female | 48 mo | Mx | Right | Swelling + pain | N/P | AM-F, AM-P, AOT-S, AOT-D, AOT-R, DM | CK19 ++ Ki-67 ++ CK8/18 − |
AAD | 5 recs; SR + radiotherapy + neck dissection | N/P |
| Sathyanarayan et al.22 (2017) | 51 | Male | 72 mo | Mx | CM | Swelling + pain | PD RL | UAM, AOT-S, AOT-D, AOT-R, DM | CK19 ++ Ki-67 ++ CK8/18 + |
AAD | 5 recs; SR | 76 mo |
| Rai et al.23 (2017) | 47 | Male | 18 mo | Mx | CM | Swelling | N/P | AM-F, AOT-S, AOT-D, DM | CK19 ++ Ki-67 ++ CK8/18 + |
AAD | 2 recs; SR + radiotherapy + neck dissection | 52 mo |
| Adorno-Farias et al.24 (2018) | 15 | Female | 12 mo | Mn | N/P | Swelling | UL RL | AM-F, AOT-S, AOT-D, DM | CK7 − CK14 − CK19 + IMP3 − Ki-67 + p53 − |
AM with adenoid features | SR | N/P |
| 37 | Male | 12 mo | Mn | Left | Swelling + pain | WD ML RL | AM-F, AOT-S, AOT-D, DM, CC | CK7 − CK14 − CK19 ++ IMP3 − Ki-67 + p53 − |
SR | N/P | ||
| 46 | Female | N/P | Mn | N/P | N/P | UL RL | CK7 − CK14 − CK19 ++ IMP3 − Ki-67 + p53 − |
SR | N/P | |||
| Adorno-Farias et al.24 (2018) | 34 | Female | N/P | Mn | N/P | Swelling | UL RL | AM-F, AOT-S, AOT-D, DM, CC | CK7 − CK14 − CK19 + IMP3 − Ki-67 + p53 − |
AM with adenoid features | SR | N/P |
| N/P | Female | N/P | N/P | N/P | UL RL | CK7 − CK14 − CK19 ++ IMP3 − Ki-67 + p53 − |
SR | N/P | ||||
| 15 | Male | 12 mo | Mn | Swelling | UL RL | AM-F, AOT-S, AOT-D, DM, CC | CK7 − CK14 ++ CK19 ++ IMP3 − Ki-67 + p53 − |
SR | N/P | |||
| 82 | Male | 36 mo | Mn | Swelling | ML RL | AM-F, AOT-D, DM, CC, GC | CK7 − CK14 ++ CK19 ++ IMP3 − Ki-67 + p53 − |
SR | N/P | |||
| 46 | Male | N/P | N/P | N/P | UL RL | AM-F, AOT-D, DM, CC | CK7 − CK14 ++ CK19 + IMP3 − Ki-67 + p53 − |
SR | N/P | |||
| Arruda et al.25 (2020) | 51 | Female | N/P | Mx | Left | None | WD UL RL | AM-P, AOT-S, AOT-D, DM | Alcian blue | AAD | 1 rec; SR | 108 mo |
N/P = not provided; Mn = mandible; Mx = maxilla; CM = crossing midline; WD = well-defined; PD = poorly defined; UL = unilocular; ML = multilocular; RL = radiolucency; RO = radiopaque; WE = wide excision; SR = surgical resection; Rec = recurrence.
Table 3.
Summary of various parameters observed following review of case reports and case series of adenoid ameloblastoma with dentinoid (N = 29)
| Parameters | n |
|---|---|
| Age in years | |
| Lowest | 15 |
| Highest | 82 |
| Mean ± SD | 38.97 ± 27.43 |
| Gender | |
| Males | 16 |
| Females | 13 |
| Mandible | 18 |
| Left | 3 |
| CM | 4 |
| Right | 6 |
| Maxilla | 9 |
| Left | 3 |
| CM | 3 |
| Right | 3 |
| Mandible and maxilla (side not provided) | 7 |
| Symptoms | |
| Asymptomatic swelling | 16 |
| Pain | 3 |
| Paresthesia/numbness | 4 |
| Radiographic | |
| Well defined unilocular radiolucency | 20 |
| Well-defined multilocular radiolucency | 2 |
| Poorly defined radiolucency | 2 |
| Radiolucent lesion with radiopaque foci | 3 |
| Features of AM component | |
| Follicular | 9 |
| Plexiform | 9 |
| Mixed | 9 |
| Unicystic ameloblastoma | 2 |
| Changes in AM component | |
| Desmoplastic | 1 |
| Granular cells | 1 |
| Squamous metaplasia | 8 |
| Features of AOT component | |
| Duct-like structures | 19 |
| Sheets/whorls of cells | 18 |
| Rosette-like structures | 6 |
| Other histological features | |
| Clear cells | 9 |
| Ghost cells | 4 |
| Recurrences | |
| Cases reporting recurrences | 12 |
| Maximum number of recurrences in a single case | 9 |
| Follow-up details not provided | 13 |
SD = standard deviation; CM = centre; AM = ameloblastoma; AOT = adenomatoid odontogenic tumour.
The first case of AAD was reported by Slabbert et al. in 1992, and till date, only 29 cases of AAD have been reported.6 The low number of reported cases are reflective of the rarity of AAD. It is possible that several cases of AAD might be overlooked by pathologists as AM or AOT, depending on the predominance of either entity in the microscopic examination. This possibility is supported by the fact that 4 out of 45 cases of AM were re-assessed and re-classified as AAD in a retrospective study conducted by Loyola et al.13 Therefore, the actual number of cases might be much higher than those reported in the literature. With increasing case reports on the tumours and subsequent increase in the awareness amongst pathologists, hybrid lesions, such as AAD, are identified accurately, which is in-line with the fact that more than half of the cases of AAD (n = 19) were reported during the years 2015–2020. Thus, more cases of AAD may be expected to be reported in the following years.
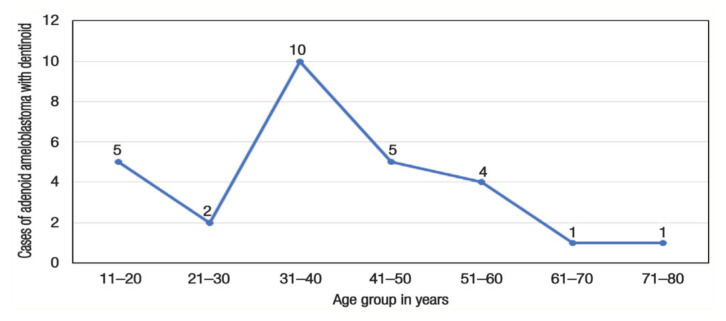
The mean age of the patients presenting the lesion was 38.97 ± 27.43 (range: 15–82) years. The maximum number of cases occurred in the fourth decade (n = 10), followed by the second and fifth decades (n = 5 each) of life, and the least number of cases were reported in the seventh decade or above [Figure 2]. The pattern of age distribution of the lesion was identical to that of AM and relatively less similar to AOT, which tends to occur in first or second decade.26 Unlike AOT, which is more common in females, AM does not exhibit gender predilection.27 In the case of AAD, the reported cases comprised of 16 males and 13 females, yielding a ratio of nearly 1:1. The absence of gender predilection in AAD was also similar to that observed in AM.
Figure 2.
Number of reported cases of adenoid ameloblastoma with dentinoid belonging to various age groups.
The cases of AAD occurred twice more frequently in the mandible (n = 18) compared to maxilla (n = 9), with a slight predilection for the right (n = 9) compared to the left side (n = 6). Considering the lower frequency of lesions in the maxilla, it may be deduced that a high percentage of lesions occurring on the left side were in the maxilla (3/6 maxillary AAD), whereas AAD occurring in the mandible exhibited a predilection for the right side (6/9 cases).
In seven instances, the lesion involved the entire arch on both sides crossing the midline, indicating the aggressive potential of the lesion. Interestingly, the tendency of the lesion to infiltrate both sides was equal in both the jaws, encompassing the entire mandible (n = 4) or maxillary jaw along with maxillary sinus and orbital floor (n = 3). Approximately two-third of AOTs have been reported to occur in the maxillary jaw with a predilection for the left side, while AM frequently tends to occur in the posterior region of the mandibular jaw with a slight predilection for the right side.26 Thus, the pattern of occurrence of AAD in the jaws is similar to AOT in the maxilla and AM in the mandible.
Similar to the clinical presentation of both AOT and AM, most of the patients presented an asymptomatic swelling (n = 16), which was accompanied by pain in only three cases. Paraesthesia and numbness were elicited in four cases, with all the lesions inevitably involving the mandibular posterior region. Pain, paraesthesia and numbness are also associated with AM lesions, albeit uncommonly, and could be attributed to the tumour mass impinging on the peripheral nerves or secondary infection.28
The radiographic evaluation revealed that the lesion presented as a well-defined unilocular radiolucency (n = 20), which was similar to that commonly noted in AOT. Loyola et al. reported only two cases, which presented a poorly defined radiolucent lesion occurring in the maxillary jaw involving the nasal fossa, nasal and paranasal sinuses, as well as the orbit.13 In cases of large AM or AOT, similar involvement of nasal and maxillary sinuses with poorly defined lesions has been reported.27 The slow, painless clinical course of the lesion as well as the thin and porous maxillary bone might be the factors that lead to lesions’ extensive, poorly defined radiolucency.29
Multilocularity was observed in only 2 out of 8 cases reported by Adorno-Farias et al.24 It is observed in the radiographic images of AM, wherein the tumour exhibits the septae of bone extending into the radiolucent tumour mass.30 In three cases, radiopaque foci were also displayed within the unilocular radiolucency in the mandible. In all these cases, ghost cells and dystrophic calcifications were noted on the histopathologic examination for focal radiopacities.10,11,17
Furthermore, the histopathology of the AM component of the tumour revealed that nine cases had a predominant plexiform pattern of ameloblast-like cell proliferation, nine cases exhibited a follicular pattern, and another nine cases comprised a mixture of both patterns. The follicular and plexiform histopathological patterns in isolated and mixed forms are similar in cases of AM, which was similar to the current findings.31 Only two cases had UAM associated with AOT, suggesting that most AADs are associated with solid/multicystic AM.
Desmoplastic changes are infrequently noted in ameloblastoma owing to the loss of expression of notch receptors representing an early stage of cell differentiation.32,33 This phenomenon is observed in AAD cases, wherein only one case, reported by Salehinejad et al., presented features of desmoplastic AM.20 In addition to the desmoplastic changes in the stroma, the lesion also comprised of large amounts of granular cells. Granular cell ameloblastoma is a rare subtype of ameloblastoma, in which granular cells are located in the centre of the follicles.34 Thus, only one case of granular cells occurring centrally within the ameloblastic follicles and desmoplastic changes within the connective tissue stroma in AAD has yet been reported.20
Squamous metaplasia occurring within the central stellate reticulum-like cells of AM follicles is an occasional histological finding termed ‘acanthomatous ameloblastoma’.27 Squamous metaplasia was observed in all the eight cases reported by Adorno-Farias et al. Amongst them, seven lesions occurred in the mandibular jaw, whereas the clinical details of one case were unreported.24 The current findings were in accordance with the inference of Bansal et al., which stated that the occurrence of squamous metaplasia is common in AM cases occurring in the mandible than maxilla.35
The other component associated with AM in the case of AAD is that of AOT. The biological mechanism underlying this mixture is yet to be elucidated. The transformation from one lesion to another seems to be a possible pathogenic mechanism.36 The term ‘adenomatoid’ is derived from ‘adḗn’, which means gland, and ‘-oma’, which means swelling or tumour. The peculiar feature of AOT that led to the derivation of the term is the presence of duct-like structures lined by cuboidal or columnar epithelial cells.26 Previous studies on the retention of extracellular matrix molecules in the duct-like structures of AOT suggested a key role of Osteonectin in the formation and maintenance of duct-like architecture.37 Duct-like structures were detected in the histopathological images of 19 out of 29 reported AAD cases in this study.
In addition to these duct-like structures, areas comprising the odontogenic epithelial cells proliferating in the form of sheets, cords, trabeculae and whorls are also observed in AOT in 18 cases. Another characteristic feature noted in AOT is the formation of nests or rosette-like structures by odontogenic epithelial cells.19 Herein, rosette-like structures were noted in only a few cases (n = 6); thus, their presence was not considered a definite criterion for the diagnosis of AAD.
An associated extracellular homogenous dentinoid material of varying amounts is invariably present in addition to the AM and AOT components in AAD. Dentinoid is defined as a non-mineralised tissue, which is collagenous in nature and intimately associated with odontogenic epithelium.38 The earliest interpretation of the eosinophilic material in AAD as dentinoid was proposed by Slabbert et al. in 1992.6 The study found that the material was positively stained for collagen via Van Gieson and Mason’s trichrome staining, negatively for amyloid staining by Congo Red and negatively for keratin by formic acid Alcian blue stain. The interpretation of the collagenous nature of dentinoid in AAD was further supported by Sonone et al. via positive Van Gieson staining and negative Congo Red staining.17 Since collagen and bone are also primarily constituted of collagen fibres, the dentinoid material is controversially considered as bone globules or cementum.26,27
The formation of dentinoid material in epithelial tumours is a result of a metaplastic process rather than epithelial–ectomesenchymal interaction. This phenomenon could be attributed to the gene products usually present in normal ectomesenchymal cells and the ameloblast-like cells of mixed odontogenic tumours.39 The outcome was the conversion of epithelial cells by subsequent interaction and co-expression of the mesenchymal phenotype. Thus, the neoplastic epithelial cells committed to ameloblastic differentiation could produce extracellular material of variable composition in a few tumours.23 The lesions represent various directions for tumour differentiation, based on the initial inductive stimulus, the degree of odontogenesis prior to the stimulus and the variation in the metaplastic process.40 The clinicopathological significance and prognostic value of the dentinoid material in AAD have not yet been determined and warrants further study.
The formation of dentinoid material has also been described in some malignant odontogenic neoplasms, such as primary intraosseous odontogenic carcinoma and odontogenic carcinoma.41 The presence of cellular atypia in concomitance with other signs of malignancy in AAD may render ameloblastic carcinoma (AMCA) or odontogenic carcinoma as an appropriate diagnosis. Furthermore, the adenoid or duct-like structure might also be present in AMCA. However, the enamel organ-like structures or buds are not observed in odontogenic carcinoma with dentinoid.42 Cellular atypia and abnormal mitosis were described in only one case of AAD reported by Khalele and Al-Shiaty.21 Presumably, the possibility of AAD with features of malignancy to be diagnosed as AMCA further adds to the challenge of acknowledging the exact frequency of reported cases of AAD. The case reports of these malignant neoplasms with dentinoid emphasising the cellular morphologies rather than the glandular component might have been missed, which pose as a limitation to the present review strategy.
Along with dentinoid, other types of extracellular materials have been identified in various studies. Matsumoto et al. reported that some of the cystic or duct-like spaces in AAD were positive for Alcian blue and Mucicarmine staining.8 Yamazaki et al. found certain areas of amyloid-based extracellular material indicated by positive Congo Red staining.11 Adorno-Farias et al. demonstrated pseudoducts with PAS-positive material.24 Loyola et al. stated that although basophilic mucoid material may be observed within the duct-like spaces, secretory component was not detected.13 In the most recent case of AAD reported by Arruda et al., Alcian blue staining revealed a significant amount of basophilic material and scarce PAS-positive eosinophilic material in duct-like spaces, indicating its mucoid nature.25
Nine cases of AAD consisted of clear cells in a varied proportion of the tumour cell population. Clear cells may be noted in tumours of the head and neck and could be a resultant product due to artifacts of fixation, lack of cell organelles and intracellular accumulation of various substances, such as glycogen, mucin, lipids, tonofilaments and immature zymogen granules.43 The clear cell changes could be attributed to tumour progression or secondary to clonal expansion.44 Furthermore, the population of neoplastic cells comprising the OTs is derived from the dental lamina, which appears to be clear in routine HE-stained sections due to the abundance of glycogen content.45
Ghost cells were detected in four reported cases of AAD. The exact nature of ghost cells remains controversial; however, these cells might be the product of abortive enamel matrix or aberrant keratin formation.46,47 The presence of variable dysplastic material along with ghost cells reflects varied productive or inductive potentiality resulting from prosoplasia of the odontogenic epithelium.48 The ghost cells are common in other odontogenic tumours, such as calcifying epithelial odontogenic tumour (CEOT), and should be differentiated from AAD as they do not comprise the adenoid areas.
Regarding the origin of cells in a tumour and subsequent diagnosis, various biomolecules are identified by immunohistochemistry (IHC).49 The odontogenic tumours, particularly odontogenic epithelial cells, are associated with various biomarkers owing to complex genetic and epigenetic factors involved in their differentiation.50 The expression of IHC markers assessed in the reported cases of AAD has been summarised [Table 4]. The most employed markers for IHC differentiation of epithelial cells are known as cytokeratins (CKs). CKs comprise a group of at least 20 polypeptides constituting specific intermediate filaments of epithelial cells. The various epithelia or carcinoma associated with these CKs are characterised by a specific pattern of polypeptides.51
Table 4.
Summary of immunohistochemical markers in reported cases of adenoid ameloblastoma with dentinoid (N = 29)
| Marker | Expression | Total | |
|---|---|---|---|
| Positive | Negative | ||
| CK14 | 6 | 8 | 14 |
| CK19 | 12 | 3 | 15 |
| CK7 | 0 | 8 | 8 |
| CK8/18 | 3 | 2 | 5 |
| CK17 | 1 | 0 | 1 |
| Calretinin | 4 | 0 | 4 |
| Ki-67 | (Low) 10 | (High) 5 | 15 |
| P53 | (High) 1 | (Low to negative) 8 | 9 |
| IMP3 | 0 | 8 | 8 |
CK14 has been identified as the primary, intermediate filament of odontogenic epithelium, present in the dental lamina, reduced enamel epithelium, duct-like structures of AOT, and in almost all the cells of the enamel organ associated with the secretory activity of the odontogenic epithelial cells.52 Strong immunopositive staining for CK14 was observed in several central and peripheral cells of tumour islands and the adenoid structures and surrounding cells in 6 out of 8 cases, as reported by Adorno-Farias et al.24 The negative expression of CK14 suggests the regions of advanced amelogenesis with the loss of cellular secretory activity, indicating the protective stage of amelogenesis.52
Strong and diffuse positive immunostaining for CK19 staining was observed in 12 out of 15 cases of AAD. CK19 is homogenously expressed in the stellate reticulum-like cells, peripheral preameloblast-like cells, areas of squamous metaplasia, some cells of the adenoid structures and areas with whorled appearance.11,13,24 It has been hypothesised that CK19 characterises ameloblasts and preameloblasts with complete differentiation. The negative immunoexpression of the molecule implied that stimuli could not activate the final differentiation process in these tumoral cells.52
CK7 has been identified in the epithelial cells of Hertwig’s epithelial root sheath and weakly in the stellate reticulum cells and dental lamina near the enamel organ.53 The strong expression in odontogenic cysts and tumours, such as glandular odontogenic cyst and CEOT, confirmed their origin from Hertwig’s epithelial root sheath cells. However, it is not expressed in tumours, such as ameloblastoma, which develop from the enamel organ.54 All the eight cases of AAD in the series reported by Adorno-Farias et al. were found to be IHC-negative for CK7.24
Although CK8/18 is present in the simple epithelium, such as ductal cells, its positive expression has been demonstrated in cases involving dysplastic epithelia, including leukoplakia and oral squamous cell carcinoma.55 The study by Wato et al. on the expression of cytokeratins in the variants of AM identified it as a component in plexiform ameloblastoma.56 The weakly positive staining of CK8/18 has also been reported previously in the epithelial cells of the ductal component in AOT.57 Furthermore, CK8/18 was found to be focally positive in 3 out of 5 cases of AAD reported by Loyola et al.13 Similarly, CK17 involved in carcinomas of stratified squamous epithelia constitutes a component of CKs in AM.56,58 Moreover, CK17 expression in AAD was detected only in one case by Yamazaki et al., wherein it was focally positive in cells containing the AM, but not the AOT component.11
The expression of calretinin, a 29-kDa calcium-binding protein, has been demonstrated in AM but not in the other types of odontogenic cysts.59 Although the underlying biological mechanism is not yet known, calretinin acts as a mediator of intracellular calcium ion signalling, i.e. a secondary messenger intervening in cellular proliferation and differentiation.60 It is also considered as a specific IHC marker for neoplastic ameloblastic epithelium, which is expressed only in AM and in odontogenic keratocyst but not in AOT.61,62 In corroboration with these findings, the IHC expression of calretinin was investigated in all AAD cases (n = 4); subsequently, focal but intense positive immunoexpression, limited to the cells in the AM component of the tumour, was noted.
In addition to the biomolecules that aid in identifying the origin or type of cells in question, specific markers indicate the proliferative activity of the cells. The expression of the human Ki-67 protein is analysed and evaluated to assess the proliferative activity in a lesion.63 The fraction of Ki-67-positive tumour cells, commonly known as Ki-67 labelling index, determines the fraction of a given cell population in the active growth phase and is often correlated with the aggressiveness of any lesion.64 Ki-67 expression was variable in AAD cases, wherein it was borderline to low in the majority of the reported cases (n = 10).11,20–25 However, the mean value of proliferative index as assessed by Ki-67 positive cells (72.4 ± 24.9 positive cells per high-power field) in the five cases of AAD reported by Loyola et al. was found to be higher than AOT and AM and was closer to that observed in AMCA. They inferred that the higher Ki-67 indices in AAD were reflective of its inherent aggressive biological nature.13
p53 is also a routinely employed proliferative marker for malignancy and acts as a regulatory checkpoint in the cell cycle.65 Normal cellular levels of wild-type p53 protein are low, and their half-life is short.66,67 Mutant p53 products have a retarded degradation and elevated stability that contributes to their nuclear accumulation.68 Thus, mutant p53 proteins are detected by IHC, rendering positive nuclear-staining signals, and have been frequently associated with malignant tumours. p53 expression was low to negative in all the cases (n = 8) reported by Adorno-Farias et al.24 The study also concluded that the lesion could be differentiated from AMCA, since the latter has a high p53 expression. However, in the case reported by Khalele et al., the lesion exhibited a strong positive expression for p53, indicating a high proliferation potential of AAD, and thus a prolonged interval of follow-up is essential in such cases.21
IGF-2 mRNA binding protein 3 (IMP3) is a post-transcriptional regulatory factor involved in embryonic development, and its aberrant expression has been associated with oncogenesis.69 IMP3 was not expressed in any of the cases (n = 8) reported by Adorno-Farias et al., which ruled out the carcinomatous nature of the lesion.24 Overall, AAD could be deemed less aggressive than AMCA because of negative staining for p53, IMP3 and low expression of Ki-67 in most cases.
The primary purpose of employing an IHC panel inclusive of proliferative markers is to correctly identify the nature of the lesion and subsequently determine the prognosis of the lesion. Once the prognosis of the lesion is determined, the surgeon can confidently decide the treatment plan. Despite low proliferative indices on IHC analysis, multiple recurrences of the lesion were reported in more than 50% of cases of AAD, including post-surgical follow-up of the patients (n = 10). Amongst these, seven cases showed occurrence in the maxilla. The reason for recurrence in most of the maxillary AADs has been suggested as the inability to achieve a complete excision with adequate margin in maxilla, owing to porous structures with high vascularity within which the lesion infiltration makes removal of all the neoplastic cells rather challenging.13 Another reason for the aggressive nature and recurrence of AAD suggested by Khalele and Al-Shiaty was the inherent aggressive biological potential of the lesion, as indicated by strong immunoexpression of p53 protein.21
The tendency to aggressively invade the local structures in AM has been attributed to the degradation of extracellular matrix, resulting from an increase in matrix metalloproteinases and receptor activator of nuclear factor-kappa B ligand along with increased mobility of neoplastic cells due to loss of syndecan-1.70 On the other hand, AOT is clinically well-contained. The lack of direct contact of neoplastic epithelium with the adjacent bone tissue and induction of reactive bone formation by Osteonectin have been suggested as factors responsible for limited destruction in AOT.70 Thus, additional studies are essential to establish a correlation between the expression of these molecular markers and the prognosis of the lesion in AAD.
In only seven cases, there was no evidence of disease on post-surgical follow-up. However, the follow-up period was 1–3 years in most of the cases, while AAD is known to recur even after nine years of treatment.9 The maximum number of recurrences in a single case of AAD was reported by Loyola et al., wherein the lesion had recurred nine times.13 Thus, it can be estimated that AAD has a recurrence rate of ≥75%, although the precise rate could not be determined owing to the lack of post-treatment follow-up in almost 33% (n = 12) of reported cases and the paucity of the available literature of the lesion. Moreover, most of the recurrences were due to the underdiagnosis of the lesion as AOT and subsequent conservative treatment. The reason for the tendency of AAD to be misdiagnosed could be attributed to the predominance of AOT-like areas in the histopathological image, which might overshadow the AM areas, thereby leading to a benign diagnosis and conservative treatment.13
Multiple recurrences of the lesion following wide excision suggested the lesion should be treated aggressively.8,9,13,16 All the cases treated with surgical resection had no evidence of recurrence for a variable follow-up period from six months to nine years. Also, the recurrences after the surgical resection of the lesion in the absence of disease until the time of the report except in one case wherein the patient had another recurrence.13 Thus, surgical resection could be deemed appropriate treatment for AAD cases, while in cases involving maxillary sinus and floor of the orbit or those recurring even after excision, radiotherapy with radical neck dissection may be preferred.13 In the case of AAD with UAM component, simple wide excision of the lesion was sufficient with no evidence of disease after a one-year follow-up, although evaluation of outcomes with prolonged follow-up period in more such cases is warranted.22
Conclusion
AAD is a rare hybrid odontogenic tumour with less than 30 cases reported to date. The lesion may occur at any age and commonly presents as an asymptomatic swelling in the mandible. Histopathologically, the lesion might vary due to follicular, plexiform, or mixed AM or UAM in conjunction with whorls of epithelial islands, duct-like structures, and infrequently, rosette-like structures of AOT along with a dentinoid component. Other features, such as granular cells, clear cells, ghost cells and desmoplasia, are seldom noted in AAD.
Furthermore, the lesion is frequently misdiagnosed as AM or AOT, and the individual entities composing the lesions owing to the abundance of either component in an incisional biopsy overshadows the other component. This leads to underdiagnosis of the lesion as AOT in several instances, and subsequent conservative treatment results in recurrence. Thus, it is imperative to identify the features of each component in the histopathological specimens of the odontogenic tumour to rule out such hybrid tumours.
Although molecular studies suggest that the lesion is relatively benign compared to AMCA, its aggressive clinical involvement cannot be overlooked as it has been reported to involve both sides of the jaws and extend to paranasal sinuses and orbital floor. Multiple recurrences following wide excision of the lesion indicated that the lesion should be treated aggressively, placing it at the aggressive end of the spectrum of benign odontogenic lesions. Therefore, an accurate diagnosis of the lesion to determine the treatment plan and the subsequent prognosis is imperative.
With the increasing number of cases reported in the last decade, AAD may be included as a distinct odontogenic neoplasm in the future. Consequently, a large number of AAD cases could be reported in the forthcoming future owing to an increase in the available literature on hybrid odontogenic tumours. This would provide clarity to the surgeons and pathologists regarding the diagnosis, management and prognosis of the entity. Moreover, future research on the genetic aspects of the tumour could elucidate the pathogenesis of AAD.
Footnotes
AUTHORS’ CONTRIBUTION
SS, TC, MS and YA conceptualised and defined the work. SS and TC conducted the initial literature review and devised the basic search strategy. MS, AS and AG modified the search strategy. All the authors contributed to performing the search and collecting data from the eligible articles. SS, TC, MA and YA analysed and interpreted the data. SS, TC and AS drafted the manuscript, while critical revision of the manuscript was done by MS and AG. All authors approved the final version of the manuscript.
References
- 1.Ruch JV, Lesot H, Begue-Kim C. Odontoblast differentiation. Int l Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- 2.Fauchard P. Tome 1-2. Paris: Pierre Jean Mariette; 1746. Le chirurgien dentiste, ou traité des dents. [Google Scholar]
- 3.Barnes L, Everson WJ, Reichart P, Sidradinsky D. Pathology and Genetics: Head and Neck Tumors. 5th ed. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Ide F, Horie N, Shimoyama T, Sakashita H. So-called hybrid odontogenic tumors: Do they really exist? Oral Med Pathol. 2001;6:13–21. doi: 10.3353/omp.6.13. [DOI] [Google Scholar]
- 5.El Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. Lyon: IARC; 2017. [Google Scholar]
- 6.Slabbert H, Altini M, Crooks J, Uys P. Ameloblastoma with dentinoid induction: Dentinoameloblastoma. J Oral Pathol Med. 1992;21:46–8. doi: 10.1111/j.1600-0714.1992.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 7.Brannon RB. Adenoid ameloblastoma with dentinoid. Washington (DC): Armed Forces Institute of Pathology; 1994. pp. 1–94. [Google Scholar]
- 8.Matsumoto Y, Mizoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: Adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30:251–4. doi: 10.1034/j.1600-0714.2001.300410.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:583–8. doi: 10.1016/j.tripleo.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Chen YU, Geng N, Bao D, Yang M. A case report of a hybrid odontogenic tumour: Ameloblastoma and adenomatoid odontogenic tumour in calcifying cystic odontogenic tumour. Oral Oncol Extra. 2006;42:287–90. doi: 10.1016/j.ooe.2006.07.003. [DOI] [Google Scholar]
- 11.Yamazaki M, Maruyama S, Abé T, Babkair H, Fujita H, Takagi R, et al. Hybrid ameloblastoma and adenomatoid odontogenic tumor: Report of a case and review of hybrid variations in the literature. Oral Surg Oral Med Oral Pathol Radiol. 2014;118:12–18. doi: 10.1016/j.oooo.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Jivan V, Altini M, Meer S, Mahomed F. Adenomatoid odontogenic tumor (AOT) originating in a unicystic ameloblastoma: A case report. Head Neck Pathol. 2007;1:146–9. doi: 10.1007/s12105-007-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loyola AM, Cardoso SV, de Faria PR, Servato JP, Eisenberg AL, Dias FL, et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:368–77. doi: 10.1016/j.oooo.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Vickers RA, Gorlin RJ. Ameloblastoma: Delineation of early histopathologic features of neoplasia. Can. 1970;26:699–710. doi: 10.1002/1097-0142(197009)26:3<699::aid-cncr2820260331>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi-Moridani S, Yazdi I. Adenoid ameloblastoma with dentinoid: A case report. Arch Iran Med. 2008;11:110–12. https://doi.org/08111/AIM.0021 . [PubMed] [Google Scholar]
- 16.Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: A selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3:18–26. doi: 10.1007/s12105-009-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonone A, Hande A, Chaudhary M, Bonde R, Sheorain A, Agni N. Adenoid ameloblastoma with dentinoid and ghost cells. A composite odontogenic tumour: A rare case report and review of the literature. Oral Surg. 2011;4:77–81. doi: 10.1111/j.1752-248X.2010.01109.x. [DOI] [Google Scholar]
- 18.Saxena K, Jose M, Chatra LK, Sequiera J. Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol. 2012;16:272–76. doi: 10.4103/jomfp.JOMFP_53_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar K, Shetty DC, Wadhwan V, Dhanapal R, Singh HP. Dentinoameloblastoma with ghost cells: A rare case report with emphasis on its biological behavior. Dent Res J. 2013;10:103–7. doi: 10.4103/1735-3327.111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salehinejad J, Gholami M, Eshghpour M, Mehri T. An infrequent histopathological subtype of ameloblastoma: Adenoid granular cell ameloblastoma with dentinoid. Dent Res J. 2016;13:376–8. doi: 10.4103/1735-3327.187878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalele BAO, Al-Shiaty RA. Adenoid ameloblastoma with dentinoid and cellular atypia: A rare case report. Ital J Med. 2016;10:238–40. doi: 10.4081/itjm.2016.639. [DOI] [Google Scholar]
- 22.Sathyanarayana VK, Srigiri H, Cheemalavagupalli M, Vankadara S, Malika G. A rare case of adenomatoid odontogenic tumour with unicystic ameloblastoma. J Clin Diagnostic Res. 2017;11:5–6. doi: 10.7860/JCDR/2017/23623.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai HK, Pai SM, Dayakar A, Supriya H. Adenoid ameloblastoma with dentinoid: A rare hybrid variant. J Oral Maxillofac Pathol. 2017;21:319. doi: 10.4103/jomfp.JOMFP_53_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adorno-Farias D, Muniz VR, Soares AP, Cury PR, Rabelo RG, Fernández-Ramires R, et al. Ameloblastoma with adenoid features: A series of eight cases. Acta Histochem. 2018;120:468–76. doi: 10.1016/j.acthis.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Arruda JAA, Noronha MS, Abreu LG, et al. Adenoid ameloblastoma in the posterior maxilla: A case report and review of the literature. Oral Maxillofac Surg. 2020;24:243–9. doi: 10.1007/s10006-020-00830-1. [DOI] [PubMed] [Google Scholar]
- 26.Reichart P, Philipsen H. Odontogenic Tumors and Allied Lesions. London, UK: Quintessence Publishing Co Ltd; 2004. pp. 41–116. [Google Scholar]
- 27.Sivapathasundharam B, Rajendran R. Sivapathasundharam B, Ed Shafer’s Textbook of Oral Pathology E-book. 8th ed. India: Elsevier Health Sciences; 2020. Odontogenic tumors; pp. 100–15. [Google Scholar]
- 28.Ueno S, Nakamura S, Mushimoto K, Shirasu R. A clinicopathologic study of ameloblastoma. J Oral Maxillofac Surg. 1986;44:361–5. doi: 10.1016/s0278-2391(86)80031-3. [DOI] [PubMed] [Google Scholar]
- 29.Iordanidis S, Makos CH, Dimitrakopoulos J, Kariki H. Ameloblastoma of the maxilla. Case report. Aus Dent J. 1999;44:51–5. doi: 10.1111/j.1834-7819.1999.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 30.White S, Pharaoh M, editors. Oral Radiology – Principles and Interpretation. 6th ed. China: Mosby Elsevier; 2009. Odontogenic epithelial tumors; pp. 373–85. [Google Scholar]
- 31.Nagi R, Sahu S, Rakesh N. Molecular and genetic aspects in the etiopathogenesis of ameloblastoma: An update. J Oral Maxillofac Pathol. 2016;20:497. doi: 10.4103/0973-029x.190954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philipsen HP, Reichart PA, Takata T. Desmoplastic ameloblastoma (including “hybrid” lesion of ameloblastoma). Biological profile based on 100 cases from the literature and own files. Oral Oncol. 2001;37:455–60. doi: 10.1016/s1368-8375(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 33.Siar CH, Nakano K, Han PP, Nagatsuka H, Ng KH, Kawakami T. Differential expression of Notch receptors and their ligands in desmoplastic ameloblastoma. J Oral Pathol Med. 2010;39:552–8. doi: 10.1111/j.1600-0714.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 34.Gardner DG, Heikinheimo K, Shear M, Philipsen HP, Coleman H. Ameloblastoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumors, Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. pp. 296–300. [Google Scholar]
- 35.Bansal M, Chaturved TP, Bansal R, Kumar M. Acanthomatous Ameloblastoma of anterior maxilla. J Ind Soc Pedodont Preventive Dent. 2010;28:209–11. doi: 10.4103/0970-4388.73797. [DOI] [PubMed] [Google Scholar]
- 36.Yoon JH, Kim HJ, Yook JI, Cha IH, Ellis GL, Kim J. Hybrid odontogenic tumour of calcifying odontogenic cyst and ameloblastic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:80–4. doi: 10.1016/j.tripleo.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Murata M, Cheng J, Horino K, Hara K, Shimokawa H, Saku T. Enamel proteins and extracellular matrix molecules are colocalized in the pseudocystic stromal space of adenomatoid odontogenic tumor. J Oral Pathol Med. 2000;29:483–90. doi: 10.1034/j.1600-0714.2000.291002.x. [DOI] [PubMed] [Google Scholar]
- 38.Gardner DG, Farquhar DA. A classification of dysplastic forms of dentin. J Oral Pathol. 1979;8:28–46. doi: 10.1111/j.1600-0714.1979.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 39.Papagerakis P, Peuchmaur M, Hotton D, Ferkdadji L, Delmas P, Sasaki S, et al. Aberrant gene expression in epithelial cells of mixed odontogenic tumors. J Dent Res. 1999;78:20–30. doi: 10.1177/00220345990780010201. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Takigawa T, Owo M, Matsumoto H, Fukumoto W. A case of malignant transformation of calcifying of odontogenic cyst. Jpn J Oral Maxiillofac. 1975;21:664. [Google Scholar]
- 41.Punnya A, Kumar GS, Rekha K, Vandana R. Primary intraosseous odontogenic carcinoma with osteoid/dentinoid formation. J Oral Pathol Med. 2004;33:121–4. doi: 10.1111/j.1600-0714.2004.00029.x. [DOI] [PubMed] [Google Scholar]
- 42.Mariano FV, Gondak RO, Scarini JF, da Silva EC, Caravina G, Scapulatempo-Neto C, Almeida OP, Altemani A, Mosqueda Taylor A. Odontogenic carcinoma with dentinoid in long-term follow-up with 2 recurrences. Int J Surg Pathol. 2020;28:181–7. doi: 10.1177/1066896919871662. [DOI] [PubMed] [Google Scholar]
- 43.Maiorano E, Altini M, Favia G. Clear cell tumors of the salivary glands, jaws and oral mucosa. Sem Diagnostic Pathol. 1997;14:203–12. [PubMed] [Google Scholar]
- 44.Nappi O, Mills S, Swanson P, Wick M. Clear cell tumours of unknown nature and origin: A systemic approach to diagnosis. Sem Diagnostic Pathol. 1997;14:164–74. [PubMed] [Google Scholar]
- 45.Jain A, Shetty DC, Juneja S, Narwal N. Molecular characterization of clear cell lesions of head and neck. J Clin Dent Res. 2016;10:ZE18. doi: 10.7860/JCDR/2016/14394.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M, Kumamoto H, Ooya K, Mayanagl H. Histopathological and immunohistochemical analysis of calcifying odontogenic cysts. J Oral Pathol. 2001;30:582–8. doi: 10.1034/j.1600-0714.2001.301002.x. [DOI] [PubMed] [Google Scholar]
- 47.Hong SP, Ellis GL, Hartman KS. Calcifying odontogenic cyst: A review of ninety-two cases with reevaluation of their nature as cysts or neoplasms, the nature of ghost cells, and subclassification. Oral Surg Oral Med Oral Pathol. 1991;72:56–64. doi: 10.1016/0030-4220(91)90190-n. [DOI] [PubMed] [Google Scholar]
- 48.Mankapure PK. Ghost cells and its histogenesis: A narrative review. IJSS Case Reports & Reviews. 2015;2:35–9. [Google Scholar]
- 49.Hornick JL. Novel uses of immunohistochemistry in the diagnosis and classification of soft tissue tumors. Modern Pathol. 2014;27:S47–63. doi: 10.1038/modpathol.2013.177. [DOI] [PubMed] [Google Scholar]
- 50.Sandoval-Basilio J, González-González R, Bologna-Molina R, Isiordia-Espinoza M, Leija-Montoya G, Alcaraz-Estrada SL, et al. Epigenetic mechanisms in odontogenic tumors: A literature review. Arch Oral Biol. 2018;87:211–7. doi: 10.1016/j.archoralbio.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 52.Crivelini MM, De Araújo VC, De Sousa SO, De Araújo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003;9:1–6. doi: 10.1034/j.1601-0825.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 53.Gratzinger D, Salama ME, Poh CF, Rouse RV. Ameloblastoma, calcifying epithelial odontogenic tumor, and glandular odontogenic cyst show a distinctive immunophenotype with some myoepithelial antigen expression. J Oral Pathol Med. 2008;37:177–84. doi: 10.1111/j.1600-0714.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Martínez M, Mosqueda-Taylor A, Carlos-Bregni R, Pires FR, Delgado-Azañero W, Neves-Silva, et al. Comparative histological and immunohistochemical study of ameloblastomas and AMCAs. Med Oral Pathol Oral Cir. Bucal. 2017;22:e324–32. doi: 10.4317/medoral.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gires O, Mack B, Rauch J, et al. CK8 correlates with malignancy in leukoplakia and carcinomas of the head and neck. Biochem Biophys Res Commun. 2006;343:252–9. doi: 10.1016/j.bbrc.2006.02.139. [DOI] [PubMed] [Google Scholar]
- 56.Wato M, Chen Y, Fang YR, He ZX, Wu LY, Bamba Y, Hida T, Hayashi H, Ueda M, Tanaka A. Immunohistochemical expression of various cytokeratins in ameloblastomas. J Oral Med Pathol. 2006;11:67–74. doi: 10.3353/omp.11.67. [DOI] [Google Scholar]
- 57.Larsson Å, Swartz K, Heikinheimo K. A case of multiple AOT-like jawbone lesions in a young patient–a new odontogenic entity? J Oral Pathol Med. 2003;32:55–62. doi: 10.1034/j.1600-0714.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 58.Cohen-Kerem R, Madah W, Sabo E, et al. Cytokeratin-17 as a potential marker for squamous cell carcinoma of the larynx. Ann Otol Rhinol Laryngol. 2004;113:821–7. doi: 10.1177/000348940411301008. [DOI] [PubMed] [Google Scholar]
- 59.Mosqueda-Taylor A. New findings and controversies in odontogenic tumors. Med Oral Patol Oral Cir Bucal. 2008;13:E555–8. [PubMed] [Google Scholar]
- 60.Sundaragiri SK, Chawda J, Gill S, Odedra S, Parmar G. Calretinin expression in unicystic ameloblastoma: An aid in differential diagnosis. J Oral Biosci. 2010;52:164–9. doi: 10.1016/s1349-0079(10)80046-5. [DOI] [Google Scholar]
- 61.Koneru A, Hallikeri K, Nellithady GS, Krishnapillai R, Prabhu S. Immunohistochemical expression of calretinin in ameloblastoma, adenomatoid odontogenic tumor, and keratocystic odontogenic tumor. Appl Immunohistochem Mol Morphol. 2014;22:762–7. doi: 10.1097/PAI.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 62.Devilliers P, Liu H, Suggs C, Simmons D, Daly B, Zhang S, et al. Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol. 2008;32:256–60. doi: 10.1097/pas.0b013e3181452176. [DOI] [PubMed] [Google Scholar]
- 63.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(sici)1097-4652(200003)182:3<311::aid-jcp1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Ueda T, Aozasa K, Tsujimoto M, Ohsawa M, Uchida A, Aoki Y, Ono K, Matsumoto K. Prognostic significance of Ki-67 reactivity in soft tissue sarcomas. Cancer. 1989;63:1607–11. doi: 10.1002/1097-0142(19890415)63:8<1607::aidcncr2820630827>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Hupp TR, Meek DW, Midgley CA, Lane DP. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–86. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 66.Montenarh M. Biochemical properties of the growth suppressor/oncoprotein p53. Oncogene. 1992;7:1673–80. [PubMed] [Google Scholar]
- 67.Oren M, Maltzman W, Levine AJ. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981;1:101–10. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083–93. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 69.Deforzh E, Vargas TR, Kropp J, Vandamme M, Pinna G, Polesskaya A. IMP-3 protects the mRNAs of cyclins D1 and D3 from GW182/AGO2-dependent translational repression. Int J Oncol. 2016;49:2578–88. doi: 10.3892/ijo.2016.3750. [DOI] [PubMed] [Google Scholar]
- 70.Modolo F, Biz MT, Martins MT, Machado de Sousa SO, De Araújo NS. Expression of extracellular matrix proteins in adenomatoid odontogenic tumor. J Oral Pathol Med. 2010;39:230–5. doi: 10.1111/j.1600-0714.2009.00846.x. [DOI] [PubMed] [Google Scholar]