To the Editor:
There is rising interest in the use of a helmet interface for noninvasive support of hypoxemic patients. Compared with a facemask, putative benefits of the helmet interface include the possibility to provide long-term treatments with high positive end-expiratory pressure (PEEP) with minimal air leaks and good tolerance, which may foster the success of the approach.1, 2, 3, 4, 5 In a single-center preliminary study on patients with acute hypoxemic respiratory failure, use of helmet support, compared with a facemask, resulted in a lower rate of endotracheal intubation and improved survival.6 Whether these results are applicable to other ICUs and among patients with COVID-19 is unknown.
CPAP has been recently shown to improve the clinical outcome of patients with respiratory failure due to COVID-19 in a large clinical trial, but no data were available to discriminate the effects of different interfaces on study results.7
We hereby report the results of a prospective single-center cohort study, which was conducted to compare the clinical outcome of patients with acute hypoxemic respiratory failure due to COVID-19 and treated with helmet vs facemask CPAP.
The study was conducted at the Hospital General de Agudos Dr Juan A. Fernández (Buenos Aires, Argentina). The institutional review board reviewed the protocol and authorized prospective data collection (code register: 2263). All consecutive patients admitted to the respiratory ICU because of COVID-19 respiratory failure from June 2020 to September 2021 were initially treated with high-flow nasal oxygen if one of the following criteria was met: Pao 2/Fio 2 ≤ 200 mm Hg, supplemental oxygen requirement ≥ 10 L/min, or respiratory rate ≥ 25 breaths/min with or without the use of accessory muscles. All the subjects underwent a 12-h trial of high-flow nasal oxygen at 60 L/min and Fio 2 to maintain Spo 2 (oxygen saturation as determined by pulse oximetry) between 92% and 96%, and, as adjuvant therapy, awake prone positioning. Subjects were considered responders to high-flow nasal oxygen when their respiratory rate was < 30 breaths/min and Spo 2 increased to > 94% with Fio 2 < 0.6% at the end of the 12-h trial. Patients who did not meet these criteria after the trial were considered nonresponders, and respiratory support was escalated to CPAP. The interface for delivering CPAP (helmet or facemask) was chosen according to the individual patient’s preference. CPAP was delivered by dedicated ventilator (Astral 150; ResMed) provided with a low-pressure oxygen source via a nonvented oronasal mask with a blue elbow (FreeMotion RT041; Fisher & Paykel) or a helmet (Ecleris). With an oronasal mask, a double-limb circuit with an expiratory valve was used; with a helmet, we used a single-limb circuit with a PEEP valve placed on the exhalation port of the interface. In both groups, CPAP was initially set at 10 cm H2O with an increase up to 14 cm H2O if needed because of worsening oxygenation, and Fio 2 to maintain Spo 2 between 92% and 96%. Continuous CPAP treatment for the initial 24 h was delivered in all studied patients. Afterward, CPAP and high-flow nasal oxygen were used alternatively within a strategy of rotation therapy to increase comfort and tolerance to treatments. No cross-over between helmet CPAP and facemask CPAP was allowed.
Patients who were pregnant, hypercapnic (Paco 2 > 45 mm Hg), or had a do-no-intubate order were not considered for inclusion in the study.
During the period of the study, to avoid delays in endotracheal intubation and to standardize the treatments delivered, we used predetermined objective criteria to define the need for intubation.1 , 8 These included (1) signs of persisting or worsening respiratory failure, defined by at least two of the following: lack of improvement or worsening oxygenation, respiratory rate > 40 breaths/min, lack of improvement in signs of respiratory muscle fatigue, development of copious tracheal secretions, acidosis with pH < 7.35, or intolerance to CPAP; or (2) one of the following: hemodynamic instability (systolic BP < 90 mm Hg, mean BP < 65 mm Hg, or requirement for vasopressor) or deterioration of neurologic status with a Glasgow Coma Scale score < 12 points.
The primary objective of this study was to compare the rate of endotracheal intubation between patients who received helmet vs facemask CPAP: secondary end points included in-hospital mortality and physiological parameters during treatment. Data are expressed as frequencies (%) or medians (interquartile range). Comparisons between groups were performed by χ2 test, Fisher exact test, repeated-measures analysis of variance, or Mann-Whitney test, as appropriate. Multivariable analyses on endotracheal intubation and mortality were conducted through a backward elimination procedure on the Cox proportional hazards regression model, considering the following predictors: Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, age, Pao 2/Fio 2 before treatment start, sex, and assigned treatment. The Cox model was iteratively fitted and the predictor with the highest nonsignificant P value was eliminated at each step, until all remaining optional predictors were simultaneously significant at the P ≤ .05 level. Results with P ≤ .05 were considered statistically significant.
Among 121 patients who failed the high-flow nasal oxygen trial during the study period, six patients were not included because of hypercapnia and three because of a do-no-intubate order. One hundred and twelve patients were included in the study: 55 were treated with helmet CPAP and 57 with facemask CPAP. Demographics, clinical characteristics, and outcomes for the study groups are displayed in Table 1 . Demographics and clinical severity at study admission were not different between the groups. Median (interquartile range) Pao 2/Fio 2 while receiving high-flow oxygen before CPAP initiation was 96 (89-106) mm Hg in patients who subsequently received helmet CPAP group and 101 (87-111) mm Hg in patients who subsequently received facemask CPAP (P = .25).
Table 1.
Baseline Characteristics of Patients and Study Outcomes, According to Study Group
| Characteristic | Helmet CPAP (n = 55) | Facemask CPAP (n = 57) | P Valueda |
|---|---|---|---|
| Age, y | 57 (48-64) | 57 (43-66) | .51 |
| Female, No. (%) | 12 (22) | 20 (35) | .12 |
| APACHE II score | 9 (7-11) | 9 (7-12) | .93 |
| SOFA score | 4 (3-4) | 4 (3-4) | .38 |
| BMI, kg/m2 | 30 (27-34) | 30 (27-35) | .41 |
| Respiratory rate at admission, breaths/min | 28 (25-32) | 30 (26-34) | .16 |
| Spo2/Fio2 at CPAP start | 118 (115-119) | 115 (113-119) | .53 |
| Pao2/Fio2 at CPAP start, % | 96 (89-106) | 101 (87-111) | .25 |
| Paco2 at CPAP start, mm Hg | 34 (33-38) | 34 (32-39) | .84 |
| Comorbidities | |||
| Obesity, No. (%) | 34 (62) | 39 (68) | .46 |
| Hypertension, No. (%) | 11 (20) | 16 (28) | .32 |
| Diabetes, No. (%) | 2 (4) | 6 (11) | .16 |
| COPD, No. (%) | 0 (0) | 2 (4) | .49 |
| Asthma, No. (%) | 2 (4) | 5 (9) | .26 |
| Cardiovascular disease, No. (%) | 1 (2) | 4 (7) | .18 |
| Concomitant medications | |||
| Dexamethasone, No. (%) | 55 (100) | 57 (100) | > .999 |
| Remdesivir, No. (%) | 0 (0) | 2 (4) | .49 |
| Prophylactic anticoagulation, No. (%) | 15 (27) | 12 (21) | .44 |
| Therapeutic anticoagulation, No. (%) | 7 (13) | 4 (7) | .31 |
| Primary outcome | |||
| Endotracheal intubation, No. (%) | 16 (29) | 28 (49) | .029 |
| Time to intubation, d | 3 (2-7) | 1 (1-5) | .18 |
| Reason for intubation | |||
| Signs of respiratory muscles fatigue, No. (%) | 9 (16) | 10 (18) | .87 |
| Hypoxemia, No. (%) | 3 (5) | 10 (18) | .05 |
| Worsening or unbearable dyspnea, No. (%) | 3 (5) | 8 (14) | .13 |
| Pulmonary thromboembolism, No. (%) | 1 (2) | 0 (0) | .49 |
| Secondary outcomes | |||
| PEEP, cm H2O | 14 (12-14) | 10 (10-12) | < .001 |
| Fio2, % | 0.4 (0.4-0.5) | 0.6 (0.4-0.6) | .099 |
| Total hours of CPAP | 211 (130-311) | 151 (42-249) | .009 |
| Respiratory rate at end of CPAP treatment | 22 (19-26) | 25 (22-30) | .002 |
| Spo2/Fio2 at end of CPAP treatment | 251 (189-320) | 187 (150-243) | .001 |
| In-hospital mortality, No. (%) | 10 (18) | 20 (35) | .049 |
| Duration of stay in the hospital, d | 16 (14-25) | 21 (13-29) | .37 |
Results are displayed as median (interquartile range), if not otherwise specified. APACHE II = Acute Physiology and Chronic Health Evaluation II; PEEP = positive end-expiratory pressure; SOFA = Sequential Organ Failure Assessment; Spo2 = oxygen saturation as determined by pulse oximetry.
All calculations are unadjusted. Endotracheal intubation and in-hospital mortality were treated as time-to-event data.
Patients in the helmet CPAP group received longer-term treatments with higher PEEP.
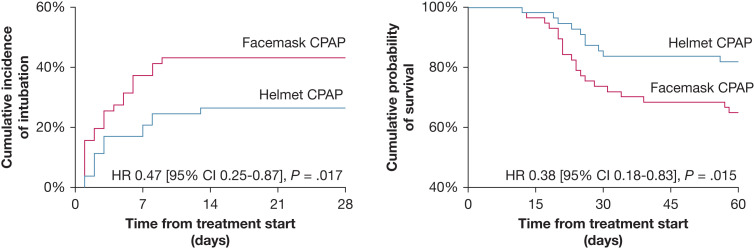
The rate of endotracheal intubation was significantly lower in the helmet group than in the facemask group: 29% vs 49%, with a hazard ratio adjusted for SOFA score and Pao 2/Fio 2 at inclusion of 0.47 (95% CI, 0.25-0.87; P = .017) (Fig 1 ). The Spo 2/Fio 2 ratio during treatment was higher, and the respiratory rate was lower, in patients treated with helmet CPAP than among those who received facemask CPAP (analysis of variance for repeated measures, P < .05 for both). Among the causes that led to endotracheal intubation, patients in the helmet group showed a significantly lower incidence of hypoxemia (5% vs 18%; P = .005). In-hospital mortality was lower in the helmet group than in the facemask group: 18% vs 35%, with a hazard ratio adjusted for sex, SOFA score, and APACHE II score at inclusion of 0.38 (95% CI, 0.18-0.83; P = .015) (Fig 1).
Figure 1.
Kaplan-Meier plots of time-to-event data for the two study groups: endotracheal intubation (left) and in-hospital mortality (right). HR = hazard ratio.
The results of the present study suggest that CPAP delivered via the helmet interface, as compared with a facemask, may prevent endotracheal intubation in patients with COVID-19 with moderate-to-severe respiratory failure. The effect on clinical outcome seems mediated mostly by improved oxygenation and the possibility to provide longer-term treatments with higher PEEP, which are known factors possibly able to improve the success of noninvasive respiratory support in hypoxemic patients.1 , 6 , 9 Use of high PEEP for longer-term treatments with good patient comfort is the most relevant advantage of using the helmet interface.10
Despite the nonrandomized design of this investigation and the small sample analyzed, these findings suggest a possible clinical benefit of helmet over facemask CPAP in patients with COVID-19 respiratory failure. Further randomized studies systematically assessing the clinical effects of helmet CPAP in COVID-19 and in other hypoxemic patients appear warranted to foster its use in other ICUs.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: J. M. A.-Í. discloses a relationship with Vygon. D. L. G. has received payments for travel expenses by Getinge and Air Liquide; speaking fees from Intersurgical, Gilead, Pfizer, General Electric Healthcare, and Fisher & Paykel; and discloses a research grant from General Electric Healthcare. None declared (N. C.-A., G. C. M., M. L. V., G. M.).
Acknowledgments
Author contributions: N. C.-A. and D. L. G. conceived the study. All authors contributed to data acquisition. N. C.-A. and G. C. M. conducted statistical analysis. N. C.-A. and G. C. M. interpreted the data and wrote the first draft of the manuscript. D. L. G. critically revised the manuscript. All the authors reviewed the final draft of the manuscript and agreed to submit it to CHEST.
References
- 1.Grieco D.L., Menga L.S., Cesarano M., et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grieco D.L., Maggiore S.M., Roca O., et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47(8):851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menga L.S., Berardi C., Ruggiero E., Grieco D.L., Antonelli M. Noninvasive respiratory support for acute respiratory failure due to COVID-19. Curr Opin Crit Care. 2022;28(1):25–50. doi: 10.1097/MCC.0000000000000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreyro B.L., Angriman F., Munshi L., et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure. JAMA. 2020;324(1):57. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rittayamai N., Grieco D.L., Brochard L. Noninvasive respiratory support in intensive care medicine. Intensive Care Med. 2022;48(9):1211–1214. doi: 10.1007/s00134-022-06762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel B.K., Wolfe K.S., Pohlman A.S., Hall J.B., Kress J.P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins G.D., Ji C., Connolly B.A., et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19. JAMA. 2022;327(6):546. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frat J.-P., Thille A.W., Mercat A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 9.Grieco D.L., Menga L.S., Cesarano M., et al. Phenotypes of patients with COVID-19 who have a positive clinical response to helmet noninvasive ventilation. Am J Respir Crit Care Med. 2022;205(3):360–364. doi: 10.1164/rccm.202105-1212LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco D.L., Patel B.K., Antonelli M. Helmet noninvasive support in hypoxemic respiratory failure. Intensive Care Med. 2022;48(8):1072–1075. doi: 10.1007/s00134-022-06737-7. [DOI] [PubMed] [Google Scholar]