Abstract
Background:
This population-based study considered the influence of rituximab on the survival of children (0–19 years), adolescents, and young adults (AYAs, 20–39 years) with diffuse large B-cell lymphoma (DLBCL), including patients with human immunodeficiency virus (HIV) infection.
Methods:
Data on 642 children and AYAs diagnosed with DLBCL during 2001–2014 were obtained from the Greater Bay Area Cancer Registry in California. Facility-level reports provided treatment details. The Kaplan–Meier method estimated survival and Cox regression models examined the association between survival and rituximab use, adjusting for sociodemographic and clinical factors.
Results:
Rituximab use increased from 2001–2007 to 2008–2014 among children (from 32% to 48%), AYAs (from 68% to 84%), and HIV patients (from 57% to 67%). Five-year survival was higher among children (91%) than AYAs (82%). On multivariable analysis, the hazard of death was 44% lower among rituximab recipients, and higher among uninsured patients, those with HIV, and those with advanced stage at diagnosis. HIV patients who received rituximab were 60% less likely to die than nonrecipients.
Conclusions:
Our study suggests a benefit of rituximab on the treatment of AYAs and HIV patients with DLBCL. The worse survival observed among HIV-positive and uninsured patients is of concern and calls for further investigation. Careful consideration should be given on whether to recommend rituximab more often on the front-line treatment of children and HIV-positive patients with DLBCL.
Keywords: AYA, children, DLBCL, population-based study, rituximab, survival
1 |. INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most frequent non-Hodgkin lymphoma (NHL) in adolescents and young adults (AYAs, 20–39 years) and the fourth most common in children (0–19 years) in the United States.1 Despite its aggressive development, most patients with DLBCL can be cured with appropriate immunochemotherapy, even when diagnosed at advanced stage. However, patients who do not respond to front-line treatment have very poor survival.2
The introduction of rituximab, a monoclonal anti-CD20 antibody, represented a major change in the treatment and survival of DLBCL. Over the two decades of its availability, rituximab use increased progressively and is now considered a gold-standard treatment for this malignancy.3 However, the survival benefit and safety profile of rituximab are less clear among pediatric and AYAs with NHL.4 Although AYAs with NHL have experienced significant survival improvement in the last 20 years, survival has not improved to the same extent in AYAs as in children.5
To our knowledge, no population-based study has considered the receipt of rituximab among children and AYAs with DLBCL. Therefore, using cancer registry data with abstracted treatment details, we investigated the influence of rituximab on the survival of children and AYAs with DLBCL, including patients with human immunodeficiency virus (HIV).
2 |. METHODS
2.1 |. Data selection
We used data from the Greater Bay Area Cancer Registry (GBACR), which is part of the Surveillance, Epidemiology and End Results (SEER) Program, and covers nine California counties (Alameda, Contra Costa, Marin, Monterey, San Bento, San Francisco, San Mateo, Santa Clara, and Santa Cruz). Eligible cases were patients aged ≤ 39 years when diagnosed with first primary DLBCL (International Classification of Diseases for Oncology, 3rd edition codes 9679–9680, 9684, and 9688) during 2001–2014. Facility-level reports for each patient were reviewed in order to abstract details on treatment regimens, identifying whether rituximab was given during the first course of treatment.
Sociodemographic and clinical variables included gender (male/female), age at diagnosis (0–19 and 20–39 years), stage at diagnosis based on the summary stage [(localized (stage I), regional (stage II), or advanced (stage III/IV)], HIV status (positive/negative), health insurance [private, public (includes medicaid, medicare, or military insurances), uninsured, or unknown], treatment facility [whether initial diagnosis and/or treatment occurred at a National Cancer Institute–designated cancer center (NCIDCC)], race/ethnicity [non-Hispanic white (white), non-Hispanic black (black), Hispanic, and Asian/Pacific Islander (Asian)], and neighborhood socioeconomic status (nSES). HIV status is a standard data item abstracted for lymphomas in the GBACR. In addition, all facility-level reports were reviewed for HIV status. Patients without evidence of HIV positivity in either source were assumed to be HIV negative. Our aggregate measure of nSES is based on census block data and captures several indicators of SES: education, unemployment, poverty, household income, rent, and home value.6 SES was classified in quintiles based on the distribution of census blocks in California. We hypothesized that rituximab use would have increased over time and categorized year of diagnosis in two equal periods (2001–2007 and 2008–2014).
Ethics approval for human subject research was obtained from the Cancer Prevention Institute of California (CPIC) Institutional Review Board. As the analysis was based on state-mandated cancer registry data, the study was conducted in accordance with the waivers of individual informed consent and Health Insurance Portability and Accountability Act authorization.
2.2 |. Statistical analysis
Survival time was estimated from the date of DLBCL diagnosis until the date of death for any cause, or until the patient was censored as alive due to loss to follow-up or end of the study (December 31, 2014), whichever occurred first. We used the Kaplan–Meier method to estimate 5-year overall survival and the log-rank test to examine differences in survival across strata of each covariate. Univariable and multivariable Cox proportional hazards models were performed to obtain the hazard ratio of death (HR) and corresponding 95% confidence interval (CI) for each covariate. We also performed separate models for patients with HIV to better investigate the influence of rituximab among these patients. In addition, we used univariable and multivariable logistic regression models to investigate the association between rituximab use and each covariate.
Results are presented as odds ratios (OR) and 95% CI.
In order to investigate whether potential changes in chemotherapy and/or radiation over time could have influenced our results, we conducted sensitivity analyses including these factors in the multivariable models. We also investigated whether lack of health insurance was associated with nSES using frequency distribution and univariate logistic regression analysis. All statistical tests were carried out using SAS software version 9.3, and all P values reported were two-sided.
3 |. RESULTS
We obtained data on 642 patients (88 children and 554 AYAs) diagnosed during 2001–2014. Patients were predominantly male (62.6%), of white race/ethnicity (43.3%) had private insurance (67.4%), lived in the highest nSES (40.3%), and were treated at non-NCIDCC (69.6%). Most patients had advanced stage at diagnosis (37.1%). Ninety patients (14.0%) had HIV infection (3 children and 87 AYAs). Approximately 40.9% of children (n = 36) and 75.8% of AYAs (n = 420) received rituximab. Rituximab use increased from 2001–2007 to 2008–2014 for both children (from 31.5% to 48.0%) and AYAs (from 68.2% to 84.4%) (Table 1). Among patients with HIV, rituximab use also increased from 57.1% in 2001–2007 to 66.7% in 2008–2014 (data not shown).
TABLE 1.
Sociodemographic and clinical characteristics of patients aged 0–39 years, 2001–2014, California, by receipt of rituximab
| Receipt of rituximab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| All |
Yes |
No |
Unknown |
||||||
| n | % | n | % | n | % | n | % | P valuea | |
| Total | 642 | 100·0 | 456 | 100·0 | 164 | 100·0 | 22 | 100·0 | |
|
| |||||||||
| Age at diagnosis, years | <0·0001 | ||||||||
| 0–19 | 88 | 13·7 | 36 | 7·9 | 50 | 30·5 | <5 | 9·1 | |
| 20–39 | 554 | 86·3 | 420 | 92·1 | 114 | 69·5 | 20 | 90·9 | |
|
| |||||||||
| Period of diagnosis | <0·0001 | ||||||||
| 2001–2007 | 330 | 51·4 | 211 | 46·3 | 111 | 67·7 | 8 | 36·4 | |
| 0–19 years | 38 | 5·9 | 12 | 2·6 | 24 | 14·6 | <5 | 9·1 | |
| 20–39 years | 292 | 45·5 | 199 | 43·6 | 87 | 53·0 | 6 | 27·3 | |
| 2008–2014 | 312 | 48·6 | 245 | 53·7 | 53 | 32·3 | 14 | 63·6 | |
| 0–19 years | 50 | 7·8 | 24 | 5·3 | 26 | 15·9 | 0 | 0·0 | |
| 20–39 years | 262 | 40·8 | 221 | 48·5 | 27 | 16·5 | 14 | 63·6 | |
|
| |||||||||
| Sex | 0·1270 | ||||||||
| Male | 402 | 62·6 | 278 | 61·0 | 111 | 67·7 | 13 | 59·1 | |
| Female | 240 | 37·4 | 178 | 39·0 | 53 | 32·3 | 9 | 40·9 | |
|
| |||||||||
| Race/ethnicity | 0·0899 | ||||||||
| Non-Hispanic white | 278 | 43·3 | 196 | 43·0 | 70 | 42·7 | 12 | 54·5 | |
| Non-Hispanic black | 58 | 9·0 | 35 | 7·7 | 20 | 12·2 | <5 | 13·6 | |
| Hispanic | 141 | 22·0 | 96 | 21·1 | 42 | 25·6 | <5 | 13·6 | |
| Asian/Pacific Islander | 155 | 24·1 | 122 | 26·8 | 29 | 17·7 | <5 | 18·2 | |
| Other/unknown | 10 | 1·6 | 7 | 1·5 | <5 | 1·8 | 0 | 0·0 | |
|
| |||||||||
| Stage at diagnosis | 0·0041 | ||||||||
| Localized | 223 | 34·7 | 146 | 32·0 | 67 | 40·9 | 10 | 45·5 | |
| Regional | 172 | 26·8 | 130 | 28·5 | 35 | 21·3 | 7 | 31·8 | |
| Advanced | 238 | 37·1 | 177 | 38·8 | 56 | 34·1 | 5 | 22·7 | |
| Unknown | 9 | 1·4 | <5 | 0·7 | 6 | 3·7 | 0 | 0·0 | |
|
| |||||||||
| Chemotherapy | <0·0001 | ||||||||
| No | 57 | 8·9 | 5 | 1·1 | 50 | 30·5 | <5 | 9·1 | |
| Yes | 577 | 89·9 | 448 | 98·2 | 114 | 69·5 | 15 | 68·2 | |
| Unknown | 8 | 1·2 | <5 | 0·7 | 0 | 0·0 | 5 | 22·7 | |
|
| |||||||||
| Treatment at a NCI-designated cancer center | 0·1983 | ||||||||
| No | 447 | 69·6 | 309 | 67·8 | 120 | 73·2 | 18 | 81·8 | |
| Yes | 195 | 30·4 | 147 | 32·2 | 44 | 26·8 | <5 | 18·2 | |
|
| |||||||||
| Neighborhood SES | 0·9476 | ||||||||
| First (low) | 43 | 6·7 | 32 | 7·0 | 10 | 6·1 | <5 | 4·5 | |
| Second | 65 | 10·1 | 47 | 10·3 | 16 | 9·8 | <5 | 9·1 | |
| Third | 119 | 18·5 | 86 | 18·9 | 28 | 17·1 | <5 | 22·7 | |
| Fourth | 156 | 24·3 | 108 | 23·7 | 43 | 26·2 | <5 | 22·7 | |
| Fifth (high) | 259 | 40·3 | 183 | 40·1 | 67 | 40·9 | 9 | 40·9 | |
|
| |||||||||
| Health insurance | 0.4889 | ||||||||
| No insurance | 54 | 8·4 | 34 | 7·5 | 18 | 11·0 | <5 | 9·1 | |
| Private | 433 | 67·4 | 311 | 68·2 | 105 | 64·0 | 17 | 77·3 | |
| Publicb | 142 | 22·2 | 103 | 22·5 | 36 | 22·0 | <5 | 13·6 | |
| Unknown | 13 | 2·0 | 8 | 1·8 | 5 | 3·0 | 0 | 0·0 | |
|
| |||||||||
| HIV status | 0.0242 | ||||||||
| Negative | 552 | 86·0 | 402 | 88·2 | 133 | 81·1 | 17 | 77·3 | |
| 0–19 years | 85 | 13·2 | 34 | 7·5 | 49 | 29·9 | <5 | 9·1 | |
| 20–39 years | 467 | 72·7 | 368 | 80·7 | 84 | 51·2 | 15 | 68·2 | |
| Positive | 90 | 14·0 | 54 | 11·8 | 31 | 18·9 | 5 | 22·7 | |
| 0–19 years | <5 | 0·5 | <5 | 0·4 | <5 | 0·6 | 0 | 0·0 | |
| 20–39 years | 87 | 13·6 | 52 | 11·4 | 30 | 18·3 | 5 | 22·7 | |
Abbreviations: HIV, human immunodeficiency virus; NCI, National Cancer Institute; SES, socioeconomic status.
Chi-squared test assessing whether the distribution of sociodemographic and clinical characteristics differ by rituximab use.
Includes Medicaid, Medicare, or Military insurances.
The median follow-up time was 5.0 years for both children and AYAs. Overall, 5-year survival improved over time, from 79.0% during 2001–2007 to 87.3% during 2008–2014, and was higher among children (90.7%) than AYAs (81.7%; Figure 1; Table 2). Among AYAs, 5-year survival was higher for those who received rituximab (84.4%) vs those who did not receive this treatment (70.2%). Five-year survival did not differ by receipt of rituximab in the pediatric group (90.0% vs 90.7% for rituximab recipients vs not recipients, respectively) (Figure 2 and Table 2). AYAs with HIV who received rituximab had higher 5-year survival (59.7%) than AYAs with HIV who did not receive this treatment (34.2%) (Table 2).
FIGURE 1.
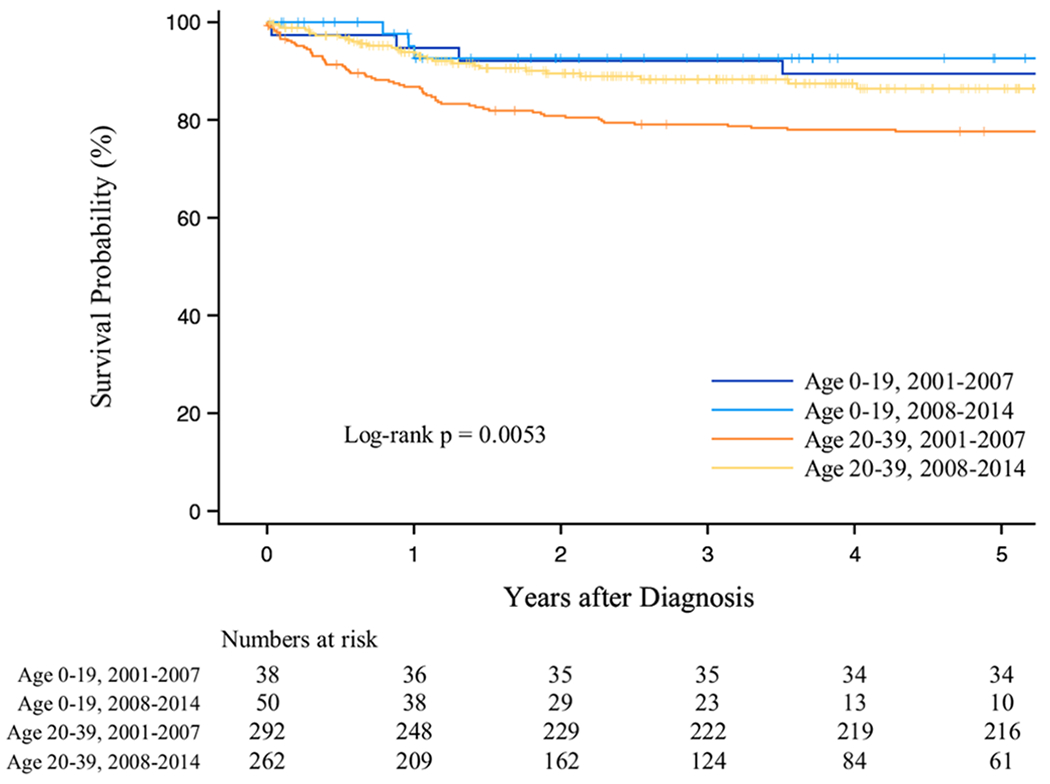
Overall survival after DLBCL by age group and calendar period of diagnosis, 2001–2014, California
TABLE 2.
Five-year overall survival after diffuse large B-cell lymphoma among patients aged 0–39 years during 2001–2014 in California
| 5-year overall survival (95% CI) |
|||
|---|---|---|---|
| All patients | HIV patients excluded | HIV patients only | |
| All patients | 82·9 (85·8–79·5) | 87·7 (90·4–84·4) | 53·6 (63·5–42·4) |
|
| |||
| Age at diagnosis, year | |||
| 0–19 | 90·7 (95·5–81·4) | 90·5 (95·4–80·9) | N/A |
| 20–39 | 81·7 (84·8–78·0) | 87·3 (90·2–83·6) | 52·4 (62·4–41·1) |
|
| |||
| Period of diagnosis | |||
| 2001–2007 | 79·0 (83·1–74·2) | 87·0 (90·5–82·2) | 45·9 (57·6–33·3) |
| 2008–2014 | 87·3 (91·1–82·2) | 88·6 (92·3–83·2) | 74·5 (87·8–51·5) |
|
| |||
| Sex | |||
| Male | 80·1 (83·8–75·5) | 86·7 (90·2–82·2) | 51·6 (62·4–39·5) |
| Female | 87·7 (91·5–82·4) | 89·3 (92·9–83·9) | 63·5 (83·0–32·8) |
|
| |||
| Race/ethnicity | |||
| Non-Hispanic white | 83·5 (87·6–78·2) | 88·0 (91·7–82·8) | 46·4 (63·3–27·6) |
| Non-Hispanic black | 79·9 (88·4–66·6) | 93·8 (98·4–77·3) | 59·0 (76·2–35·9) |
| Hispanic | 72·1 (79·2–63·2) | 77·3 (84·7–67·1) | 52·9 (68·9–33·7) |
| Asian/Pacific Islander | 92·3 (95·7–86·5) | 93·3 (96·5–87·5) | 74·1 (93·0–28·9) |
| Other/unknown | 90·0 (98·5–47·3) | 90·0 (98·5–47·3) | N/A |
|
| |||
| Stage at diagnosis | |||
| Localized | 90·1 (93·5–85·0) | 92·6 (95·7–87·6) | 68·2 (83·4–44·6) |
| Regional | 88·6 (92·8–82·2) | 90·7 (94·7–84·2) | 70·6 (86·6–43·0) |
| Advanced | 72·3 (77·8–65·7) | 80·3 (85·7–73·4) | 39·5 (53·6–25·1) |
| Unknown | 75·0 (93·1–31·5) | 83·3 (97·5–27·3) | 50·0 (91·0–0·6) |
|
| |||
| Rituximab use | |||
| No | 76·5 (82·5–68·9) | 85·9 (91·1–78·3) | 36·6 (53·7–19·6) |
| 0–19 years | 90·7 (96·4–76·7) | 90·4 (96·4–76·2) | N/A |
| 20–39 years | 70·2 (78·0–60·5) | 83·2 (89·9–72·8) | 34·2 (51·8–17·4) |
| Yes | 84·8 (88·0–80·8) | 88·1 (91·1–84·1) | 60·6 (72·5–45·7) |
| 0–19 years | 90·0 (96·7–72·1) | 89·7 (96·5–71·3) | N/A |
| 20–39 years | 84·4 (87·7–80·2) | 87·9 (91·1–83·7) | 59·7 (71·8–44·7) |
| Unknown | 90·9 (97·6–68·3) | 94·1 (99·1–65·0) | 80·0 (96·9–20·4) |
|
| |||
| Treatment at an NCI-designated cancer center | |||
| No | 82·9 (86·2–78·9) | 87·7 (90·8–83·7) | 54·5 (66·0–41·2) |
| Yes | 82·6 (87·7–75·7) | 87·5 (92·1–80·3) | 51·3 (68·8–30·3) |
|
| |||
| Neighborhood SES | |||
| First (low) | 70·1 (82·5–51·9) | 66·4 (81·4–44·1) | 78·8 (94·3–38·1) |
| Second | 80·6 (89·3–66·3) | 81·1 (90·3–65·0) | 79·5 (94·5–39·3) |
| Third | 77·5 (84·4–68·3) | 87·3 (93·0–77·5) | 44·0 (61·9–24·5) |
| Fourth | 85·0 (89·9–78·1) | 90·5 (94·5–83·8) | 44·4 (65·1–21·6) |
| Fifth (high) | 86·7 (90·4–81·6) | 90·5 (93·8–85·6) | 52·6 (69·8–31·9) |
|
| |||
| Health insurance | |||
| Private | 87·4 (90·4–83·6) | 90·1 (92·8–86·4) | 58·9 (73·4–40·5) |
| No insurance | 74·7 (84·5–60·4) | 84·1 (92·6–67·8) | 41·7 (66·5–15·2) |
| Publica | 72·0 (79·0–63·2) | 79·6 (86·8–69·3) | 53·3 (67·6–36·5) |
| Unknown | 83·1 (95·5–47·2) | 90·0 (98·547·3) | N/A |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; N/A, not applicable; NCI, National Cancer Institute; SES, socioeconomic status.
Includes Medicaid, Medicare, or Military insurance.
FIGURE 2.
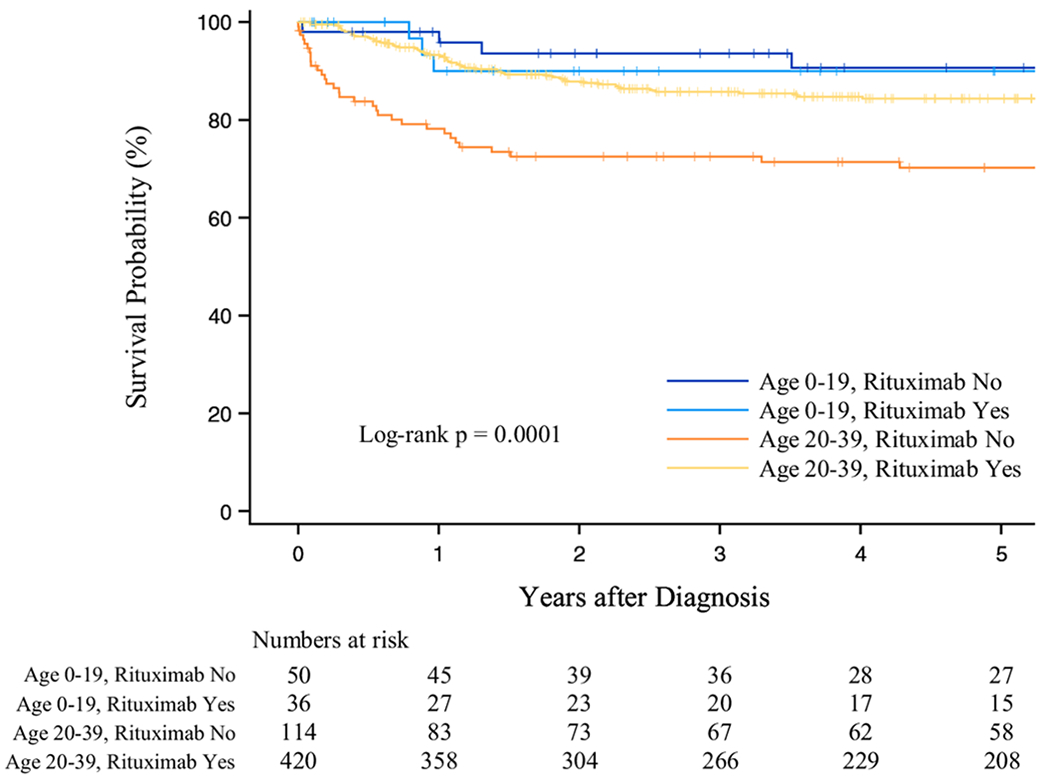
Overall survival after DLBCL by age group and rituximab use, 2001–2014, California
On univariable analysis, patients diagnosed during 2008–2014 were nearly 50% less likely to die than patients diagnosed during 2001–2007 (HR = 0.53; 0.35–0.81). The hazard of death was significantly lower among patients who received rituximab (HR = 0.57; 0.38–0.84), females (HR.0.65; 0.43–0.99), and Asians (vs. whites; HR = 0.49; 0.26–0.94). Hispanics were more likely to die than whites (HR = 2.03; 1.32–3.13), as were patients with advanced stage at diagnosis (vs. localized; HR = 3.09; 1.92–4.96), those who lived in the lowest nSES (vs. highest; HR = 2.37; 1.23–4.57), non-privately insured patients (HR = 2.25; 1.25–4.06 for uninsured and 2.67; 1.77–4.03 for publicly insured patients), and those with HIV (HR = 5.73; 3.91–8.40; Table 3).
TABLE 3.
Hazard ratios of death for patients aged 0–39 years, diagnosed with diffuse large B-cell lymphoma, during 2001–2014 in California
| Univariable HR (95% CI) | Adjusted HR (95% CI)a | |
|---|---|---|
| Age at diagnosis, years | ||
| 0–19 | Reference | Reference |
| 20–39 | 2.04 (0.99–4.19) | 1.94 (0.91–4.12) |
|
| ||
| Period of diagnosis | ||
| 2001–2007 | Reference | Reference |
| 2008–2014 | 0.53 (0.35–0.81) | 0.71 (0.45–1.12) |
|
| ||
| Sex | ||
| Male | Reference | Reference |
| Female | 0.65 (0.43–0.99) | 0.95 (0.60–1.50) |
|
| ||
| Race/ethnicity | ||
| Non-Hispanic white | Reference | Reference |
| Non-Hispanic black | 1.60 (0.86–2.98) | 0.92 (0.46–1.83) |
| Hispanic | 2.03 (1.32–3.13) | 1.44 (0.90–2.32) |
| Asian/Pacific Islander | 0.49 (0.26–0.94) | 0.75 (0.39–1.45) |
| Other/unknown | 0.57 (0.08–4.16) | 0.62 (0.08–4.63) |
|
| ||
| Stage at diagnosis | ||
| Localized | Reference | Reference |
| Regional | 1.05 (0.57–1.94) | 1.04 (0.56–1.96) |
| Advanced | 3.09 (1.92–4.96) | 2.47 (1.50–4.05) |
| Unknown | 2.74 (0.65–11.63) | 1.47 (0.32–6.71) |
|
| ||
| Rituximab use | ||
| No | Reference | Reference |
| Yes | 0.57 (0.38–0.84) | 0.56 (0.37–0.85) |
| Unknown | 0.54 (0.17–1.76) | 0.58 (0.17–1.93) |
|
| ||
| Treatment at an NCI-designated cancer center | ||
| No | Reference | Reference |
| Yes | 0.96 (0.63–1.46) | 1.12 (0.72–1.74) |
|
| ||
| Neighborhood SES (quintiles) | ||
| First (low) | 2.37 (1.23–4.57) | 1.11 (0.55–2.25) |
| Second | 1.48 (0.75–2.92) | 1.15 (0.57–2.34) |
| Third | 1.66 (1.00–2.76) | 1.36 (0.79–2.36) |
| Fourth | 1.24 (0.74–2.08) | 1.21 (0.71–2.05) |
| Fifth (high) | Reference | Reference |
|
| ||
| Health insurance | ||
| Private | Reference | Reference |
| No insurance | 2.25 (1.25–4.06) | 1.89 (1.03–3.46) |
| Publicb | 2.67 (1.77–4.03) | 1.43 (0.90–2.26) |
| Unknown | 1.22 (0.30–5.00) | 1.47 (0.35–6.25) |
|
| ||
| HIV status | ||
| Negative | Reference | Reference |
| Positive | 5.73 (3.91–8.40) | 3.48 (2.20–5.50) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; NCI, National Cancer Institute; SES, socioeconomic status.
Adjusted for all variables in the table.
Includes Medicaid, Medicare, or Military insurance.
On multivariable analyses, the hazard of death remained about 44% lower for patients who received rituximab compared with those who did not receive it (HR = 0.56; 0.37–0.85). After adjustment for covariates, the hazard of death remained higher among patients who were uninsured (HR = 1.89; 1.03–3.46) or publicly insured (HR = 1.43; 0.90–2.26) versus privately insured individuals, those with advanced versus localized disease at diagnosis (HR = 2.47; 1.50–4.05), and HIV patients (HR = 3.48; 2.20–5.50) (Table 3). In separate models, limited to HIV patients, those who received rituximab were 60% less likely to die than those who did not receive this treatment (HR = 0.40; 0.21–0.78) (Supporting Information Table S1). Although AYAs had a higher hazard of death than children in both univariable and multivariable models, the results did not reach statistical significance (Table 3).
On univariable analysis, the odds of receipt of rituximab were significantly lower among HIV patients (OR = 0.58; 0.36–0.93). Patients more likely to receive rituximab were AYAs (vs. children, OR = 5.12; 3.18–8.23) and those diagnosed in the later calendar period (2008–2014 vs 2001–2007; OR = 2.43; 1.67–3.54) (Table 4).
TABLE 4.
Odds ratios of receipt of rituximab for patients aged 0–39 years, diagnosed with diffuse large B-cell lymphoma during 2001–2014 in California
| Univariable OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Age at diagnosis | ||
| 0–19 years | Reference | Reference |
| 20–39 years | 5.12 (3.18–8.23) | 8.33 (4.77–14.56) |
|
| ||
| Period of diagnosis | ||
| 2001–2007 | Reference | Reference |
| 2008–2014 | 2.43 (1.67–3.54) | 2.94 (1.89–4.59) |
|
| ||
| Sex | ||
| Male | Reference | Reference |
| Female | 1.34 (0.92–1.96) | 1.14 (0.74–1.75) |
|
| ||
| Race/ethnicity | ||
| Non-Hispanic white | Reference | Reference |
| Non-Hispanic black | 0.62 (0.34–1.15) | 0.52 (0.25–1.07) |
| Hispanic | 0.82 (0.52–1.29) | 0.71 (0.41–1.23) |
| Asian/Pacific Islander | 1.50 (0.92–2.45) | 1.72 (0.99–2.98) |
| Other/unknown | 0.83 (0.21–3.31) | 0.71 (0.16–3.07) |
|
| ||
| Stage at diagnosis | ||
| Localized | Reference | Reference |
| Regional | 1.70 (1.06–2.73) | 1.48 (0.87–2.49) |
| Advanced | 1.45 (0.96–2.20) | 1.63 (1.01–2.64) |
| Unknown | 0.23 (0.06–0.95) | 0.27 (0.06–1.31) |
|
| ||
| Treatment at an NCI-designated cancer center | ||
| No | Reference | Reference |
| Yes | 1.30 (0.87–1.93) | 1.27 (0.79–2.02) |
|
| ||
| Neighborhood SES (quintiles) | ||
| First (low) | 1.17 (0.55–2.51) | 1.38 (0.57–3.35) |
| Second | 1.08 (0.57–2.02) | 1.26 (0.59–2.69) |
| Third | 1.12 (0.68–1.87) | 1.54 (0.84–2.80) |
| Fourth | 0.92 (0.59–1.44) | 0.96 (0.58–1.60) |
| Fifth (high) | Reference | Reference |
|
| ||
| Health insurance | ||
| Private | Reference | Reference |
| No insurance | 0.64 (0.35–1.18) | 0.66 (0.33–1.32) |
| Publicb | 0.96 (0.62–1.48) | 1.19 (0.68–2.07) |
| Unknown | 0.54 (0.17–1.69) | 0.59 (0.17–2.02) |
|
| ||
| HIV status | ||
| Negative | Reference | Reference |
| Positive | 0.58 (0.36–0.93) | 0.55 (0.31–0.99) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NCI, National Cancer Institute; OR, odds ratio; SES, socioeconomic status.
Adjusted for all variables in the table.
Includes Medicaid, Medicare, or Military insurance.
On multivariable analyses, the odds of receiving rituximab remained lower among HIV patients (OR = 0.55; 0.31–0.99). The odds ratios were higher for AYAs versus children (OR = 8.33; 4.77–14.56), for patients diagnosed more recently (2008–2014 vs 2001–2007; OR = 2.94; 1.89–4.59), and among those with advanced versus localized disease (OR = 1.63; 1.01–2.64) (Table 4).
We found that the proportion of patients who received CHOP was similar in the two calendar periods of diagnosis (69% during 2001–2007 vs 63% during 2008–2014, P = 0.078), whereas the use of radiation decreased over time from 44.2% during 2001–2007 to 26% during 2008–2014 (P < 0.001). However, neither chemotherapy regimen nor use of radiation significantly changed the associations we found between survival and rituximab use or survival and other demographic or clinical variables in models, so these variables were not included in the multivariable models. In addition, we found that health insurance status was independent of nSES (data not shown).
4 |. DISCUSSION
Our study revealed that rituximab use increased in California in the more recent years of diagnosis (2008–2014) for both children and AYAs, which corresponds to the period post-rituximab approval by the US Food and Drug Administration as front-line treatment for DLBCL. AYAs were over eight times more likely to receive rituximab than children, which reflects the more general practice of treating AYAs with DLBCL with adult regimens compared with children who are usually treated with intensive “Burkitt-like” lymphoma protocols, without rituximab.1 Our findings suggest a survival benefit for AYAs who received rituximab compared with those who receive chemotherapy alone. Interestingly, we found that children had similar survival whether they received rituximab or not. Future studies are necessary to investigate whether the addition of rituximab to pediatric DLBCL regimens could be beneficial in reducing late chemotherapy-related toxicity. Recently, a randomized phase III trial that assessed the addition of rituximab to standard therapy for high-risk children and adolescents with B-cell NHL and Burkitt lymphoma revealed superior results with a 1-year event-free survival of 94·2% in the rituximab arm versus 81·5% in the control arm.7
Treatment outcomes for patients with HIV-related lymphoma have improved in the era of effective antiretroviral therapy (ART), in particular for DLBCL and Burkitt lymphoma, and curability of HIV-related lymphoma is considered similar to that of HIV-unrelated lymphoma.8 However, we found that patients with HIV-related DLBCL had worse survival, consistent with a recent US study.9 A previous study conducted by the Eastern Cooperative Oncology Group showed that rituximab plus chemotherapy is very effective to treat HIV-associated B-cell NHL among patients aged ≥18 years, and no increase of infection-related deaths was observed.10 Based on this study and a clinical trial conducted by Dunleavy et al,11 colleagues from the US NCI recommend the use of rituximab with chemotherapy in the front-line treatment of all patients with HIV-related aggressive lymphomas.8 A later study, which used data pooled from 19 prospective clinical trials, revealed that the use of rituximab with concurrent ART and chemotherapy was associated with longer survival among patients with HIV-associated CD20-positive lymphomas.12 Although we observed that rituximab use increased over time by approximately 30% for patients with HIV, HIV patients were still less likely to receive rituximab as part of their initial treatment than those without HIV. Notably, HIV patients who received rituximab had 60% better survival than HIV-negative patients who did not receive this treatment. We did not observe increased incidence of infection-related deaths among HIV patients who received rituximab. These relevant findings reinforce the need to enroll HIV patients in prospective clinical trials13 and carefully consider the use of rituximab on the front-line treatment of HIV-related DLBCL patients.
The survival differences we found between AYAs and children were not statistically significant, which may be explained by the relative modest number of children in our sample. However, an important question is whether the borderline differences in survival between children and AYAs could be related to disease biology. It has been shown that distinct molecular characteristics of DLBCL influence survival. For example, among adult patients, those with germinal-center B-cell–like (GCB) tumor have better survival following treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) than those with activated B-cell–like (ABC) disease. The GCB is the primary subtype observed in childen,14 whereas the ABC subtype increases with age.15 Other biologic features that are associated with increasing age are chromosomal translocations involving both cMyc and BCL2 proteins, leading to double-hit lymphoma; or involvement of cMyc, BCL2, and BCL6, leading to triple-hit lymphoma, both entities related to poor survival.16 The extent to which these biologic characteristics impact survival among AYAs is unclear and merits further research.
Medical care at hospitals that enroll AYAs in clinical trials is crucial to timely and effective treatment. However, enrollment of AYAs in these trials is usually very low in the United States (approximately 1%−2% for patients aged 20–39 years).17 Consequently, crucial information on tumor biology and treatment outcomes is lost, preventing fundamental investigation focused on protocol improvement. In our study, we did not observe significant difference in survival between patients treated at NCIDCC and those treated at other centers. However, this should be interpreted with caution because we did not have information on whether patients initially diagnosed and/or treated at non-NCIDCC were later referred to NCIDCC or vice-versa.
We observed that the majority of AYAs were diagnosed with advanced disease, and these patients had about 2·5 times higher hazard of death than those with localized disease, consistent with previous studies.18 We also found that patients who lacked health insurance had substantially higher hazard of death than those privately insured. In the United States, lack of insurance has been associated with being diagnosed at advanced cancer stage and receiving suboptimal medical care, leading to worse survival.19 Several factors can influence the access to high-quality care, at the patient, medical provider, and/or health system levels. According to previous reports, AYAs were the largest and fastest-growing population in the United States who lacked insurance or were underinsured.20 Yet, a recent study showed that among nonelderly patients diagnosed with cancer, the proportion of those without insurance decreased substantially after the implementation of the Affordable Care Act, mainly among low-income and vulnerable patients. Interestingly, a trend toward early-stage diagnosis was found for some types of cancers.21
Residence in the lowest nSES at time of diagnosis was associated with lower survival on the univariable analysis, but this association did not remain on the multivariable analysis. This result differs from previous studies of AYAs and older adults with NHL in California that observed worse survival among those residing in lower SES neighborhoods.18,22,23 Our findings may have differed from these prior studies as we have used more recent data and adjusted our models for rituximab receipt. It is possible that the greater use of rituximab observed in the later years outweighed the detrimental effects of lower nSES. To support this hypothesis, our study suggested that access to rituximab was not different for patients living in lower versus higher nSES.
Although we observed a survival disadvantage for Hispanic compared with white patients on the univariable analysis, the association was reduced and no longer statistically significant on the multivariable analysis. A previous study in the United States,24 which examined survival from NHL among patients aged ≥18 years over 40 years using SEER data, found that white patients with DLBCL had better median survival than nonwhite patients. Remarkably, the largest survival gap was between whites and Hispanics (median survival was 5–8 vs 2–8 years, respectively). However, this study did not consider the use of rituximab, health insurance, or nSES. In our study, the likelihood of receiving rituximab did not differ by race/ethnicity.
Our study was limited in that we lacked data on tumor molecular characteristics, detailed chemotherapy and radiotherapy regimens, occurrence of relapse, and whether a patient received hematopoietic stem cell transplantation or participated in clinical trials, information not routinely or completely collected by population-based cancer registries. Despite these limitations, we were able to access important treatment information at the facility level and examine survival among children and AYAs by rituximab use, including those with HIV-related DLBCL. In addition, we concomitantly investigated the influence of multiple factors on survival such as health insurance, stage at diagnosis, race/ethnicity, nSES, and treatment facility.
In summary, to our knowledge, this is the first population-based study to examine the receipt of rituximab among children and AYAs with DLBCL. Our findings suggest a benefit of using rituximab on the treatment of AYAs with DLBCL and raise a relevant question on whether the conventional high-dose chemotherapy strategies for children with DLBCL could be reduced when integrated with rituximab. At the population level, our findings suggest that the use of rituximab associated with chemotherapy is beneficial for HIV-related DLBCL patients. The worse survival observed among HIV and uninsured patients is of concern and warrants further investigation. Moreover, late stage at diagnosis continues to be an independent strong predictor of outcome and highlights the paramount importance of early cancer diagnosis to improve cancer survival.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Li Tao for her statistical support in the initial phase of this study.
FUNDING
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Abbreviations:
- ABC
activated B-cell-like
- ART
antiretroviral therapy
- AYA
adolescent and young adult
- CI
confidence interval
- CPIC
Cancer Prevention Institute of California
- DLBLC
diffuse large B-cell lymphoma
- GBACR
Greater Bay Area Cancer Registry
- GCB
germinal center B-cell–like
- HIV
human immunodeficiency virus
- HR
hazard ratio
- NCIDCC
National Cancer Institute–designated cancer center
- NHL
non-Hodgkin lymphoma
- nSES
neighborhood socioeconomic status
- OR
odds ratio
- R-CHOP
rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- SAS
statistical analysis system
- SEER
Surveillance, Epidemiology, and End Results
- US
United States
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Hochberg J, El-Mallawany NK, Brugieres L, McMillan A, Cairo MS. Non-Hodgkin lymphoma. In: Bleyer A, Barr R, Ries L, Whelan J, Ferrari A, eds. Cancer in Adolescents and Young Adults Pediatric Oncology. Cham: Springer; 2017. [Google Scholar]
- 2.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Murawski N, Zeynalova S, et al. Optimization of rituximab for the treatment of DLBCL: increasing the dose for elderly male patients. Br J Haematol. 2017;179:410–420. [DOI] [PubMed] [Google Scholar]
- 4.Kobos R, Terry W. Advances in therapies for non-Hodgkin lymphoma in children. Hematology Am Soc Hematol Educ Program. 2015;2015: 522–528. [DOI] [PubMed] [Google Scholar]
- 5.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016. [DOI] [PubMed] [Google Scholar]
- 6.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control: CCC. 2001;12:703–711. [DOI] [PubMed] [Google Scholar]
- 7.Minard-Colin V, Auperin A, Pillon M, Burke A, Anderson JR, Barkauskas DA. Results of the randomized intergroup trial inter-B-NHL Ritux 2010 for children and adolescents with high risk B-cell non-Hodgkin’s lymphoma and mature acute leukemia: evaluation of rituximab efficacy in addition to standard LMB chemotherapy regimen. J Clin Oncol. 2016;34. [Google Scholar]
- 8.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119:3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X, Jemal A, Hulland E, et al. HIV infection and survival of lymphoma patients in the era of highly active antiretroviral therapy. Cancer Epidemiol Biomarkers Prev. 2017;26:303–311. [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunleavy K, Little RF, Pittaluga S, et al.The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122:3251–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. J Clin Oncol. 2017;35:3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oschlies I, Klapper W, Zimmermann M, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin–Frankfurt–Munster) multicenter trial. Blood. 2006;107:4047–4052. [DOI] [PubMed] [Google Scholar]
- 15.Klapper W, Kreuz M, Kohler CW,et al. Patient age at diagnosis is associated with the molecular characteristics of diffuse large B-cell lymphoma. Blood. 2012;119:1882–1887. [DOI] [PubMed] [Google Scholar]
- 16.Aukema SM, Siebert R,Schuuring E,et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. [DOI] [PubMed] [Google Scholar]
- 17.Bleyer A, O’Leary M, Barr R, Ries L. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Institutes of Health, National Cancer Institute; 2006. NIH Pub. No. 06-5767:1-14-173-190. [Google Scholar]
- 18.Kent EE, Morris RA, Largent JA, Ziogas A, Sender LS, Anton-Culver H. Socioeconomic impacts on survival differ by race/ethnicity among adolescents and young adults with non-Hodgkin’s lymphoma. J Cancer Epidemiol. 2010;2010:824691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. [DOI] [PubMed] [Google Scholar]
- 20.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110:2385–2393. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Lin CC, Davidoff AJ, Han X. Changes in insurance coverage and stage at diagnosis among nonelderly patients with cancer after the Affordable Care Act. J Clin Oncol. 2017;35:3906–3915. [DOI] [PubMed] [Google Scholar]
- 22.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. 2014;123:3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao C, Chiu V, Xu L, Cooper RM. Survival differences by race/ethnicity and neighborhood socioeconomic status in adolescents and young adults diagnosed with non-Hodgkin lymphoma. J Adolesc Young Adult Oncol. 2015;4:76–83. [DOI] [PubMed] [Google Scholar]
- 24.Crozier JA, Sher T, Yang D, et al. Persistent disparities among patients with T-cell non-Hodgkin lymphomas and B-cell diffuse large cell lymphomas over 40 years: a SEER database review. Clin Lymphoma Myeloma Leuk. 2015;15:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


