ABSTRACT
Exposure to severe stress can lead to the development of neuropsychiatric disorders, including post-traumatic stress disorder (PTSD). The cause of PTSD is dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis and an imbalance of monoamines. Fruits and vegetables contain large amounts of luteolin (LU; 3′,4′,5,7-tetrahydroxylflavone), which has various pharmacological activities such as anti-inflammatory, antioxidant, and anti-allergic effects. We investigated the effects of LU on fear, depression, and anxiety following monoamine imbalance and hyperactivation of the HPA axis in rats exposed to single prolonged stress (SPS). Male rats were dosed with LU (10 and 20 mg/kg) once daily for 14 days after exposure to SPS. Administration of LU reduced fear freezing responses to extinction recall and depression- and anxiety-like behaviors, and suppressed increases in plasma corticosterone and adrenocorticotropic hormone levels. Also, administration of LU restored the increased norepinephrine and decreased serotonin levels in the structures within the fear circuit, medial prefrontal cortex, and hippocampus. Our results showed that administration of LU improved freezing behavior according in a situation-dependent manner, and showed anti-depressant and anxiolytic effects. Thus, LU may be a useful therapeutic agent to prevent traumatic stress such as PTSD.
KEYWORDS: Luteolin, post-traumatic stress disorder, anxiety, hypothalamic-pituitary-adrenal (HPA) axis, monoamine
Introduction
Post-traumatic stress disorder (PTSD) is a serious physical and mental disorder that is often comorbid with panic disorder, depression, and anxiety disorders and occurs after exposure to severe stressors (Kirkpatrick and Heller 2014). The core symptoms of PTSD are intrusive memories, avoidance, re-experiencing trauma, and cognitive and mood disorders (Kirkpatrick and Heller 2014). PTSD has a high prevalence rate that is expected to increase by 45% within the next 5 years, it causes considerable stress that severely interferes with daily life (Francati et al. 2007).
PTSD is caused by activation of the serotonergic and norepinephrinergic systems, changes in the neuroendocrine system, and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis (Cohen et al. 2009). Serotonin (5-HT) is decreased, and noradrenaline (NE) increased in the medial prefrontal cortex (PFC) and hippocampus (HIP) in PTSD patients (Krystal and Neumeister 2009). Some studies have suggested that PTSD is underpinned by disturbances of the major monoaminergic neurotransmitters that mediate rapid excitatory synaptic responses in the central nervous system, and increase inflammatory cytokines in response to traumatic stimuli following disruption of the fear circuit (Wang et al. 2018). Traumatic stress causes an imbalance of neurotransmitters in the PFC, HIP, and amygdala (AMY), which are structures within the fear circuit and induce emotional responses, such as fear and anxiety, through activation of the hypothalamus (Krishnamurthy et al. 2013). Elevated corticosterone (CORT) changes the expression of the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) in the HIP and AMY, resulting in alterations in negative feedback mechanisms and dysfunction of the HPA axis (Sherin and Nemeroff 2011). Serum levels of CORT and corticotropin-releasing hormone (CRH), and adrenocorticotropic hormone (ACTH) are elevated by dysregulation of the HPA axis in rats exposed to chronic stress, resulting in anxiety-like behavioral alterations (Gao et al. 2019). In clinical studies, anxiety symptoms are alleviated by drugs that target dysregulation of the HPA axis or modulate serotonergic systems (Faye et al. 2018).
5-HT and NE reuptake inhibitors, which are currently used as therapeutics, have very limited efficacy and can also have various side effects, such as withdrawal, sedation, dependence, and cognitive dysfunction (Berger et al. 2009). Therefore, there is a need for new drugs that are safe and effective.
Luteolin (LU, 3′,4′,5,7-tetrahydroxylflavone) is a flavonoid found in foods consumed on a daily basis including fruits, vegetables, nuts, and herbs (Kim et al. 2000). Preclinical studies have shown that LU has various biological and pharmacological activities, such as anti-inflammatory, antitumor, antihepatotoxic, antioxidant, and anti-allergic effects (Chen et al. 2017; Albarakati et al. 2020; Dong et al. 2021). LU reduces oxidative stress in diabetic rats and exerts cardioprotective effects by inhibiting the mitochondrial permeability transition pore (Xiong et al. 2017). LU also exerts anti-inflammatory and antioxidant effects in acute pancreatitis caused by oxidative stress and retinal pigment epithelial cells (Hytti et al. 2016; Li et al. 2019; Rajapriya and Geetha 2021). LU crosses the blood–brain barrier (Zhang et al. 2017) and has been shown to reduce inflammatory cytokines, improve short-term memory, and promote synaptic plasticity in a cognitive deficit animal model following a decline in chronic cerebral perfusion (Yao et al. 2018). LU also improves spatial learning and memory in streptozotocin-induced Alzheimer’s disease (Wang et al. 2016). In addition, LU protects PC12 cells from 6-hydroxydopamine, suppresses Bcl-2-associated increases in X protein mRNA expression, and decreases B-cell lymphoma 2 expression (Guo et al. 2013). Because many studies have suggested the neuroprotective effects of LU, it is reasonable to expect that LU will be effective for treating PTSD.
In this study, animals were exposed to single prolonged stress (SPS), which caused them to exhibit PTSD-like symptoms due to the damage of fear extinction. This model is based on the premise that people with multiple or early traumas are more likely to develop PTSD after a traumatic event.
Therefore, this study investigated the ability of LU to alleviate the fear response and anxiety-like symptoms caused by dysregulation of the HPA axis and neurotransmitter imbalance in an animal model of PTSD caused by SPS.
Materials and methods
Animals and LU administration
As experimental animals, 6∼7 week-old male Sprague–Dawley rats (200∼220 g; Samtaco Animal Co., Seoul, Korea) were used. All experimental methods and procedures were approved by the Animal Care and Use Committee of Kyung Hee University (KHUASP(SE)-21-045). All laboratory procedures and animal room environments were performed in accordance with the guidelines for the Care and Use of Laboratory Animals.
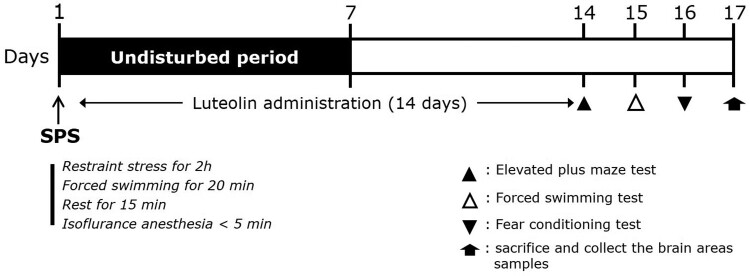
For the experiment, the standard doses of LU (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and paroxetine hydrochloride (PAX, positive control; Sigma-Aldrich) were determined based on previous studies (Wang et al. 2016). LU (10 and 20 mg/kg) and PAX (15 mg/kg) were dissolved in 0.9% saline before use and injected intraperitoneally (i.p.) once daily for 14 days. The experimental schedule is shown in Figure 1.
Figure 1.
Experimental protocols for inducing anxiety-like behaviors via SPS and treating rats with luteolin. Different groups of rats (n = 7 per group) were used in each experimental condition.
SPS
In the PTSD animal model, rats were immobilized for 2 h, forced to swim for 20 min, and then rested for 15 min. After rest, they were exposed to isoflurane (2∼3%) until losing consciousness. For sensitization, rats were left alone in cages for 7 days. In the sucrose preference test, intake of water and sucrose solution (1%, w/v) was measured for 3 h, as previously described (Lee et al. 2020).
Behavioral test
To evaluate the fear response (fear conditioning test, FCT), rats were acclimatized to a conditioned chamber (conditioned stimuli [CS, audible alarm]; 30 s, 85 dB) for 5 min followed by a single electric foot shock (unconditioned stimuli [US]; 2 s, 0.5 mA). After 24 h, when the rats were briefly re-exposed to the CS, the freezing response was measured for 5 min as previously described (Lee et al. 2020).
To evaluate depression-like behavior (forced swimming test, FST), rats were forced to swim in a water bath (20 cm diameter × 50 cm height) for 5 min. Rats that did not swim and were floating were considered to be in a depressed state, as previously described (Lee et al. 2020).
To evaluate anxiety-like behavior using the apparatus detailed in a previous study (elevated plus maze [EPM] test) (Lee et al. 2020), rats were placed in the center of a maze and tested for 5 min. The time spent in, and number of visits to, two open and two closed arms were measured. Behavior in the maze was measured by the S-MART program (PanLab, Barcelona, Spain).
Enzyme-linked immunosorbent assay to assess neurotransmitter levels
Plasma levels of CORT (Novus Biologicals, LLC., Littleton, CO, USA), ACTH (Abcam, Cambridge, UK), and CRH (Biocompare, South San Francisco, CA, USA), and concentrations of 5-HT (Abcam) and NE (Novus Biologicals) in the PFC, HIP, and AMY, were measured by competitive enzyme-linked immunosorbent assay (ELISA), as previously described (Lee et al. 2020).
Total RNA preparation and reverse transcription-polymerase chain reaction
TRIzol reagent (Sigma-Aldrich) was added to the HIP isolated from the brain to recover total RNA. After the RNA had been synthesized to cDNA by reverse transcription reaction, polymerase chain reaction (PCR) was performed as previously described (Lee et al. 2020). To confirm the results of the PCR reaction, electrophoresis was performed on a 1.5% agarose gel.
Western blot analysis
Total protein was extracted from the brain to detect GR protein in the HIP. The protein concentration was measured using a colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). After incubation with a mouse GR antibody (1:500; Cell Signaling, Danvers, MA, USA), the membrane was incubated with horseradish peroxidase conjugated goat anti-mouse IgG secondary antibody (Santa Cruz Biotech, Santa Cruz, CA, USA). A chemiluminescent kit (Super Signal West Pico; Pierce, Rockford, IL, USA) was used to detect GR protein. Protein content was analyzed using an enhanced chemiluminescence detection system (Santa Cruz), and the density was measured using the Tina 2.1 program.
Statistical analyses
Data are presented as the mean ± standard error. To test for statistical differences between groups, one-way analysis of variance was performed using SPSS software (version 23.0; SPSS, Inc., Chicago, IL, USA), followed by Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
Effects of body weight and sucrose intake according to SPS
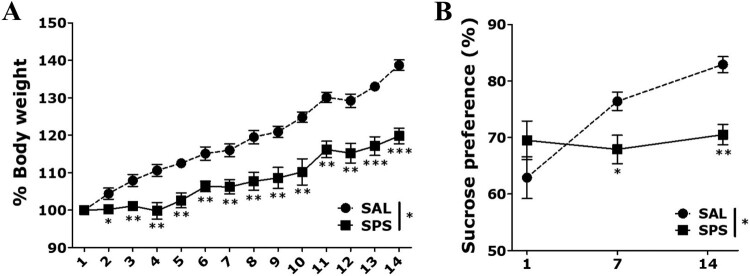
Rats exposed to SPS showed a significant decrease in the change of body weight over time compared to the saline-treated normal (SAL) group (t = 7.455, p < 0.05, p < 0.01 and p < 0.001, respectively; Figure 2(A)). This change in body weight showed a significant difference from day 2 after exposure to SPS.
Figure 2.
Results of body weight and sucrose intake analyses of rats subjected to 14 days of SPS. Body weights and food intake were significantly lower in SPS-exposed than SAL-treated rats (n = 7 per group, mean ± SEM). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. SAL group.
Rats exposed to SPS showed a significant decrease in sucrose intake on days 7 and 14 compared to the SAL group (t = 4.884, p < 0.05 and p < 0.01, respectively; Figure 2(B)). The rats exhibited sufficient physiological changes and anhedonia due to traumatic stress following SPS exposure.
Effects of LU on behavioral changes by SPS
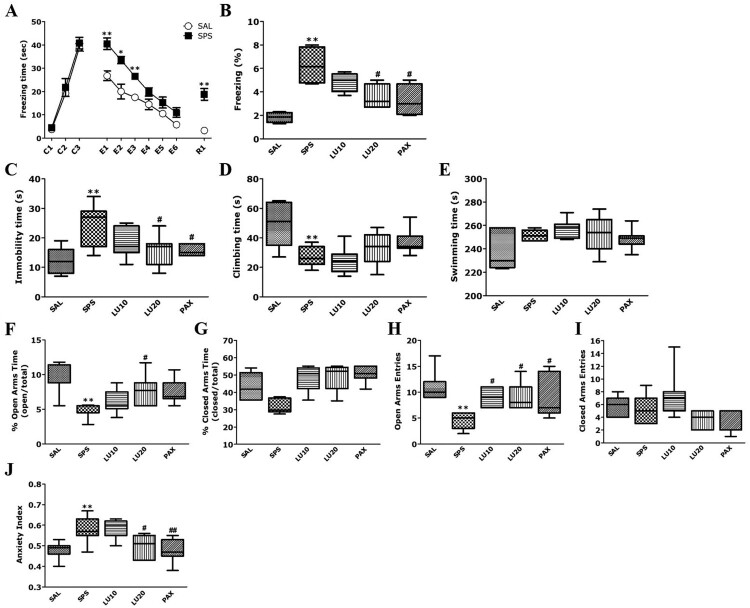
In the FCT, rats exposed to SPS showed a similar pattern of freezing responses to the fear stimulus (conditioning trials; C1∼C3) to the SAL group (t = 0.623, p = 0.575; Figure 3(A)). During fear both extinction (E1∼E6) and fear extinction recall (R1; short-term recall), the rats exposed to SPS showed a significantly increased freezing response compared to the SAL group (p < 0.05 and p < 0.01, respectively). However, administration of 20 mg/kg LU significantly decreased the freezing response during fear extinction recall compared to the SPS group (p < 0.05; Figure 3(B)). The freezing response of the LU20 group were similar to those of the PAX group.
Figure 3.
Effect of luteolin (LU) on freezing behavior in response to conditioning (C1∼C3), extinction (E1∼E6), and short-term recall (R1)(A) and the percentage of time spent frozen during short-term recall (B) in the FCT; on the immobility time (C), climbing behavior (D) and swimming time (E) in the FST; on the time spent in the open (F) and closed (G) arms, numbers of entries into the open (H) and closed (I) arms, and anxiety index (J) in the EPM test (n = 7 per group, mean ± SEM). *p < 0.05 and **p < 0.01 vs. SAL group; #p < 0.05 and ##p < 0.01 vs. SPS group.
In the FST, rats exposed to SPS had a significantly increased immobility time compared to the SAL group (p < 0.01; Figure 3(C)). Administration of 20 mg/kg LU significantly decreased the immobility time compared to the SPS group (p < 0.05). The immobility time of the LU20 group were similar to those of the PAX group. However, administration of 20 mg/kg LU increased the climbing time compared to the SPS group, although not statistically significantly (p = 0.795; Figure 3(D)). Also, there was no difference in swimming time among all groups (F4,34 = 2.621, p = 0.054; Figure 3(E)).
In the EPM test, the percentage of time spent in, and the number of entries into, the open arms of rats exposed to SPS was significantly decreased compared to the SAL group (p < 0.01; Figure 3(F,H)). However, administration of 20 mg/kg LU significantly increased the percentage of time spent in, and number of entries into, the open arms compared to the SPS group (p < 0.05). The number of entries into the open arms of the LU20 group were similar to those of the PAX group. Among all groups, the time spent in, and the number of entries into, in the closed arms did not differ (F4,34 = 1.163, p = 0.347 and F4,34 = 1.352, p = 0.274, respectively; Figure 3(G,I)). The administration of 20 mg/kg LU significantly decreased the anxiety index compared to the SPS group (p < 0.05; Figure 3(J)).
Effects of LU on HPA axis activation and neurotransmitter changes induced by SPS
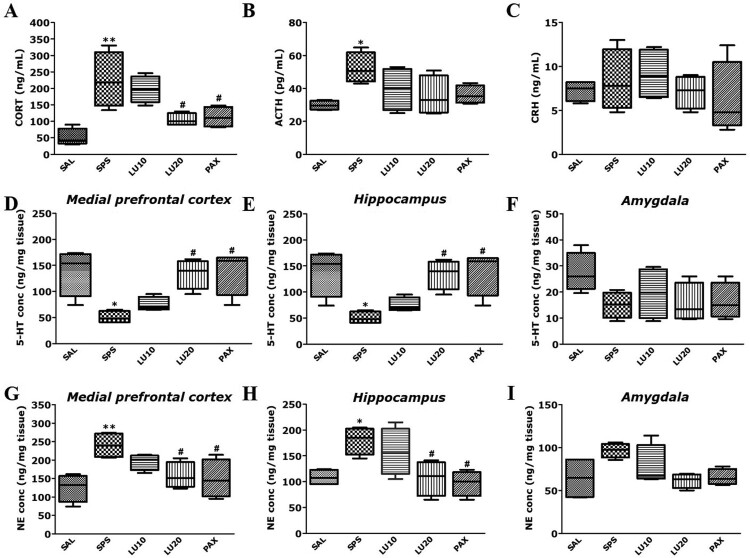
Rats exposed to SPS had significantly increased plasma CORT level compared to the SAL group (p < 0.01; Figure 4(A)). Compared to the SAL group, rats exposed to SPS had significantly increased plasma ACTH level (p < 0.05; Figure 4(B)), but there was no significant difference in plasma CRH level (p = 0.983; Figure 4(C)). However, the administration of 20 mg/kg LU significantly decreased the plasma CORT level compared to the SPS group (p < 0.05). Similarly, rats in the PAX group significantly decreased the plasma CORT level compared to the SPS group (p < 0.05).
Figure 4.
Effect of LU on CORT (A), ACTH (B), CRH (C) levels in the plasma, the 5-HT concentrations in the PFC (D), HIP (E), and AMY (F) and the NE concentrations in the PFC (G), HIP (H), and AMY (I) of rats exposed to SPS for 14 consecutive days are shown (n = 3∼4 per group, mean ± SEM). *p < 0.05 and **p < 0.01 vs. SAL group; #p < 0.05 vs. SPS group.
Rats exposed to SPS had significantly decreased 5-HT concentrations in the PFC and HIP compared to the SAL group (p < 0.05; Figure 4(D,E)), but there was no significant difference in 5-HT concentration in the AMY (p = 0.195; Figure 4(F)). However, the administration of 20 mg/kg LU significantly increased the 5-HT concentrations in the PFC and HIP compared to the SPS group (p < 0.05). Similarly, rats in the PAX group significantly increased the 5-HT concentrations in the PFC and HIP compared to the SPS group (p < 0.05).
Rats exposed to SPS had significantly increased NE concentrations in the PFC and HIP compared to the SAL group (p < 0.05 and p < 0.01, respectively; Figure 4(G,H)), but there were no significant changes in NE concentration in the AMY (p = 0.102; Figure 4(I)). The administration of 20 mg/kg LU significantly decreased the NE concentrations in the PFC and HIP compared to the SPS group (p < 0.05). Similarly, rats in the PAX group significantly decreased the NE concentrations in the PFC and HIP compared to the SPS group (p < 0.05).
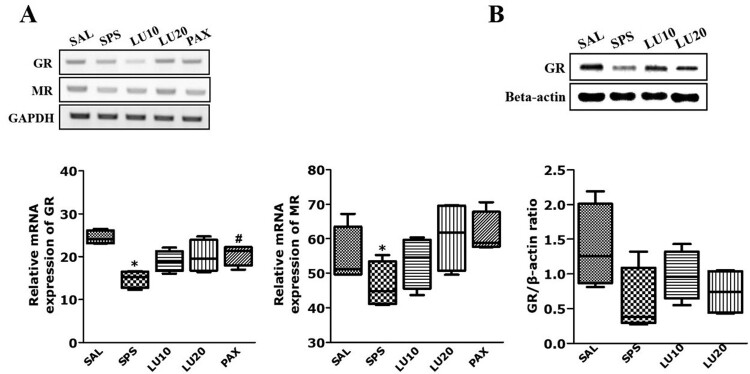
Effects of LU on the GR and MR mRNA expression induced by SPS
Rats exposed to SPS had significantly reduced GR and MR mRNA expression in the HIP compared to the SAL group (p < 0.05; Figure 5(A)). However, the administration of 20 mg/kg LU increased GR and MR mRNA expression in the HIP compared to the SPS group, although not statistically significantly (p = 0.428 and p = 0.071, respectively).
Figure 5.
Effect of LU of GR and MR mRNA expression in rats with SPS-induced hippocampal impairment (A). PCR bands on agarose gels and relative intensities are shown (n = 3 per group, mean ± SEM) Activation of GR in the HIP after LU treatment (B). Western blot analysis of protein expression levels of GR (n = 3∼4 per group, mean ± SEM). *p < 0.05 vs. SAL group; #p < 0.05 vs. SPS group.
Effect of LU on GR activation in the hippocampus by SPS
The effect of LU on the protein expression level of GR in hippocampal tissue were determined by Western blotting. The SPS group showed decrease in GR protein expression level in the HIP compared to the SAL group, although not statistically significantly (p = 0.126; Figure 5(B)). However, LU treatment (20 mg/kg) attenuated the SPS-induced decrease in GR protein expression level, although not statistically significantly (p = 0.965).
Discussion
In this study, we showed that administration of LU can ameliorate PTSD-like behaviors following fear, depression, and anxiety. Also, administration of LU suppressed the increase in CORT and ACTH by controlling the HPA axis, and ameliorated the imbalance of NE and 5-HT in the structures within the fear circuit, such as the PFC and HIP. Therefore, the anti-PTSD-like effect of LU may be exerted via regulation of the HPA axis and monoamine balance.
PTSD is an anxiety disorder that occurs when people experience trauma, such as a serious accident or violence (Fokkens et al. 2015). In this study, after exposure to SPS, rats showed a decrease in body weight and sucrose intake compared to the control group. After exposure to SPS, an increase of anhedonia induced depression in association with traumatic stress. Also, although body weight loss is not a direct indicator of fear, depression, and anxiety, physiological changes caused by these maladaptive responses to traumatic stress occur after exposure to SPS.
PTSD patients experience recurrent psychological distress due to fear memories of traumatic events (Corley et al. 2012). These fear responses and memories are key features of PTSD (Careaga et al. 2016). Rats exposed to SPS in this study showed a fear response in association with the fear memory (sound + electric foot shock). However, if the rat was put in the same place again without an electric foot shock, the fear memory was suppressed to some extent. However, on the following day, when the same sound was played without an electric foot shock, the fear response reappeared. Reinstatement of extinct freezing behaviors reflects the impairment of fear extinction due to dysregulation of the HPA axis and activation of the PFC and HIP, which are structures within the fear circuit (Feng et al. 2021; Wang et al. 2018). Also, the increased freezing behavior of our rats was highly spatially and context-dependent. However, administration of LU led to a decrease in freezing behavior following the attenuation of fear and short-term extinction. Therefore, LU can improve PTSD-like behavior mediated by fear memory due to enhanced fear extinction.
Depression and anxiety-like behaviors have been identified as comorbid symptoms in both human and animal studies of PTSD (Careaga et al. 2016; Jang et al. 2021). After SPS exposure, our rats exhibited increased immobility time in the FST, which indicated more fear and behavioral despair due to traumatic stress (i.e. forced swimming). In addition, after exposure to SPS in the EPM test, the rats spent significantly less time in, and made fewer entries into, the open arms. This reflected an increase in anxiety-like behavior on exposure to an aversive experience novel and threatening situation (Gao et al. 2019). Increased depression and anxiety-like behaviors are associated with CORT level and changes in monoamine levels in various brain regions (Gao et al. 2019). Our results showed that administration of LU helped to suppress SPS-induced depression and anxiety-like behavior.
Similar to the unpredictable chronic stress model, the SPS model is associated with HPA axis sensitization; elevated CORT is increased due to dysfunction of the HPA axis and alterations in negative feedback mechanisms (Cohen et al. 2009). In PTSD, excessive CORT secretion may disrupt homeostasis due to dysregulation of the HPA axis in response to stress (Krishnamurthy et al. 2013). Accordingly, in this study, exposure to SPS led to increased plasm CORT and ACTH levels, although there was no significant change in CRH level. Elevated CORT level cause behavioral changes, such as anxiety, following the processing of traumatic stimuli (Gao et al. 2019). However, administration of LU may reduce plasma CORT level and excitability of the HPA axis, as well as ameliorate dysregulation of the HPA axis, thereby improving fear, depressive, and anxiety behaviors.
After SPS exposure, the plasma CRH level did not significantly change, as stated above. Because CRH neurons are widely distributed in the central nervous system, including in the hypothalamus, changes in the concentration of CRH can be affected by some of the numerous factors should be stated (Walker et al. 2009). For example, expression of CRH in the AMY is associated with stress-induced anxious behavior in rats (Walker et al. 2009). CRH neurons in the paraventricular hypothalamic nucleus of the hypothalamus are important in the activation of the HPA axis associated with PTSD, although changes in plasma CRH level are inconsistent.
PTSD involves the AMY, which processes primitive emotions, and the PFC, which suppresses emotional response and impulses, such as fear, through reason (Schöner et al. 2017). In patients with PTSD, disturbances in the adrenergic system in the structures within the fear circuit cause behavioral dysregulation (Schöner et al. 2017). An increase in NE in PTSD is associated with avoidance and re-experience of traumatic stress (Krystal and Neumeister 2009). This leads to depression and anxiety, ever after the memory of the traumatic event is extinguished (Krystal and Neumeister 2009). In our study, SPS-induced rats showed increased levels of NE in the PFC and HIP. However, administration of LU suppressed the increase of NE in the PFC and HIP caused by SPS. This suggests that LU can prevent an imbalance of catecholamines, which is an important pathophysiological factor in PTSD.
5-HT regulates behavior and emotions, and suppresses aggressive behavior on exposure to new environments (Krystal and Neumeister 2009; Wang et al. 2018). Reduced levels of 5-HT increase fear, leading to aggressive behavior, emotions such as sadness and depressive-like symptoms (Krystal and Neumeister 2009; Wang et al. 2018). Our SPS-induced rats had decreased 5-HT in the structures within the fear circuit, including the PFC and HIP (Krystal and Neumeister 2009; Gao et al. 2019). However, administration of LU restored normal 5-HT levels in the PFC and HIP, suggesting that LU can prevent an imbalance of 5-HT, which is an important pathophysiological factor in PTSD.
In our study, mRNA expression of GR and MR was decreased in the HIP of SPS-exposed rats. However, plasma CORT levels increased in the HIP. Elevation of CORT due to dysregulation of the HPA axis is associated with co-regulation of GR and MR mRNA in the HIP. The decreased GR mRNA expression in the HIP of our SPS-exposed rats may have been caused by excessive CORT secretion due to dysregulation of the HPA axis (Han et al. 2014). Decreased GR and MR mRNA expression in the HIP is correlated with immobility time in the FST, and with the amount of time spent in, and number of entries into, the open arms in the EPM test. Thus, after SPS exposure, the decrease of GR mRNA seen in the HIP is directly related to maintenance of the fear memory, which can lead to fear, depression, and anxiety (Han et al. 2014). The decreased GR and MR mRNA expression in the HIP associated with the administration of LU is in turn associated with improvements in anxiety-like behavior and negative feedback in the HPA axis in rats (Han et al. 2014). However, administration of LU suppressed the decreased GR and MR mRNA expression associated with SPS, but did not lead to complete recovery. Therefore, the antidepressant and anti-anxiety effects of administration of LU is related to the modulation of HPA axis, but it does not appear to be directly related to the reduction of GR protein. Therefore, our results suggested that antidepressant and anti-anxiety effects caused by LU is mediated via HPA axis-independent mechanism.
Finally, our results showed that administration of LU in an SPS-induced PTSD animal model reduced the dysregulation of the HPA axis, thus improving freezing behavior, and also exerted antidepressant and anti-anxiety effects by modulating modulation of monoamine neurotransmitters. These results suggest that LU may be a useful therapeutic agent to prevent traumatic stress, such as PTSD.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2020R1A2C1100975).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Albarakati AJA, Baty RS, Aljoudi AM, Habotta OA, Elmahallawy EK, Kassab RB, Abdel Moneim AE.. 2020. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep. 47:2591–2603. [DOI] [PubMed] [Google Scholar]
- Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, Figueira I.. 2009. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 33:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga MBL, Girardi CEN, Suchecki D.. 2016. Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neurosci Biobehav Rev. 71:48–57. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun XB, Lu HE, Wang F, Fan XH.. 2017. Effect of luteoin in delaying cataract in STZ-induced diabetic rats. Arch Pharm Res. 40:88–95. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Savion N, Natar MA, Loewenthal U, Loewenthal N, Zohar J, Kaplan Z.. 2009. An association between stress-induced disruption of the hypothalamic-pituitary-adrenal axis and disordered glucose metabolism in an animal model of post-traumatic stress disorder. J Neuroendocrinol. 21:898–909. [DOI] [PubMed] [Google Scholar]
- Corley MJ, Caruso MJ, Takahashi LK.. 2012. Stress-induced enhancement of fear conditioning and sensitization facilitates extinction-resistant and habituation-resistant fear behaviors in a novel animal model of posttraumatic stress disorder. Physiol Behav. 105:408–416. [DOI] [PubMed] [Google Scholar]
- Dong J, Xu O, Wang J, Shan C, Ren X.. 2021. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-kappaB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol. 43:319–327. [DOI] [PubMed] [Google Scholar]
- Faye C, Mcgowan JC, Denny CA, David DJ.. 2018. Neurobiological mechanisms of stress resilience and implications for the aged population. Curr Neuropharmacol. 16:234–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JH, Sim SM, Park JS, Hong JS, Suh HW.. 2021. Modulation of corticosterone and changes of signal molecules in the HPA axis after cold water swimming stress. Anim Cells Syst. 25:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokkens AS, Groothoff JW, van der Klink JJ, Popping R, Stewart RE, van de Ven L, Brouwer S, Tuinstra J.. 2015. The mental disability military assessment tool: a reliable tool for determining disability in veterans with post-traumatic stress disorder. J Occup Rehabil. 25:569–576. [DOI] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner J.. 2007. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 24:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZW, Ju RL, Luo M, Wu SL, Zhang WT.. 2019. The anxiolytic-like effects of ginsenoside Rg2 on an animal model of PTSD. Psychiatry Res. 279:130–137. [DOI] [PubMed] [Google Scholar]
- Guo DJ, Li F, Yu PHF, Chan SW.. 2013. Neuroprotective effects of luteolin against apoptosis induced by 6-hydroxydopamine on rat pheochromocytoma PC12 cells. Pharm Biol. 51:190–196. [DOI] [PubMed] [Google Scholar]
- Han F, Ding JL, Shi YX.. 2014. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single prolonged stress rats. BMC Neurosci. 77:1471–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytti M, Piippo N, Korhonen E, Honkakoski P, Kaarniranta K, Kauppinen A.. 2016. Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation. Sci Rep. 5:17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Jung T, Kang M, Kim J, Noh J.. 2021. Oxytocin-induced anxiogenic behavior in juvenile male rats. Anim Cells Syst. 25:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kwon CS, Son KH.. 2000. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 64:2458–2461. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick HA, Heller GM.. 2014. Post-traumatic stress disorder: theory and treatment update. Int J Psychiatry Med. 47:337–346. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Garabadu D, Joy KP.. 2013. Risperidone ameliorates post-traumatic stress disorder-like symptoms in modified stress re-stress model. Neuropharmacology. 75:62–77. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A.. 2009. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 1293:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sur B, Oh S.. 2020. Neuroprotective effect of Korean red ginseng against single prolonged stress-induced memory impairments and inflammation in the rat brain associated with BDNF expression. J Gin Res. 44:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Luo W, Qian Y, Zhu W, Qian J, Li J, Jin Y, Xu X, Liang G.. 2019. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 59:152774. [DOI] [PubMed] [Google Scholar]
- Rajapriya S, Geetha AJ.. 2021. Effect of luteolin on the gene level expression of apoptosis-associated speck-like protein containing a caspase recruitment domain of NLRP3 inflammasome and NF-kappaB in rats subjected to experimental pancreatitis – influence of HSP70. Basic Clin Physiol Pharmacol. 24:1–10. [DOI] [PubMed] [Google Scholar]
- Schöner J, Heinz A, Endres M, Gertz K, Kronenberg G.. 2017. Post-traumatic stress disorder and beyond: an overview of rodent stress models. J Cell Mol Med. 21:2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB.. 2011. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 13:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M.. 2009. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 33:1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang H, Cheng H, Che Z.. 2016. Ameliorating effect of luteolin on memory impairment in an Alzheimer's disease model. Mol Med Rep. 13:4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Lin CC, Chen CC, Tzeng NS, Liu YP.. 2018. Effects of oxytocin on fear memory and neuroinflammation in a rodent model of posttraumatic stress disorder. Int J Mol Sci. 19:3848–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Wang K, Yuan C, Xing R, Ni J, Hu G, Chen F, Wang X.. 2017. Luteolin protects mice from severe acute pancreatitis by exerting HO1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med. 39:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu JC.. 2018. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem Res. 43:806–820. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Xing JG, Wang LL, Jiang HL, Guo SL, Liu R.. 2017. Luteolin inhibits fibrillary beta-amyloid(1-40)-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-kappaB signaling pathways. Molecules. 22:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]