Abstract
The field of paper-based microfluidics has experienced rapid growth over the past decade. Microfluidic paper-based analytical devices (μPADs), originally developed for point-of-care medical diagnostics in resource-limited settings, are now being applied in new areas, such as environmental analyses. Low-cost paper sensors show great promise for on-site environmental analysis; the theme of ongoing research complements existing instrumental techniques by providing high spatial and temporal resolution for environmental monitoring. This review highlights recent applications of μPADs for environmental analysis along with technical advances that may enable μPADs to be more widely implemented in the field.
Graphical Abstract
Recent outbreaks of food- and water-borne illnesses and sudden releases of toxic compounds into surface waters offer reminders of the importance of environmental monitoring for public health protection. 1-3 Efforts to advance pollution monitoring have led to the development of many instrumented techniques capable of detecting parts per million (ppm) to parts per trillion (ppt) analyte concentrations in diverse sample matrices. To meet the needs of the monitoring community – namely, high sensitivity and low detection limits – most analytic methods rely on expensive equipment that require a high level of training to operate reliably. As a result, few methods have been developed that enable rapid, in-field detection. Furthermore, sampling and measurement costs associated with environmental monitoring often limit the sample size of the measurement, which in turn constrains our ability to define spatial and temporal patterns of contaminant release, transport, and fate. Ultimately, this lack of measurement resolution (due largely to cost, timeliness, and deployment limitations) hinders decision-making.
There is a growing need for low-cost technologies that can detect and monitor environmental contaminants concentrations quickly, easily, and in-field to provide timely data regarding the extent and magnitude of pollution. An enhanced understanding of the source, transport, and persistence of environmental contaminants could help prevent both human illness and ecosystem damage. Low-cost microfluidic paper-based analytical devices (μPADs) offer an opportunity to address this need by increasing the frequency and geographic coverage of environmental monitoring while also reducing analytic costs and complexity of the measurement.
1. The Rise of Lab on Paper
Various definitions of “microfluidic” exist throughout the literature; however, one common theme involves manipulating small volumes of fluids within micrometer-scale channels in engineered devices (e.g., not simple tubing).4 Microfluidic devices include those that are considered micro total chemical analysis systems (μTAS), as well as more recent examples that range in capability from protein crystallization to point-of-care diagnostics.5-9 Chemically patterned paper was re-introduced in the last decade as an alternative to traditional microfluidic substrates (e.g. glass, silicon, and polymers) as a simple, low-cost platform. Paper and related porous hydrophilic materials offer many unique advantages over traditional microfluidic technologies such as power-free fluid transport via capillary action, high surface area to volume ratios for chemical reactions and detection, lightweight designs (~10 mg cm−2), and the capacity for storing reagents in active form within the fiber network.10, 11
Paper has played a significant role in chemical analysis for many years; noted examples include litmus paper, home pregnancy tests, sample filtration, and chromatography.12-16 As early as 23 to 79 A.D., paper saturated with extract from gallnuts was used to detect ferrous sulfate in verdigris, the blue-green patina that follows the oxidation of copper, brass, and bronze surfaces.17 In the twentieth century, paper sensors were developed for chromatographic and electrophoretic separations as well as metals detection.18, 19 In 2007, Martinez et al. reported the first μPAD for multiplexed chemical analysis.20 Such devices differ from traditional litmus paper or lateral-flow immunoassay methods in that chemical printing or cutting are used to define flow paths for conducting multiplexed analysis. For this review, we define a μPAD as a device in which microliter volumes of sample are manipulated through a fiberous network by capillary action and where flow paths are defined by impermeable barriers or where the paper has been cut to create flow channels. Although the earliest μPADs were developed for point-of-care clinical diagnostics, in recent years μPADs have been used in other fields including environmental science. One of the first reported applications of μPAD technology for an environmental sample was by Nie et al. in 2010 for electrochemical detection of Pb(II) and Zn(II).21 Since then, new μPADs have been reported for a diverse range of contaminants in soil, water, air, and food.
Sensing devices made from paper have been featured in several reviews on microfluidics and advances in point-of-care diagnostics.4, 22-27 The general science of μPADs including theory, fabrication techniques, applications, and detection modes has been discussed in detail in a number of recent reviews dedicated specifically to μPAD research.28-38 Other reviews have focused on applications for point-of-care medical diagnostics,39-44 electrochemical detection on paper,45, 46 and on μPADs as micro total analysis systems.28, 47, 48 To date, however, no review has focused exclusively on environmental applications, and this review seeks to address this important gap. We showcase multiple environmental applications of μPADs, categorized by analyte class. Next, the discussion is broadened to include recent trends in μPAD technology toward field deployment and how these developments might impact environmental monitoring. Finally, we revisit some of the challenges of employing μPADs for environmental monitoring and include a perspective on future directions in the field.
2. Applications
Analytes for environmental μPADs can be roughly grouped into three classes – inorganic (metals, non-metals such as phosphate), organic (small molecules, pesticides, etc), and biological (bacteria, etc) – or by specific applications (explosives, oxidative reactivity, etc), based on the literature published to date. For each analyte class or application presented below, a general overview is provided followed by an in-depth discussion of seminal works.
Analyte quantification with μPADs is commonly achieved using colorimetric (intensity/hue-, count-, distance-, or time-based), electrochemical, fluorescent, or electrochemiluminescent methods. Although colorimetric analysis is most common because of its simplicity, the method can suffer from low signal sensitivity and can be inadequate for point-of-need environmental analysis without additional sample preparation steps (e.g. analyte preconcentration, matrix simplification, etc). Low-cost fabrication methods for more sensitive electrochemical techniques (employing carbon paste or metal micro-wire electrodes) have opened the door for this technology to become a viable alternative to colorimetric detection on paper sensors.46 The sensitivity and specificity of electrochemistry on paper can compete with traditional benchtop techniques like UV-Vis spectroscopy, liquid chromatography, and inductively coupled plasma-mass spectrometry. Developing multiplexed tests may be key for electrochemical systems to find success in future commercial markets. However, one drawback of electrochemical sensing is the need for potentiostats, however small they may be , which can lead to increased costs for analysis relative to colorimetric systems read by the naked eye.
2.1. Metals
Human exposure to metals has been established as a contributor to morbidity and mortality, especially in regions lacking strict regulations for metal contamination of water, soil, and/or air.49, 50 Redox active metals like Fe, Cu, Cr, and Co possess the ability to generate free radicals that can generate oxidative stress in organisms, 51 while metals like Pb and Cd are well known neurotoxins.52, 53 Human exposure to metals is associated with many diseases, but ongoing efforts to identify exposure sources are hindered by the cost of measurement; routine analysis often exceeds $100 per sample, resulting in limited measurement campaigns. The spatial and temporal distribution of measurement can also be important for understanding the source (or spread) of a pollutant and for tracking its impact on people, wildlife, and the environment. After three million gallons of polluted mine waste containing Co, As, Ni, and Cr from the Gold King Mine was accidently released into the Animas River in Colorado (2015), reliable information regarding pollution levels was scarce for many days. At the time of the spill, little was known about the concentrations of the metals in the river, which generated a significant public outcry.2
Since 2010, metal quantification with paper-based sensors has attracted attention because colored metal-ligand complexes are easily discernable with the unaided eye and/or can be quantified inexpensively with other optical motifs (e.g. scanner or camera-phone). Additionally, much of the complexation chemistry is well-characterized.54 One of the first examples of μPAD-based quantification of metals was a sensor comprised of four detection zones for simultaneously measuring Fe, Cu, and Ni from medical incineration ash.55 Detection limits for this type of particulate matter ranged from 1-1.5 μg (total mass on device) for each analyte. Colorimetric detection of total Cr and Cr(VI) from ash and welding fume samples has also been reported in devices with similar architecture.56, 57 Paper devices for measuring Zn, Cu, Ag, Cd, Pb, Ni, Hg, and Cr(VI) colorimetrically have been developed with detection limits in the tens to hundreds of ppm using metal-to-ligand charge-transfer chemistry.58-70 A fluorogenic method for measuring Hg and Cr(III) has also been reported.71 In the absence of sample preconcentration, however, many colorimetric methods are limited to ppm-level detection limits, which can be inadequate for some point-of-need scenarios requiring ppb-level detection limits. Electrochemical detection, on the other hand, has demonstrated the capability to quantify metals in water at sub-ppb levels.21, 72
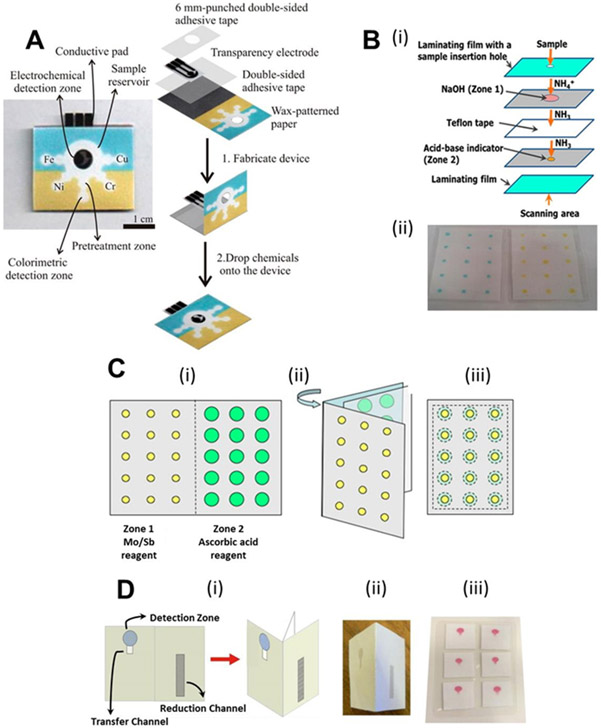
Hybrid μPADs that combine detection motifs, such as colorimetry and electrochemistry, are potentially advantageous because environmental contaminants are often present at concentrations that differ by several orders of magnitude through space and time; a single technique may not be suitable for measuring all analytes in the same matrix. 72 In a recent publication, Rattanarat et al.73 created a three-dimensional μPAD (Figure 1A) that combined colorimetric and electrochemical detection on separate layers for quantifying Ni, Cu, Fe, Pb, Cr(VI), and Cd using a small punch (10 mm diameter) taken from an air sampling filter. The technique was developed for contaminants present in airborne particulate matter. Sample flowed laterally on the top layer of the device in four segregated channels enabling colorimetric determination of Cu, Ni, Fe, and total Cr. These metals were measured colorimetrically because they are often present at higher (ppm) concentrations in the environment. Sample also flowed vertically to a second layer where Pb and Cd were quantified electrochemically. Separating the detection modes via multiple layers was necessary to minimize cross-contamination; distinct reaction chemistry (e.g. agent masking, pH adjustments) also enhanced analyte selectivity and sensitivity. For example, interference from Cu during anodic stripping voltammetry of Cd and Pb was minimized by adding a Cu complexing agent, ferricyanide, to the electrochemical layer without impeding the colorimetric detection of Cu in the top layer. Detection limits as low as 0.75 μg (15 ppm) for Fe, Ni, and Cu, 0.12 μg (2.4 ppm) for Cr(VI) and 0.25 ng (50 ppb) for Cd and Pb were reported.
Figure 1:
Examples of μPADs for inorganic species. (A) Three-dimensional hybrid colorimetric/electrochemical μPAD for simulataneously determining Fe, Ni, Cu, Cr, Cd, and Pb. Reproduced with permission from Ref. 73. Copyright 2014 American Chemical Society. (B) Multilayer device for quantifying ammonia using pH indicators. Adapted with permission from Ref. 93. Copyright 2015 American Chemical Society. (C) μPAD for measuring reactive phosphate; a removable sheet was placed between paper layers to improve shelf life. Adapted from Ref. 82 with permission from Elsevier. (D) μPAD for measuring nitrate and nitrite. Adapted with permission from Ref. 89. Copyright 2014 American Chemical Society.
2.2. Non-Metals
Health concerns associated with exposure to many non-metal inorganic compounds have led to environmental regulations and policies for establishing permissible exposure concentrations. For example, excess nitrogen and phosphorus in surface and groundwater are precursors to algal blooms (cyanobacterial toxins)74, 75 and conditions such as Methaemoglobinaemia (blue baby syndrome).76, 77 These inorganic contaminants are released into the environment as combustion byproducts, in agricultural runoff, and in other sample matrices from animal production facilities. Chloride and fluoride, which are intentionally added to drinking water for health reasons, can negatively impact human health if ingested concentrations are too high.78, 79 Cyanide, which is extremely toxic, is released into the environment from natural deposits, mining operations, orchards, biomass combustion, and waste streams from glass and electronics production.80, 81
A number of low-cost assays, including μPADs, have been developed for measuring inorganic compounds in the environment. Numerous μPAD papers have described the colorimetric determination of phosphate,82 nitrate,83-89 nitrite,83-91 ammonia,92, 93 arsenic,94 and cyanide.95 A paper device capable of performing an acid-base titration has been applied to the measurement of water pH in an acidic hot spring.96 Electrochemical detection of iodide,97 bromide,97 chloride,97-100 potassium,100 and ammonium100 has been demonstrated on a μPAD using potentiometry, where the potential difference between a reference and indicator electrode was indicative of analyte concentration. The majority of these publications only focus on the development of novel μPAD designs and benchtop fabrication techniques; few publications have reported on method validation in the field (or with real-world samples analyzed in the lab).
Jayawardane et al. published a series of papers describing novel approaches for detecting reactive ammonia/ammonium cation,93 phosphate,82 and nitrite/nitrate89 in water using three-dimensional μPADs. Sodium hydroxide was used to convert ammonium cations to ammonia, and a hydrophobic microporous Teflon membrane separated ammonia from the remaining ammonium cations by gaseous diffusion (Figure 1B). The ammonia content was quantified using the acid-base indicators 3-nitrophenol and bromothymol blue giving detection limits of 0.8 and 1.8 mg N L−1, respectively. The phosphate μPAD (Figure 1C) used phosphoantimonylmolybdenum blue complex and had a working detection range of 0.2–10 mg L∓1. Notable to this design was the inclusion of a PTFE or cellulose acetate sheet that was removed immediately prior to use for improving device shelf-life. The nitrate/nitrite μPAD (Figure 1D) was able to detect nitrite concentrations down to 1.0 μM using the Griess reaction. Total nitrate/nitrite levels were also quantifiable using zinc particles to reduce nitrate to nitrite. To their knowledge, this was the first application of solid-phase reagents in a modern μPAD. Each of these devices and their detection methodologies were tested with lab standards and environmental water and wastewater samples. The μPAD assays were also validated against traditional spectroscopic methods, ion chromatography, or flow injection analysis.
2.3. Organic Molecules
Exposure to environmentally persistent organic pollutants has numerous adverse health effects depending on the mechanism of action (e.g., xenoestrogenic, carcinogenic, mutagenic, etc.) or the impacted organs.101-103 Paper-based sensors have been reported for the detection of chemical warfare agents,104 recreational drugs,105 volatile organic compounds (VOCs),106-109 and phenolic compounds.110-113 Detection of chemical warfare agents and recreational drugs is more appropriately described as a forensic rather than an environmental application and will not be discussed here. Several μPAD procedures for the detection of VOCs have been published using both colorimetric106, 108, 109 and electrochemical detection.107 Notably, Soga et al. developed a colorimetric sensor capable of selectively discriminating volatile primary amines from other common VOCs.108
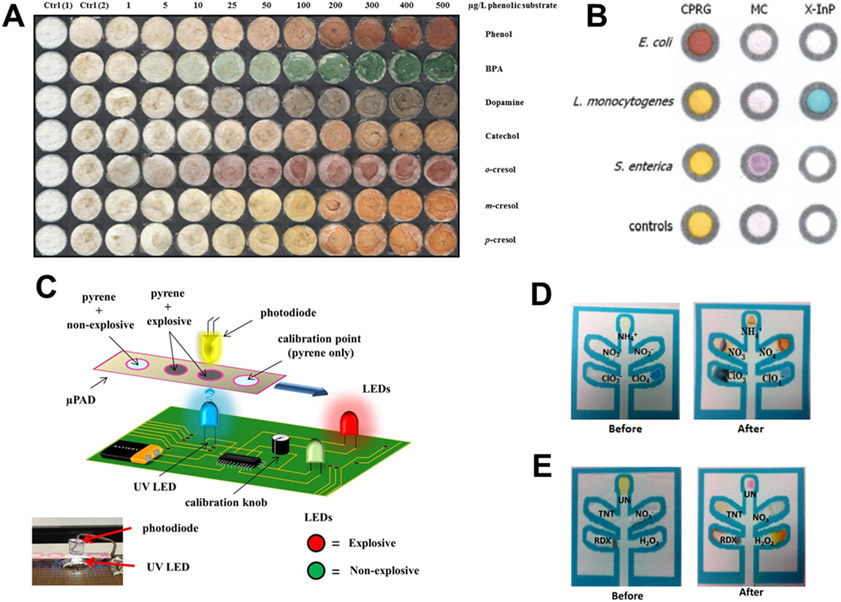
Although VOCs are environmentally important contaminants, μPADs for detecting these compounds are in the early stages of development. Phenolic compounds, on the other hand, have been more widely measured using both electrochemical111-113 and colorimetric113 detection techniques. Due to their potential bioaccumulation in the environment and their wide-ranging human and ecological health impacts, phenolic compounds pose a significant risk.114-117 The first reported μPAD for the detection of environmental phenolic compounds was published by Alkasir et al (Figure 2A).110 Their device was constructed using layer-by-layer deposition of chitosan, tyrosinase, and sodium alginate onto filter paper. The alginate and chitosan layers electrostatically trapped tyrosinase. The immobilized enzyme oxidized the aromatic phenol to a corresponding ketone which, in turn, selectively bound to the amino functionality of the chitosan layer producing a color change. The device was applied to the detection of phenol, bisphenol-A (BPA), m-cresol, p-cresol, catechol, and dopamine. Most reagents were identified by the formation of a red-brown product; however, addition of BPA results in a blue-green color. The number of layers, pH, and reagent amounts were optimized resulting in a detection limit of 0.86 ±1 μg L−1 for the phenolic compounds tested. Cross-reactivity was not observed for ascorbic acid, uric acid, and phenyalanine. Devices were tested with BPA spiked tap and river water samples to evaluate their effectiveness in real matrices. Furthermore, the device showed room temperature stability over 260 days. A scaled-up manufacturing method was presented in which the layer-by-layer deposition was achieved using inkjet printing. In a subsequent work,118 the authors presented a portable device using the same sensor to conduct field measurements of BPA in indoor dust. The device consisted of an air-sampler interfaced with a test zone containing interchangeable paper sensors. The procedure was validated alongside traditional gas chromatography methods. The detection limit for the sensor was 0.28 μg g−1 which is similar to the established United States Environmental Protection Agency reference dose of 50 μg kg−1 BW/day.118
Figure 2:
Examples of μPADs for organic species, bacteria, and explosive compounds. (A) Tyrosinase-based μPAD for detecting phenolic compounds. Reprinted with permission from Ref. 110. Copyright 2012 American Chemical Society. (B) Spot tests for three bacterial strains showing lack of interference. Reprinted with permission from Ref. 141. Copyright 2012 American Chemical Society. (C) Schematic of a handheld fluorescence detector used to measure fluorescence quenching of pyrene by explosive compounds. Reproduced from Ref. 147 with permission from The Royal Society of Chemistry. (D) Colorimetric μPAD for detecting inorganic explosives. Adapted from Ref. 150 with permission from The Royal Society of Chemistry. (E) Colorimetric μPAD for organic and high explosives. Adapted from Ref. 150 with permission from The Royal Society of Chemistry.
2.4. Pesticides
Pesticides are well-known toxins found in air, water, soil, food, and feed products.119 Exposure can occur via ingestion, inhalation, and dermal absorption and is associated with neurotoxicity, hepatotoxicity, renal toxicity, dermatitis, and cancer.120-122 Separation methods like gas and high-pressure liquid chromatography are commonly applied techniques for sensitive and selective determination of pesticides, but analysis is expensive and not well suited for on-site measurement, particularly in remote regions or for situations requiring rapid screening. Alternatively, μPADs offer a practical, cost-effective means of rapidly analyzing food products for pesticides.
Many μPAD-based efforts for identifying or detecting pesticides are based on the inhibition of acetylcholinesterase (AChE), an enzyme critical for controlling normal transmission of nerve impulses.123-125 Normally, acetylcholine is broken down by AChE into choline and acetic acid, but the presence of organophosphate, organophosphorus, and carbamate pesticides is inhibitory.126-128 Measuring AChE inhibition levels by pesticides has been accomplished using chemiluminescence,129, 130 electrochemistry,131 and colorimetry with immobilized organic molecules132-134 and semiconductor quantum dots.123, 135 Dichlorvos (2,2-dichlorovinyl dimethylphosphate), a highly toxic organophosphate pesticide widely used for crop protection, 136 has been measured with μPADs at levels as low as 0.8 ppb.129, 137 In 2009, Hossain et al.138 developed a multiplexed sensor for rapidly (~5 min) measuring acutely toxic organophosphate insecticides like bendiocarb (0.2 ppb), carbaryl (2 ppb), paraoxon (0.3 ppb), and malathion (3 ppb) based on AChE inhibition. The colorimetric reagent, indophenyl, produced a yellow to blue color shift in basic solution upon AChE-catalyzed hydrolysis of indophenyl, forming the indophenoxide anion. Color intensity was inversely proportional to analyte concentration. Lateral-flow and dipstick formats were developed where the analyte was measured with and without incubation. In the lateral-flow format, a pipette was used to deliver the analyte to the device. For the dipstick device, one end of the sensor was immersed in a container filled with the sample. Pesticide residues collected from apples and lettuce were in agreement with conventional analytical methods and matrix effects from spiked milk and juice were negligible.
2.5. Bacteria
The use of μPADs for the detection of bacterial contamination in environmental samples, notably food and water, is driven by the need for faster, simpler, lower cost methods in both developing and developed nations.139-143 Traditional measurements involve sending samples to a centralized laboratory for culture analysis or molecular detection (polymerase chain reaction, enzyme-linked immunosorbent assay), neither of which is suitable for routine monitoring due to the cost, analysis times, and need for highly trained personnel. A μPAD platform, on the other hand, offers low-cost, on-site, analysis with relatively rapid turnaround relative to traditional methods. Three main strains of bacteria have been investigated in environmental samples with μPADs: Escherichia coli,139-141, 143 Listeria monocytogenes,141, 142, 144 and Salmonella.141, 145 Frequently reported detection motifs use colored enzymatic assays on modified bioactive paper,139-143 light scattering,145 and chemilluminescence.139, 144
In 2012, Jokerst et al. developed a series of paper-based spot tests for the colorimetric enzymatic determination of E. coli, L. monocytogenes, and S. enterica in ready-to-eat meat (Figure 2B).141 In this report, enzymes produced by bacteria during growth in culture media reacted with compounds pre-deposited (and dried) on the μPAD. E. coli determination was based on the yellow to red color change produced between β-galactosidase and chlorophenol red β-galactopyranoside. L. monocytogenes detection was achieved by the reaction between phosphatidylinositol-specific phospholipase C and 5-bromo-4-chloro-3-indolyl-myo-inositol phosphate, which was characterized by the appearance of a blue color. Finally, S. enterica was determined using the reaction between esterase and 5-bromo-6-chloro-3-indolyl caprylate to produce a purple color. Limits of detection were on the order of 10 colony forming units/cm2 (cfu cm−2) from a surface swab after 4-12 hours of enrichment which is significantly faster than standard culture techniques. The devices were optimized based on enzyme and substrate concentrations, total well volume, sonication time, and cross-reactivity. Finally, the authors inoculated ready-to-eat meats141 and samples of irrigation water146 to demonstrate method applicability for food safety and environmental monitoring.
In another notable paper from the same year, Hossain et al. demonstrated a multiplexed device for the specific detection of non-pathogenic and pathogenic E. coli.140 Non-pathogenic E. coli was detected from the reaction of 5-bromo-4-chloro-3-indolyl-β-D-glucuronide sodium salt in the presence of β-glucuronidase, which produced the highly colored indolyl species and glucuronide. Total E. coli was indicated using the same β-galactosidase chemistry described above. Moreover, the authors demonstrated the use of immunomagnetic nanoparticles to concentrate E. coli by means of a magnetic separation step without the need for external cell culturing. The influence of other components in the sample matrix was tested with artificially contaminated samples of orange juice and milk. The specificity of the device was confirmed against the presence of Bacillus subtilis and S. enterica in the liquid test samples. The detection limits for pathogenic and non-pathogenic E. coli were 5 and 20 cfu mL−1, respectively.
2.6. Explosives
Pre- and post-detonation screening of explosives is interesting to environmental science because compounds in explosives have relatively slow decomposition in soil, which can lead to water contamination.147 Several articles have been published on explosives analysis with μPADs using fluorescence,147, 148 colorimetry,149-152 and surface-enhanced Raman spectroscopy.153 Most μPADs for detecting explosives have focused on nitroaromatics;148, 149, 153 however, recent efforts have focused on the development of μPADs capable of simultaneous, multiplexed detection of a wide range of compounds including both inorganic and organic explosives.147, 150, 151
In 2013, Taudte et al. developed a μPAD for measuring compounds in explosives via fluorescence quenching of pyrene.147 The poor water solubility of pyrene coupled with the incompatibility of organic solvents and wax barriers were addressed by testing different waxes (e.g. colors) and barrier thicknesses along with mixed solvent systems. A 80:20 methanol:water mixture was suitable for dissolving pyrene and showed minimal penetration of the wax barriers. Device performance was evaluated with 10 different compounds including nitrate esters, nitroaromatics, and nitro amines, all of which led to fluorescence quenching of pyrene. While not specific to a particular compound, the method was selective for explosive versus non-explosive samples such as water, milk, or coffee, based on differences in fluorescence quenching. The authors also developed a small, portable fluorescence detector for in-field use (Figure 2C). The prototype reader had comparable performance to the benchtop instrument used for method validation.
Peters et al. detailed simultaneous colorimetric detection of a series of inorganic and organic compounds found in improvised explosive devices.150 Previously reported μPADs focused on a subset of explosives; however, this was the first device capable of multiplexed detection of different explosive compounds. Two distinct μPADs were prepared (Figure 2D, E) – the first for detection of inorganic explosives (chlorate, nitrate, ammonium, nitrite, and perchlorate) and the second for detecting a mixture organic and inorganic explosive compounds (nitroamines, trinitroaromatics, urea nitrate, nitrate, and hydrogen peroxide). Several interferences were tested and no false positives or false negatives were observed. Additionally, black powder and fireworks were also tested to demonstrate the device performance on real samples.
2.7. Oxidative Activity
Oxidative stress in living organisms, a phenomenon that has been linked to aging and disease, can occur following exposure to reactive oxygen species (ROS).154-157 Common ROS are redox active metals, superoxides, hydroxide radicals, small organic molecules (e.g. quinone family), and enzymes. Some ROS are generated within living cells as product of natural metabolism, but more recent interest has focused on exposure to ROS from environmental sources as these compounds may overwhelm the body’s natural ability to manage oxidative stress. Given the wide range of species that fall under the category of ROS it is necessary to find an assay that can serve as an approximate gauge of the total ROS activity found in environmental samples.
Mani et al. developed a field-deployable μPAD to detect oxidative DNA damage using Cytochrome P450 enzymes to metabolize environmental toxins into reactive metabolites.158 The metabolites reacted with co-immobilized DNA causing it to partially unfold. The degree of unfolding was proportional to the toxin level. An electrochemiluminescent signal is generated when (bis-2,2′-bipyridyl) ruthenium polyvinylpyridine ([Ru(bpy)2(PVP)10]2+ polymer reacts with exposed guanines. The signal increases based on the extent of DNA damage, which is explained by the increased availability of guanines in DNA to react with the Ru(III). As is common with ROS studies, a suitable external standard was needed to compare the oxidative activity among heterogeneous samples. Mani et al found that benzo-[a]-pyrene was a suitable model oxidant and converted genotoxicity of their samples to a benzo-[a]-pyrene equivalent. The technique was demonstrated by analyzing smoke, water, and cooked food samples.
Dithiothreitol (DTT) oxidation is also used to gauge the oxidative reactivity of analytes in environmental samples.155, 159-162 In this assay, DTT is oxidized by ROS in samples (e.g. fluid extracts from air filter media) for a fixed period of time. After reacting with the sample, remaining (unconsumed) DTT is reacted with 5,5'-dithiobis-2-nitrobenzoic acid (Ellman’s Reagent) to yield the yellow 2-nitro-5-thiobenzoate dianion. The color intensity of this dianion is inversely related to the ROS activity of the sample. The DTT assay has been demonstrated on μPADs for air samples collected in close proximity to a wildfire, on a clear day, and from a commercial kitchen.162 Other colorimetric assays based on tris(2-carboxyethyl)phosphine (TCEP) or the aggregation of silver nanoparticles in the presence of reduced glutathione have been reported. 163, 164
3. Current Limitations and Recent Trends
Despite the promises of low-cost, small sample and reagent volumes, portability, and rapid response time, μPADs, in their current state, must overcome several limitations that inhibit their widespread application in the field. Large-scale field demonstrations of μPAD capabilities have been limited; one of the few examples to date was a biomedical test for the detection of transaminase as an indicator of drug-induced liver injury.165 Although some studies using environmental μPADs have reported the analysis of real-world samples brought back to the lab, these were, for the most part, small-scale studies, and the devices have not yet reached the same state of field readiness as their biomedical counterparts. One notable exception is the recent work of Sicard et al.133 Their preliminary experiments coupled the detection of organophosphate pesticides on a paper sensor with analysis using a smartphone. After each analysis, the result, date, and location were uploaded to a central web server using the smartphone’s GPS system. The advantages afforded by this technique for collaborative mapping may be applied to long-term or large-scale environmental monitoring of contamination hotspots or as a means for early detection.
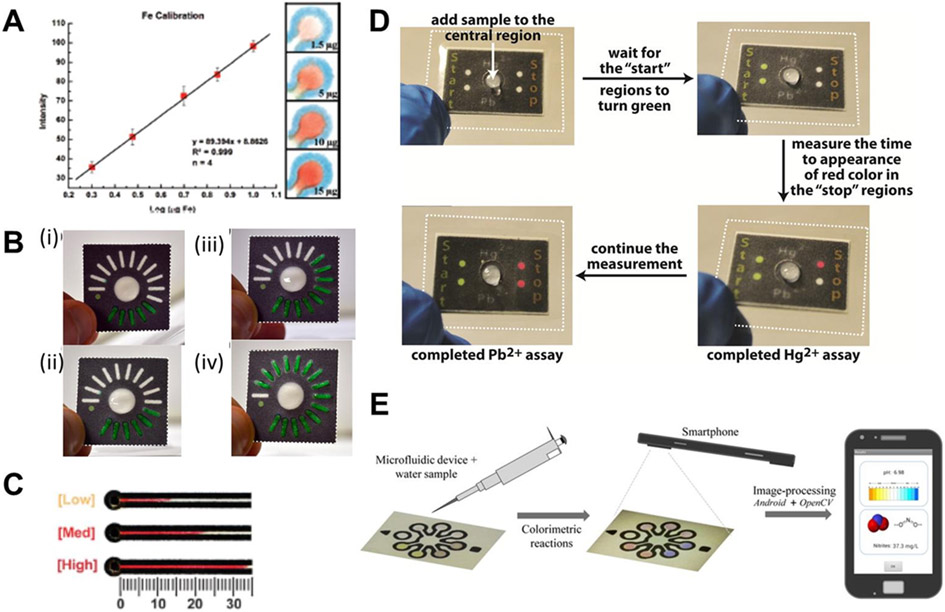
On-site analysis is important for widespread deployment of a rapid response test. Perhaps the most straightforward (and inexpensive) method for target determination has been colorimetric detection. If performed entirely visually (i.e. without any optical instrumentation), differentiation of color intensity is semi-quantitative and has been shown to vary from person-to-person64, 166 Colorimetric detection aided by a scanner or camera for image acquisition can improve method accuracy (Figure 3A), but increases analysis costs and could limit field use. Recently, groups have reported semi-quantitative alternatives to intensity-based colorimetry such as count-, distance-, and time-based μPADs that eliminate the need for imaging. In count-based detection, concentration is determined by counting the number of bars, tabs, or spots that develop color (Figure 3B).167 Distance-based quantification is achieved by measuring the distance of continuous color development along a channel with an on- or off-device ruler (Figure 3C).68, 69 Time-based quantification correlates concentration to the time difference for color development between a test zone and control zone (Figure 3D).168, 169
Figure 3:
Quantitative methods commonly used with colorimetric assays on μPADs. (A) Calibration curve for intensity-based analysis of a scanned device using ImageJ. Reprinted with permission from Ref. 55. Copyright 2012 American Chemical Society. (B) Count-based quantitation achieved by counting the number of colored tabs. Adapted from Ref. 167 with permission from John Wiley and Sons. (C) Distance-based detection by measuring the length of color development along a channel. Reproduced from Ref. 68 with permission from The Royal Society of Chemistry. (D) Time-based quantification. Reproduced from Ref. 169 with permission from The Royal Society of Chemistry. (E) Use of a smartphone to conduct intensity-based measurements. Reprinted with permission from Ref. 88. Copyright 2014 American Chemical Society.
Similarly, many μPAD-based electrochemical assays rely on expensive (>$10,000) desktop potentiostats for analysis. If the capabilities of μPADs are to be reached, the cost of electrochemical detectors will need to be reduced. Fortunately, in the last few years, several groups have reported new handheld units capable of measuring single ppb levels of analyte for a fraction of the price of traditional units.170-173 One example is the CheapStat, which is an inexpensive (<$80), open-source hardware and software potentiostat.170 On-going work will continue to drive down the cost of handheld sensors which will rapidly expand the utility and reach of μPAD-based electrochemical systems.
Worldwide distribution of smartphones and camera phones has significantly decreased the cost of sensor development because the phone becomes the detector and source of data transmission. For example, new software programs have been developed for conducting intensity-based colorimetric measurements in the field using a smartphone.88, 133, 166, 170, 174-182 Lopez-Ruiz et al. recently described a colorimetric μPAD for simultaneously evaluating pH and nitrite concentration in water (Figure 3E).88 A custom smartphone application was used to photograph the μPAD, and a software algorithm correlated hue-saturation-brightness indices with solution pH and nitrite concentration. Park et al. expanded this method by developing an application to indicate the optimal angle and distance from the user to the completed device.145 Smartphone analysis has also been applied to electrochemical measurements on μPADs. For example, Delaney et al. recently reported a procedure in which they used a smartphone as a potentiostat by controlling the amplitude and waveform of the audio output.175 Additionally, quick response (QR) codes have been implemented in some devices and could be used in the future to share vital diagnostic/identifier information about the sample without requiring user input. This sample identification would help automated and improve field data collection.111, 183 Future examples utilizing smartphone technology will continue to revolutionize μPAD application, enabling further field deployment.
4. Conclusions
Although μPADs were originally developed as biomedical assays for point-of-care diagnostics,20 their use has expanded into environmental research. By comparison to the progress achieved in biomedical devices, environmental monitoring with μPADs is still a nascent field of research. Guidance for the development of new applications may be taken from environmental and public health concerns highlighted by the World Health Organization (WHO),184 the Environmental Protection Agency (EPA),185-187 and other similar organizations from around the world.
The research highlighted in this review demonstrates that μPADs can successfully detect environmental contaminants; however, there is still a need for further developments to improve sensitivity and for field validation. The field-readiness of existing μPAD assays can be assessed in terms of established regulatory limitations and standards. For example, the Occupational Safety and Health Administration (OSHA) limits personal exposure to Cu in air at 1.0 mg/m3.188 The corresponding EPA regulation for Cu in drinking water is 1.3 mg/L (1.3 ppm).189 Rattanarat, et al.73 reported a LOD of 15 ppm for Cu by colorimetric methods indicating a need for improvement since method sensitivity falls above regulatory limits for drinking water. By contrast, the LOD for nitrite by Jayawardane, et al.89 of 1.0 uM (0.046 ppm) is well within the EPA limit of 1 mg/L (1 ppm).189 Due to the diversity of μPAD techniques and variability in regulatory limits for a given analyte, which depend on the sample matrix and the regulating agency, the field-readiness of environmental μPADs needs to be considered on a case-by-case basis, which is beyond the scope of the present review.
In addition to meeting regulatory limits, the successful commercialization of μPAD technologies may require the design of devices capable of measuring multiple analytes.10, 69, 70, 73, 140 One particular challenge presented by environmental monitoring is that analyte concentrations can vary by orders of magnitude in space and time. Devices, such as the dual colorimetric-electrochemical μPAD designed by Rattanarat et al. for quantifying metals show promise for meeting this challenge.73 Multimodal detection strategies are useful because colorimetric analysis, can achieve ppm sensitivity whereas electrochemistry can detect analytes with ppb sensitivity. In certain situations, it may be more advantageous to utilize one detection motif over others. By integrating portable electrochemical analyzers (described above), hybrid-detection mode μPADs have the potential to become very powerful tools for in-field, long-term environmental monitoring.
Environmental μPADs are not likely to replace existing instrumented environmental monitoring techniques, rather they will likely serve as a facile means of complementing current methodology. The low cost and rapid detection time of μPADs have the potential for widespread routine monitoring over large geographic areas and for long time periods providing thousands of individual measurements. Moreover, the recent developments towards improving the distribution and usability of environmental μPADs may provide the growing field of citizen science with a new set of tools and more readily involve citizens in the protection and improvement of human and environmental health. These measurements will ultimately provide better profiling of spatial and temporal variations in pollutants on a scale that has not been possible previously due to the lower cost of analysis.
References
- 1.Centers for Disease Control and Prevention, Listeria Outbreaks, http://www.cdc.gov/listeria/outbreaks/, (accessed December, 2015).
- 2.United States Environmental Protection Agency, Emergency Response to August 2015 Release from Gold King Mine, http://www.epa.gov/goldkingmine, (accessed December, 2015).
- 3.United States Environmental Protection Agency, Deepwater Horizon – BP Gulf of Mexico Oil Spill, http://www.epa.gov/enforcement/deepwater-horizon-bp-gulf-mexico-oil-spill, (accessed December, 2015).
- 4.Gencoglu A and Minerick AR, Microfluid. Nanofluid, 2014, 17, 781–807. [Google Scholar]
- 5.Manz A, Graber N and Widmer H. á., Sens. Actuators, B, 1990, 1, 244–248. [Google Scholar]
- 6.Haeberle S and Zengerle R, Lab Chip, 2007, 7, 1094–1110. [DOI] [PubMed] [Google Scholar]
- 7.Whitesides GM, Nature, 2006, 442, 368–373. [DOI] [PubMed] [Google Scholar]
- 8.Mark D, Haeberle S, Roth G, von Stetten F and Zengerle R, Chem. Soc. Rev, 2010, 39, 1153–1182. [DOI] [PubMed] [Google Scholar]
- 9.Chin CD, Linder V and Sia SK, Lab Chip, 2007, 7, 41–57. [DOI] [PubMed] [Google Scholar]
- 10.Abe K, Suzuki K and Citterio D, Anal. Chem, 2008, 80, 6928–6934. [DOI] [PubMed] [Google Scholar]
- 11.Delaney JL, Hogan CF, Tian J and Shen W, Anal. Chem, 2011, 83, 1300–1306. [DOI] [PubMed] [Google Scholar]
- 12.Davy J, Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London, 1800. [Google Scholar]
- 13.United States Pat., US 691249 A, 1902.
- 14.Yagoda H, Ind. Eng. Chem. Anal. Ed, 1937, 9, 79–82. [Google Scholar]
- 15.West PW, Ind. Eng. Chem. Anal. Ed, 1945, 17, 740–741. [Google Scholar]
- 16.Weil H, Kolloid-Z., 1953, 132, 149–162. [Google Scholar]
- 17.Partington J, J. Roman Stud, 1929, 19, 267–268. [Google Scholar]
- 18.Kunkel HG and Tiselius A, J. Gen. Physiol, 1951, 35, 89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rheinboldt H, Die Methoden der Organischen Chemie, Thieme Publishers, Leipzig, 3 edn., 1925. [Google Scholar]
- 20.Martinez AW, Phillips ST, Butte MJ and Whitesides GM, Angew. Chem. Int. Ed, 2007, 46, 1318–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie Z, Nijhuis CA, Gong J, Chen X, Kumachev A, Martinez AW, Narovlyansky M and Whitesides GM, Lab Chip, 2010, 10, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aragay G, Pons J and Merkoçi A, Chem. Rev, 2011, 111, 3433–3458. [DOI] [PubMed] [Google Scholar]
- 23.Gubala V, Harris LF, Ricco AJ, Tan MX and Williams DE, Anal. Chem, 2011, 84, 487–515. [DOI] [PubMed] [Google Scholar]
- 24.Jokerst JC, Emory JM and Henry CS, Analyst, 2012, 137, 24–34. [DOI] [PubMed] [Google Scholar]
- 25.Mao X and Huang TJ, Lab Chip, 2012, 12, 1412–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahavandi S, Baratchi S, Soffe R, Tang S-Y, Nahavandi S, Mitchell A and Khoshmanesh K, Lab Chip, 2014, 14, 1496–1514. [DOI] [PubMed] [Google Scholar]
- 27.Sackmann EK, Fulton AL and Beebe DJ, Nature, 2014, 507, 181–189. [DOI] [PubMed] [Google Scholar]
- 28.Martinez AW, Phillips ST, Whitesides GM and Carrilho E, Anal. Chem, 2009, 82, 3–10. [DOI] [PubMed] [Google Scholar]
- 29.Ballerini DR, Li X and Shen W, Microfluid. Nanofluid, 2012, 13, 769–787. [Google Scholar]
- 30.Liana DD, Raguse B, Gooding JJ and Chow E, Sensors, 2012, 12, 11505–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie J, Zhang Y, Lin L, Zhou C, Li S, Zhang L and Li J, Anal. Chem, 2012, 84, 6331–6335. [DOI] [PubMed] [Google Scholar]
- 32.Tomazelli Coltro WK, Cheng CM, Carrilho E and Jesus DP, Electrophoresis, 2014, 35, 2309–2324. [DOI] [PubMed] [Google Scholar]
- 33.Mace CR and Deraney RN, Microfluid. Nanofluid, 2014, 16, 801–809. [Google Scholar]
- 34.Cate DM, Adkins JA, Mettakoonpitak J and Henry CS, Anal. Chem, 2015, 87, 19–41. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Wu Y, Fu J-Z and Wu W-B, RSC Adv., 2015, 5, 78109–78127. [Google Scholar]
- 36.Yamada K, Henares TG, Suzuki K and Citterio D, Angew. Chem. Int. Ed, 2015, 54, 5294–5310. [DOI] [PubMed] [Google Scholar]
- 37.Mahadeva SK, Walus K and Stoeber B, ACS Appl. Mater. Interfaces, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed S, Bui M-PN and Abbas A, Biosens. Bioelectron, 2016, 77, 249–263. [DOI] [PubMed] [Google Scholar]
- 39.Pelton R, Trac-Trends Anal. Chem, 2009, 28, 925–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong F and Hu YF, Anal. Bioanal. Chem, 2012, 403, 7–13. [DOI] [PubMed] [Google Scholar]
- 41.Nery EW and Kubota LT, Anal. Bioanal. Chem, 2013, 405, 7573–7595. [DOI] [PubMed] [Google Scholar]
- 42.Yetisen AK, Akram MS and Lowe CR, Lab Chip, 2013, 13, 2210–2251. [DOI] [PubMed] [Google Scholar]
- 43.Rozand C, Eur. J. Clin. Microbiol, 2014, 33, 147–156. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, Wang S, Wang L, Li F, Pingguan-Murphy B, Lu TJ and Xu F, Biosens. Bioelectron, 2014, 54, 585–597. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Du D, Hua X, Yu XY and Lin Y, Electroanal., 2014, 26, 1214–1223. [Google Scholar]
- 46.Adkins J, Boehle K and Henry C, Electrophoresis, 2015, 36, 1811–1824. [DOI] [PubMed] [Google Scholar]
- 47.Jeong S-G, Kim J, Nam J-O, Song YS and Lee C-S, Int. Neurourl. J, 2013, 17, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisowski P and Zarzycki PK, Chromatographia, 2013, 76, 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun G, Crissman K, Norwood J, Richards J, Slade R and Hatch GE, Am. J. Physiol.-Lung C, 2001, 281, L807–L815. [DOI] [PubMed] [Google Scholar]
- 50.Dreher KL, Jaskot RH, Lehmann JR, Richards JH, Ghio JKMAJ and Costa DL, J. Toxicol. Env. Heal. A, 1997, 50, 285–305. [PubMed] [Google Scholar]
- 51.Jomova K and Valko M, Toxicology, 2011, 283, 65–87. [DOI] [PubMed] [Google Scholar]
- 52.Mehra R and Juneja M, Indian J. Biochem. Bio, 2004, 41, 53–56. [PubMed] [Google Scholar]
- 53.Lin J-H, Wu Z-H and Tseng W-L, Anal. Methods, 2010, 2, 1874–1879. [Google Scholar]
- 54.Pickering W, Ore Geol. Rev, 1986, 1, 83–146. [Google Scholar]
- 55.Mentele MM, Cunningham J, Koehler K, Volckens J and Henry CS, Anal Chem, 2012, 84, 4474–4480. [DOI] [PubMed] [Google Scholar]
- 56.Rattanarat P, Dungchai W, Cate DM, Siangproh W, Volckens J, Chailapakul O and Henry CS, Anal. Chim. Acta, 2013, 800, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cate DM, Nanthasurasak P, Riwkulkajorn P, L’Orange C, Henry CS and Volckens J, Ann. Occup. Hyg, 2014, 58, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almeida MIG, Chan C, Pettigrove VJ, Cattrall RW and Kolev SD, Environ. Pollut, 2014, 193, 233–239. [DOI] [PubMed] [Google Scholar]
- 59.Chaiyo S, Siangproh W, Apilux A and Chailapakul O, Anal. Chim. Acta, 2015, 866, 75–83. [DOI] [PubMed] [Google Scholar]
- 60.Hossain SZ and Brennan JD, Anal. Chem, 2011, 83, 8772–8778. [DOI] [PubMed] [Google Scholar]
- 61.Jayawardane BM, Cattrall RW and Kolev SD, Anal. Chim. Acta, 2013, 803, 106–112. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Cao R, Nilghaz A, Guan L, Zhang X and Shen W, Anal. Chem, 2015, 87, 2555–2559. [DOI] [PubMed] [Google Scholar]
- 63.Sadollahkhani A, Hatamie A, Nur O, Willander M, Zargar B and Kazeminezhad I, ACS Appl. Mater. Interfaces, 2014, 6, 17694–17701. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Zhou C, Nie J, Le S, Qin Q, Liu F, Li Y and Li J, Anal. Chem, 2014, 86, 2005–2012. [DOI] [PubMed] [Google Scholar]
- 65.Apilux A, Siangproh W, Praphairaksit N and Chailapakul O, Talanta, 2012, 97, 388–394. [DOI] [PubMed] [Google Scholar]
- 66.Ratnarathorn N, Chailapakul O, Henry CS and Dungchai W, Talanta, 2012, 99, 552–557. [DOI] [PubMed] [Google Scholar]
- 67.Elavarasi M, Rajeshwari A, Chandrasekaran N and Mukherjee A, Anal. Methods, 2013, 5, 6211–6218. [Google Scholar]
- 68.Cate DM, Dungchai W, Cunningham JC, Volckens J and Henry CS, Lab Chip, 2013, 13, 2397–2404. [DOI] [PubMed] [Google Scholar]
- 69.Cate DM, Noblitt SD, Volckens J and Henry CS, Lab Chip, 2015, 15, 2808–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L and Lin H, Anal. Chem, 2014, 86, 8829–8834. [DOI] [PubMed] [Google Scholar]
- 71.Patidar R, Rebary B and Paul P, J. Fluoresc, 2015, 25, 387–395. [DOI] [PubMed] [Google Scholar]
- 72.Apilux A, Dungchai W, Siangproh W, Praphairaksit N, Henry CS and Chailapakul O, Anal. Chem, 2010, 82, 1727–1732. [DOI] [PubMed] [Google Scholar]
- 73.Rattanarat P, Dungchai W, Cate D, Volckens J, Chailapakul O and Henry CS, Anal. Chem, 2014, 86, 3555–3562. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization, Cyanobacterial Toxins, http://www.who.int/water_sanitation_health/diseases/cyanobacteria/en/, (accessed September, 2015).
- 75.Sellner KG, Doucette GJ and Kirkpatrick GJ, J. Ind. Microbiol. Biotechnol, 2003, 30, 383–406. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization, Methaemoglobinemia, http://www.who.int/water_sanitation_health/diseases/methaemoglob/en/, (accessed September, 2015).
- 77.Fewtrell L, Environ. Health Persp, 2004, 112, 1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization, Fluorosis, http://www.who.int/water_sanitation_health/diseases/fluorosis/en/, (accessed September, 2015).
- 79.Ozsvath DL, Rev. Environ. Sci. Biotechnol, 2009, 8, 59–79. [Google Scholar]
- 80.United State Environmental Protection Agency, Cyanide Compounds, http://www3.epa.gov/ttn/atw/hlthef/cyanide.html, (accessed January, 2016).
- 81.Egekeze JO and Oehme FW, Vet. Quart, 1980, 2, 104–114. [DOI] [PubMed] [Google Scholar]
- 82.Jayawardane BM, McKelvie ID and Kolev SD, Talanta, 2012, 100, 454–460. [DOI] [PubMed] [Google Scholar]
- 83.He Q, Ma C, Hu X and Chen H, Anal. Chem, 2013, 85, 1327–1331. [DOI] [PubMed] [Google Scholar]
- 84.Kao PK and Hsu CC, Anal. Chem, 2014, 86, 8757–8762. [DOI] [PubMed] [Google Scholar]
- 85.Kao P-K and Hsu C-C, Microfluid. Nanofluid, 2014, 16, 811–818. [Google Scholar]
- 86.Li B, Fu L, Zhang W, Feng W and Chen L, Electrophoresis, 2014, 35, 1152–1159. [DOI] [PubMed] [Google Scholar]
- 87.Weng CH, Chen MY, Shen CH and Yang RJ, Biomicrofluidics, 2014, 8, 066502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez-Ruiz N, Curto VF, Erenas MM, Benito-Lopez F, Diamond D, Palma AJ and Capitan-Vallvey LF, Anal. Chem, 2014, 86, 9554–9562. [DOI] [PubMed] [Google Scholar]
- 89.Jayawardane BM, Wei S, McKelvie ID and Kolev SD, Anal. Chem, 2014, 86, 7274–7279. [DOI] [PubMed] [Google Scholar]
- 90.Cardoso TM, Garcia PT and Coltro WK, Anal. Methods, 2015, 7, 7311–7317. [Google Scholar]
- 91.He Y, Wu W.-b. and Fu J.-z., RSC Adv., 2015, 5, 2694–2701. [Google Scholar]
- 92.Chen Y, Zilberman Y, Mostafalu P and Sonkusale SR, Biosens. Bioelectron, 2015, 67, 477–484. [DOI] [PubMed] [Google Scholar]
- 93.Jayawardane BM, McKelvie ID and Kolev SD, Anal. Chem, 2015, 87, 4621–4626. [DOI] [PubMed] [Google Scholar]
- 94.Nath P, Arun RK and Chanda N, RSC Adv., 2014, 4, 59558–59561. [Google Scholar]
- 95.Hao Y, Chen W, Wang L, Zhou B, Zang Q, Chen S and Liu Y-N, Anal. Methods, 2014, 6, 2478. [Google Scholar]
- 96.Karita S and Kaneta T, Anal. Chem, 2014, 86, 12108–12114. [DOI] [PubMed] [Google Scholar]
- 97.Cuartero M, Crespo GA and Bakker E, Anal. Chem, 2015, 87, 1981–1990. [DOI] [PubMed] [Google Scholar]
- 98.Lan WJ, Zou XU, Hamedi MM, Hu J, Parolo C, Maxwell EJ, Buhlmann P and Whitesides GM, Anal. Chem, 2014, 86, 9548–9553. [DOI] [PubMed] [Google Scholar]
- 99.Lisak G, Cui J and Bobacka J, Sens. Actuators, B, 2015, 207, 933–939. [Google Scholar]
- 100.Novell M, Parrilla M, Crespo GA, Rius FX and Andrade FJ, Anal. Chem, 2012, 84, 4695–4702. [DOI] [PubMed] [Google Scholar]
- 101.Jones KC and De Voogt P, Environ. Pollut, 1999, 100, 209–221. [DOI] [PubMed] [Google Scholar]
- 102.Abelsohn A, Gibson BL, Sanborn MD and Weir E, Can. Med. Assoc. J, 2002, 166, 1549–1554. [PMC free article] [PubMed] [Google Scholar]
- 103.El-Shahawi M, Hamza A, Bashammakh A and Al-Saggaf W, Talanta, 2010, 80, 1587–1597. [DOI] [PubMed] [Google Scholar]
- 104.Pardasani D, Tak V, Purohit AK and Dubey D, Analyst, 2012, 137, 5648–5653. [DOI] [PubMed] [Google Scholar]
- 105.Wei WY and White IM, Analyst, 2013, 138, 1020–1025. [DOI] [PubMed] [Google Scholar]
- 106.Eaidkong T, Mungkarndee R, Phollookin C, Tumcharern G, Sukwattanasinitt M and Wacharasindhu S, J. Mater. Chem, 2012, 22, 5970–5977. [Google Scholar]
- 107.Mirica KA, Weis JG, Schnorr JM, Esser B and Swager TM, Angew. Chem. Int. Ed, 2012, 51, 10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soga T, Jimbo Y, Suzuki K and Citterio D, Anal. Chem, 2013, 85, 8973–8978. [DOI] [PubMed] [Google Scholar]
- 109.Yoon B, Park IS, Shin H, Park HJ, Lee CW and Kim JM, Macromol. Rapid Comm, 2013, 34, 731–735. [DOI] [PubMed] [Google Scholar]
- 110.Alkasir RS, Ornatska M and Andreescu S, Anal. Chem, 2012, 84, 9729–9737. [DOI] [PubMed] [Google Scholar]
- 111.Santhiago M, Henry CS and Kubota LT, Electrochim. Acta, 2014, 130, 771–777. [Google Scholar]
- 112.Sun G, Wang P, Ge S, Ge L, Yu J and Yan M, Biosens. Bioelectron, 2014, 56, 97–103. [DOI] [PubMed] [Google Scholar]
- 113.Tee-ngam P, Nunant N, Rattanarat P, Siangproh W and Chailapakul O, Sensors, 2013, 13, 13039–13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Staples CA, Dome PB, Klecka GM, Oblock ST and Harris LR, Chemosphere, 1998, 36, 2149–2173. [DOI] [PubMed] [Google Scholar]
- 115.Tsai W-T, J. Environ. Sci. Heal. C, 2006, 24, 225–255. [DOI] [PubMed] [Google Scholar]
- 116.Golub MS, Wu KL, Kaufman FL, Li LH, Moran-Messen F, Zeise L, Alexeeff GV and Donald JM, Birth Defects Res. B, 2010, 89, 441–466. [DOI] [PubMed] [Google Scholar]
- 117.Flint S, Markle T, Thompson S and Wallace E, J. Environ. Manage, 2012, 104, 19–34. [DOI] [PubMed] [Google Scholar]
- 118.Alkasir RS, Rossner A and Andreescu S, Environ. Sci. Technol, 2015, 49, 9889–9897. [DOI] [PubMed] [Google Scholar]
- 119.Casarett LJ, Klaassen CD and Amdur MO, Casarett and Doull's Toxicology: The Basic Science of Poisons, McGraw-Hill, New York, 7th edn., 2008. [Google Scholar]
- 120.Binukumar B, Bal A, Sunkaria A and Gill KD, Toxicology, 2010, 270, 77–84. [DOI] [PubMed] [Google Scholar]
- 121.Xu Z, Fang G and Wang S, Food Chem., 2010, 119, 845–850. [Google Scholar]
- 122.Duarte T, Martin C, Baud FJ, Laprévote O and Houzé P, Toxicol. Lett, 2012, 213, 142–150. [DOI] [PubMed] [Google Scholar]
- 123.Zheng Z, Zhou Y, Li X, Liu S and Tang Z, Biosens. Bioelectron, 2011, 26, 3081–3085. [DOI] [PubMed] [Google Scholar]
- 124.Pohanka M, Anal. Lett, 2012, 45, 367–374. [Google Scholar]
- 125.Hossain SZ, Luckham RE, Smith AM, Lebert JM, Davies LM, Pelton RH, Filipe CD and Brennan JD, Anal. Chem, 2009, 81, 5474–5483. [DOI] [PubMed] [Google Scholar]
- 126.Van Dyk JS and Pletschke B, Chemosphere, 2011, 82, 291–307. [DOI] [PubMed] [Google Scholar]
- 127.Liang H and Hay MT, Water Environ. Res, 2011, 83, 956–982. [Google Scholar]
- 128.Arduini F and Amine A, in Biosensors Based on Aptamers and Enzymes, Springer, 2014, pp. 299–326. [DOI] [PubMed] [Google Scholar]
- 129.Liu W, Kou J, Xing H and Li B, Biosens. Bioelectron, 2014, 52, 76–81. [DOI] [PubMed] [Google Scholar]
- 130.Wang S, Ge L, Li L, Yan M, Ge S and Yu J, Biosens. Bioelectron, 2013, 50, 262–268. [DOI] [PubMed] [Google Scholar]
- 131.Pohanka M, Hrabinova M, Fusek J, Hynek D, Adam V, Hubalek J and Kizek R, Int. J. Electrochem. Sci, 2012, 7, 50–57. [Google Scholar]
- 132.Badawy ME and El-Aswad AF, Int. J. Anal. Chem, 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sicard C, Glen C, Aubie B, Wallace D, Jahanshahi-Anbuhi S, Pennings K, Daigger GT, Pelton R, Brennan JD and Filipe CDM, Water Res., 2015, 70, 360–369. [DOI] [PubMed] [Google Scholar]
- 134.Pohanka M, Karasova JZ, Kuca K, Pikula J, Holas O, Korabecny J and Cabal J, Talanta, 2010, 81, 621–624. [DOI] [PubMed] [Google Scholar]
- 135.Su Y, Ma S, Jiang K and Han X, Chinese J. Chem, 2015, 33, 446–450. [Google Scholar]
- 136.Pimsen R, Khumsri A, Wacharasindhu S, Tumcharern G and Sukwattanasinitt M, Biosens. Bioelectron, 2014, 62, 8–12. [DOI] [PubMed] [Google Scholar]
- 137.Liu W, Guo Y, Luo J, Kou J, Zheng H, Li B and Zhang Z, Spectrochim. Acta A, 2015, 141, 51–57. [DOI] [PubMed] [Google Scholar]
- 138.Hossain SZ, Luckham RE, McFadden MJ and Brennan JD, Anal. Chem, 2009, 81, 9055–9064. [DOI] [PubMed] [Google Scholar]
- 139.Burnham S, Hu J, Anany H, Brovko L, Deiss F, Derda R and Griffiths MW, Anal. Bioanal. Chem, 2014, 406, 5685–5693. [DOI] [PubMed] [Google Scholar]
- 140.Hossain SZ, Ozimok C, Sicard C, Aguirre SD, Ali MM, Li Y and Brennan JD, Anal. Bioanal. Chem, 2012, 403, 1567–1576. [DOI] [PubMed] [Google Scholar]
- 141.Jokerst JC, Adkins JA, Bisha B, Mentele MM, Goodridge LD and Henry CS, Anal. Chem, 2012, 84, 2900–2907. [DOI] [PubMed] [Google Scholar]
- 142.Liu H, Zhan F, Liu F, Zhu M, Zhou X and Xing D, Biosens. Bioelectron, 2014, 62, 38–46. [DOI] [PubMed] [Google Scholar]
- 143.Ma S, Tang Y, Liu J and Wu J, Talanta, 2014, 120, 135–140. [DOI] [PubMed] [Google Scholar]
- 144.Liu F and Zhang C, Sens. Actuators, B, 2015, 209, 399–406. [Google Scholar]
- 145.San Park T, Li W, McCracken KE and Yoon J-Y, Lab Chip, 2013, 13, 4832–4840. [DOI] [PubMed] [Google Scholar]
- 146.Bisha B, Adkins JA, Jokerst JC, Chandler JC, Perez-Mendez A, Coleman SM, Sbodio AO, Suslow TV, Danyluk MD and Henry CS, J. Vis. Exp, 2014, e51414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taudte RV, Beavis A, Wilson-Wilde L, Roux C, Doble P and Blanes L, Lab Chip, 2013, 13, 4164–4172. [DOI] [PubMed] [Google Scholar]
- 148.Szram J, Schofield SJ, Cosgrove MP and Cullinan P, Eur. Respir. J, 2013, 42, 1186–1193. [DOI] [PubMed] [Google Scholar]
- 149.Pesenti A, Taudte RV, McCord B, Doble P, Roux C and Blanes L, Anal. Chem, 2014, 86, 4707–4714. [DOI] [PubMed] [Google Scholar]
- 150.Peters KL, Corbin I, Kaufman LM, Zreibe K, Blanes L and McCord BR, Anal. Methods, 2015, 7, 63–70. [Google Scholar]
- 151.Salles M, Meloni G, de Araujo W and Paixão T, Anal. Methods, 2014, 6, 2047–2052. [Google Scholar]
- 152.Xu M, Bunes BR and Zang L, ACS Appl. Mater. Interfaces, 2011, 3, 642–647. [DOI] [PubMed] [Google Scholar]
- 153.Fierro-Mercado PM and Hernández-Rivera SP, Int. J. Spectrosc, 2012, 2012, 716527. [Google Scholar]
- 154.Finkel T and Holbrook NJ, Nature, 2000, 408, 239–247. [DOI] [PubMed] [Google Scholar]
- 155.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J and Nel A, Environ. Health Persp, 2003, 111, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Møller P, Folkmann JK, Forchhammer L, Bräuner EV, Danielsen PH, Risom L and Loft S, Cancer Lett., 2008, 266, 84–97. [DOI] [PubMed] [Google Scholar]
- 157.Risom L, Møller P and Loft S, Mutat. Res.-Fund. Mol. M, 2005, 592, 119–137. [DOI] [PubMed] [Google Scholar]
- 158.Mani V, Kadimisetty K, Malla S, Joshi AA and Rusling JF, Environ. Sci. Technol, 2013, 47, 1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eiguren-Fernandez A, Di Stefano E, Schmitz DA, Guarieiro ALN, Salinas EM, Nasser E, Froines JR and Cho AK, J. Air Waste Manage, 2015, 65, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koehler KA, Shapiro J, Sameenoi Y, Henry C and Volckens J, Aerosol Sci. Tech, 2014, 48, 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sameenoi Y, Koehler K, Shapiro J, Boonsong K, Sun Y, Collett J Jr, Volckens J and Henry CS, J. Am. Chem. Soc, 2012, 134, 10562–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sameenoi Y, Panymeesamer P, Supalakorn N, Koehler K, Chailapakul O, Henry CS and Volckens J, Environ. Sci. Technol, 2012, 47, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ciriello F, Gualtieri M, Longhin E, Ruffo R, Camatini M and Parenti P, Environ. Sci. Pollut. Res, 2015, 22, 12469–12478. [DOI] [PubMed] [Google Scholar]
- 164.Dungchai W, Sameenoi Y, Chailapakul O, Volckens J and Henry CS, Analyst, 2013, 138, 6766–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pollock NR, Rolland JP, Kumar S, Beattie PD, Jain S, Noubary F, Wong VL, Pohlmann RA, Ryan US and Whitesides GM, Sci. Transl. Med, 2012, 4, 152ra129–152ra129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yetisen AK, Martinez-Hurtado J, Garcia-Melendrez A, da Cruz Vasconcellos F and Lowe CR, Sens. Actuators, B, 2014, 196, 156–160. [Google Scholar]
- 167.Lewis GG, DiTucci MJ and Phillips ST, Angew. Chem, 2012, 124, 12879–12882. [DOI] [PubMed] [Google Scholar]
- 168.Lewis GG, Robbins JS and Phillips ST, Anal. Chem, 2013, 85, 10432–10439. [DOI] [PubMed] [Google Scholar]
- 169.Lewis GG, Robbins JS and Phillips ST, Chem. Commun, 2014, 50, 5352–5354. [DOI] [PubMed] [Google Scholar]
- 170.Rowe AA, Bonham AJ, White RJ, Zimmer MP, Yadgar RJ, Hobza TM, Honea JW, Ben-Yaacov I and Plaxco KW, PloS One, 2011, 6, e23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nemiroski A, Christodouleas DC, Hennek JW, Kumar AA, Maxwell EJ, Fernández-Abedul MT and Whitesides GM, Proc. Natl. Acad. Sci, 2014, 111, 11984–11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramfos I, Vassiliadis N, Blionas S, Efstathiou K, Fragoso A, O'Sullivan CK and Birbas A, Biosens. Bioelectron, 2013, 47, 482–489. [DOI] [PubMed] [Google Scholar]
- 173.Coltro WK, Mora MF, Garcia CD, Escarpa A, González MC and López MÁ, Agricultural and Food Electroanalysis, 2015, 443–477. [Google Scholar]
- 174.Chang B-Y, B. Korean Chem. Soc, 2012, 33, 549–552. [Google Scholar]
- 175.Delaney JL, Doeven EH, Harsant AJ and Hogan CF, Anal. Chim. Acta, 2013, 790, 56–60. [DOI] [PubMed] [Google Scholar]
- 176.Fronczek CF, San Park T, Harshman DK, Nicolini AM and Yoon J-Y, RSC Adv., 2014, 4, 11103–11110. [Google Scholar]
- 177.Martinez AW, Phillips ST, Carrilho E, Thomas III SW, Sindi H and Whitesides GM, Anal. Chem, 2008, 80, 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mudanyali O, Dimitrov S, Sikora U, Padmanabhan S, Navruz I and Ozcan A, Lab Chip, 2012, 12, 2678–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li X, Ballerini DR and Shen W, Biomicrofluidics, 2012, 6, 11301–1130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thom NK, Lewis GG, Yeung K and Phillips ST, RSC Adv., 2014, 4, 1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thom NK, Yeung K, Pillion MB and Phillips ST, Lab Chip, 2012, 12, 1768–1770. [DOI] [PubMed] [Google Scholar]
- 182.Wang H, Li Y.-j., Wei J.-f., Xu J.-r., Wang Y.-h. and Zheng G.-x., Anal. Bioanal. Chem, 2014, 406, 2799–2807. [DOI] [PubMed] [Google Scholar]
- 183.Myers NM, Kernisan EN and Lieberman M, Anal. Chem, 2015, 87, 3764–3770. [DOI] [PubMed] [Google Scholar]
- 184.World Health Organization, Water-related diseases: information sheets, http://www.who.int/water_sanitation_health/diseases/diseasefact/en/, (accessed September, 2015).
- 185.United States Environmental Protection Agency, Drinking Water Contaminants, http://water.epa.gov/drink/contaminants/, (accessed September, 2015).
- 186.United States Environmental Protection Agency, Air Pollutants, http://www.epa.gov/air/airpollutants.html, (accessed September, 2015).
- 187.United States Environmental Protection Agency, Nutrient Pollution Policy and Data, http://www2.epa.gov/nutrient-policy-data, (accessed September, 2015).
- 188.Centers for Disease Control and Prevention, NIOSH Pocket Guide to Chemical Hazards, http://www.cdc.gov/niosh/npg/, (accessed January, 2016).
- 189.United States Environmental Protection Agency, Table of Regulated Drinking Water Contaminants, http://www.epa.gov/your-drinking-water/table-regulated-drinking-water-contaminants, (accessed January, 2016).