Abstract
A spinal cord injury (SCI) occurs when the spinal cord is deteriorated or traumatized, leading to motor and sensory functions lost even totally or partially. An imbalance within the generation of reactive oxygen species and antioxidant defense levels results in oxidative stress (OS) and neuroinflammation. After SCI, OS and occurring pathways of inflammations are significant strenuous drivers of cross-linked dysregulated pathways. It emphasizes the significance of multitarget therapy in combating SCI consequences. Polyphenols, which are secondary metabolites originating from plants, have the promise to be used as alternative therapeutic agents to treat SCI. Secondary metabolites have activity on neuroinflammatory, neuronal OS, and extrinsic axonal dysregulated pathways during the early stages of SCI. Experimental and clinical investigations have noted the possible importance of phenolic compounds as important phytochemicals in moderating upstream dysregulated OS/inflammatory signaling mediators and axonal regeneration's extrinsic pathways after the SCI probable significance of phenolic compounds as important phytochemicals in mediating upstream dysregulated OS/inflammatory signaling mediators. Furthermore, combining polyphenols could be a way to lessen the effects of SCI.
1. Introduction
Neurodegenerative disorders (NDDs) progressively affect millions worldwide as significant causes of disability and death, despite progress in considering various dysregulated routes in the pathophysiology of NDDs. The main pathophysiological processes of NDDs are still unknown [1–4]. Spinal cord injury (SCI) is an NDD that causes sensory-motor impairment and significantly lowers the standard of living. SCI is becoming more common among people aged 14.6 to 67.6 years old, and men are four times more likely than women [5, 6]. SCI has primary and secondary phases from a pathophysiological standpoint. The secondary step comprises inherent oxidative stress (OS), autophagic, apoptotic, and inflammatory routes. Direct injuries occur after spinal mechanical trauma [7].
In contrast, extrinsic routes have an essential role in SCI, such as glial scar development and destruction [8]. Extrinsic pathways are coupled with intrinsic processes such as OS, neuroinflammation, and neuroapoptosis (e.g., axonal signaling). Thus, the preceding pathogenic pathways negatively affect neurodegeneration and neurodegenerative mechanisms, eventually leading to apoptosis. Antioxidant defenses modulate neuroinflammatory and neuroapoptosis responses, which influence microglia, astrocytes, and related mediators and have a considerable position in the initiation and development of SCI [9, 10].
It is crucial to highlight that developing new plant medications has a compelling track record in producing unconventional therapeutics. Incidentally, the plant kingdom has demonstrated encouraging outcomes in that against SCI. Polyphenols/phenolic combinations are obtainable phytochemicals and can act as multiple targeted drugs with excellent selectivity and minimal toxicity among natural substances, because of their broad biological activity and therapeutic properties are now used in contemporary medications to construct and acquire novel treatments. In many NDDs, these substances have been regarded as reliable nutritional mediators with potent repressive impacts on OS and inflammation [11]. Emerging research has recently focused on utilizing organic neuroprotective polyphenols with putative antioxidant properties to treat SCI and NDDs [12]. This review discussed about the oxidative-mediated polyphenols' role in controlling and managing SCI.
2. Methodology
PubMed, Scopus, and Web of Science were all used to conduct this literature review. The terms polyphenols, SCI, oxidative stress, reactive oxygen species, preclinical studies, and clinical studies were utilized. We selected and analyzed English-published research papers, narrative review articles, and primary research articles until June 2022. An algorithm used the flowchart imposed in Figure 1 (according to Page et al.'s guidelines [13]) and contained all of the steps/selection constraints for the required literature.
Figure 1.
The stages of picking data for inclusion in the existing research are illustrated in a flow chart; n = number of literature reports.
3. Spinal Cord Injury Pathophysiology
SCI is categorized into primary, secondary, and chronic [14, 15]. The first stage is the physical forces related to the original traumatic event, often the essential factors of injury severity, causing the first stage. Compression, shearing, laceration, and severe stretch/distraction are examples of these forces [16]. Following the original injury, a series of subsequent occurrences occur. The damage worsens in the second stage, and neurological impairments and consequences worsen [17, 18]. After the first injury, secondary SCI is a gradual and progressive injury (Figure 2).
Figure 2.
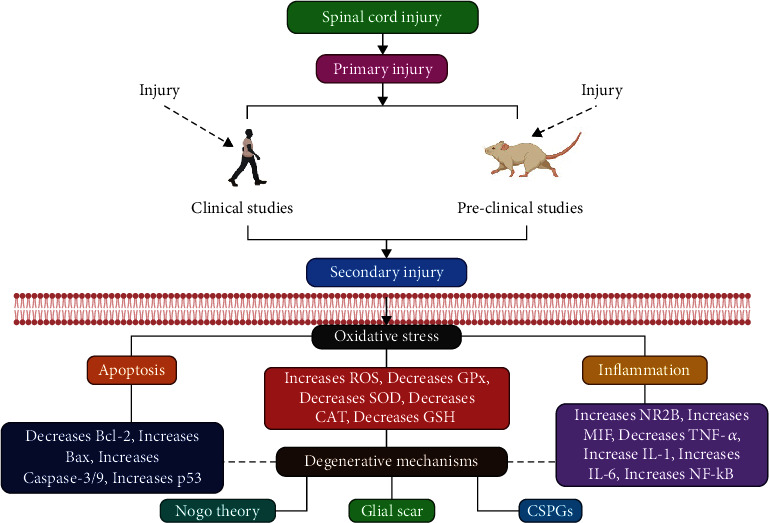
Pathophysiology of SCI: spinal cord injury. ROS: reactive oxygen species; GPx: glutathione peroxidase; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; MIF: macrophage migration inhibitory factor; TNF-α: tumor necrosis factor-alpha; and NF-κB: nuclear factor kappa B.
Furthermore, a chronic stage could affect the orthograde and retrograde routes and brian-specific regions; moreover, according to the time scale, chronic stages can start from days to years following the damage [19, 20]. Several vascular alterations are detected during the secondary cascade [21]—neutrophils and macrophages and role in releasing superoxide anion and hydrogen peroxide to sanitize the wounded area. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a significant superoxide anion originator of superoxide anion that plays a role in activating the hematogenous phagocytic cells [22]. Moreover, the phagocytic inflammatory cells work as reactive oxygen species (ROS) producers. At the same time, the free radicals respond to polyunsaturated fatty acids, which lead to a phospholipid structural design disruption of cellular and subcellular organelle membranes. Furthermore, aldehyde molecules produced by lipid peroxidation prevent metabolic enzymes, such as Na+/K+-ATPase, from working precisely [23].
SCI causes an increase in cytokines containing tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), as well as overexpression of nuclear factor kappa B (NF-κB), activator protein 1 (AP-1), c-Jun N-terminal Kinase (JNK), and other inflammatory and apoptotic factors like p38, mitogen-activated protein kinase (MAPK), and prostaglandin E2 (PGE2) [24]. The generation of excitation amino acids involving glutamate from damaged cells increases the discharge of excitation amino acids after SCI [25, 26].
Additionally, the glial scar formation, microglia/macrophages, reactive astrocytes, and extracellular matrix molecules—particularly chondroitin sulfate proteoglycans—at the chronic phase play a vital part in preventing axon growth by acting as a protective border [27–29]. Therefore, developing reliable methods and treatments for SCI patients becomes imperative. Reduced ROS levels are an essential approach for SCI management, which can be accomplished by employing antioxidants or drugs that standardize or modulate ROS signaling routes [30, 31].
4. Spinal Cord Injury and Oxidative Stress
Reactive nitrogen species (RNS) and ROS are frequently formed endogenously. However, an increased ROS construction may outpace the antioxidant defense capability, leading to OS and oxidative destruction (Figure 3) [32–35]. Superoxide is created by the NADPH oxidase (NOX), mitochondrial electron transport chain, and xanthine oxidase (XO), which response to nitric oxide (NO) manufactured by the nitric oxide synthase (NOS) to generate the peroxynitrite (ONOO) [36, 37].
Figure 3.
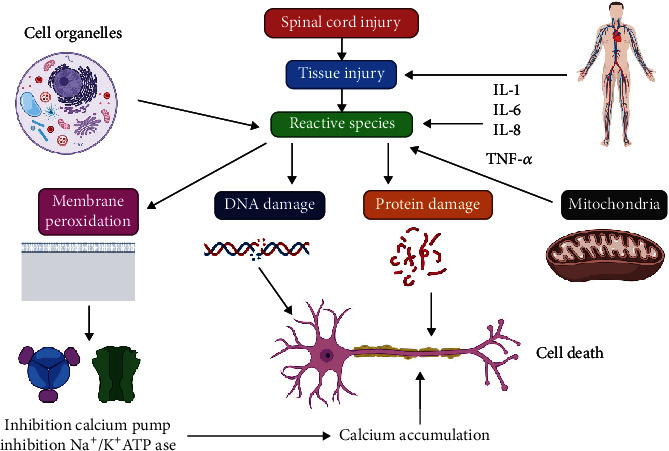
SCI can be facilitated by oxidative stress. TNF-α: tumor necrosis factor-alpha.
Superoxide dismutase (SOD) is an enzyme that transforms oxygen (O2) into hydrogen peroxide (H2O2). There are two similar forms of SOD: (1) copper (Cu)/zinc(Zn)-SOD and (2) manganese(Mn)-SOD. Zn plays a considerable part in the antioxidant defense scheme. According to the databases, the Zn condition and time-dependent modifications following SCI are still unknown [38–42]. The analysis of Zn dynamics in 38 cervically damaged SCI patients yielded a prediction prototype for continuing functional prediction [41]. Heller and colleagues [42] looked at the vigorous variations in serum Zn intensity in short periods throughout the preliminary 72 hours after injury to see a link between early changes in total Zn serum levels and NDDs and patient outcomes. They discovered that patients with the cognitive disease have higher median Zn concentrations in the initial 9 hours after injury than patients with vertebral fractures who do not have neurological dysfunction. They established that the result is associated with early Zn level dynamics and could be an investigative tool for these patients. Alterations in serum Zn levels allow early assessing the risk of neurological damage [42].
In this context, it was discovered that Zn therapy aided motor control restoration in the 28 days that followed SCI and reduced ROS and increased antioxidant potential [43]. The Fenton reaction allows H2O2 to produce the highly reactive hydroxyl radical (HO•), that considers the leading cause of lipid peroxidation in the presence of iron. Catalase (CAT) and glutathione peroxidase (GPx) convert H2O2 to water and oxygen [44]. SOD, CAT, GPX, and glutathione reductase are the primary endogenous antioxidant enzymes [34].
The enzyme GPX is selenium (Se) dependent. By neutralizing reactive oxygen species (ROS) via GPX and reversible oxidation to glutathione disulfide, GSH acts as an antioxidant (GSSG). Glutathione reductase transforms into GSH. Meanwhile, XO produces superoxide but catalyzes the conversion of xanthine to UA, a compound that may scavenge superoxide. HO is the primary antioxidant in biological fluids. In rats, Se nanoparticles were shown to treat OS-induced SCI [45]. According to Seelig et al., Cu and Se concentrations upon intake and Se and ceruloplasmin levels after one day were indications of likely SCI clearance [46]. Within the secondary injury stage, magnesium (Mg) is assumed to play an important role. A better probability of neurological recovery has been associated with reduced Mg serum concentrations during the first seven days [47]. Mg acts by blocking ROS generation and lipid peroxidation precisely [48].
Acrolein, a reactive aldehyde generated endogenously by lipid peroxidation and involved in SCI, is more responsive than the other HNEs and causes glutathione deprivation [49]. To investigate the antioxidant potential of SCI patients, Bastani et al. examined a vast scope of antioxidant and OS markers. When evaluating persons with SCI to controls, they observed that urine F2-IsoP and specific enzymes (NOX and XO) in vastus lateralis biopsies enhanced while SOD decreased [50, 51].
5. Polyphenols in Spinal Cord Injury
To reduce OS after SCI, many natural polyphenolic combinations have been used [52]. These compounds impede the restoration of molecules following free radical damage and control various dysregulated pathways/mediators, such as blocking production. OH. Such polyphenols have formerly been prospective neuroprotective therapeutics in other OS-related NDDs (Figure 4) [53–55].
Figure 4.
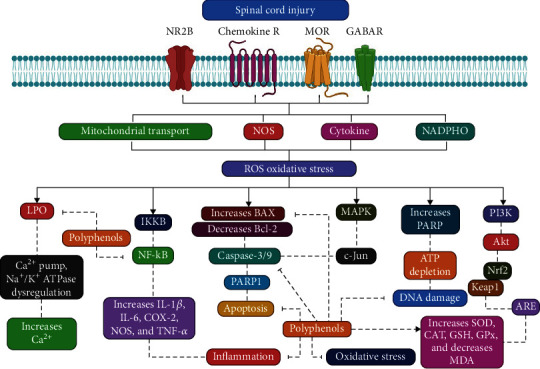
Action mechanism illustration of polyphenols blocking spinal cord injury. LPO: lactoperoxidase; TNF-α: tumor necrosis factor-alpha; NF-κB: nuclear factor kaa-B; GPx: glutathione peroxidase; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; COX-2: cyclooxygenase-2; MDA: malondialdehyde; Nrf2: nuclear factor erythroid 2–related factor 2; PARP1: poly-ADP ribose polymerase 1 (PARP-1).
5.1. Epigallocatechin Gallate
The primary compound of tea catechins is epigallocatechin gallate (EGCG) (Figure 5), often called epicatechin. This composition is related to the biological functions of green tea extracts [56]. EGCG's anti-apoptotic, anti-inflammatory, and antioxidant actions have been demonstrated to prevent against NDDs [57], brain injury [58], SCI [59], and peripheral nerve damage [60] in many experiments conducted. The hydroxyl groups in the catechins ring B and D cause them to interact with free radicals [61]. For 24 hours, various doses of green tea polyphenols (Table 1) (50–200 μg/mL) prevented spinal neurons from oxidative damage caused by H2O2 [62].
Figure 5.
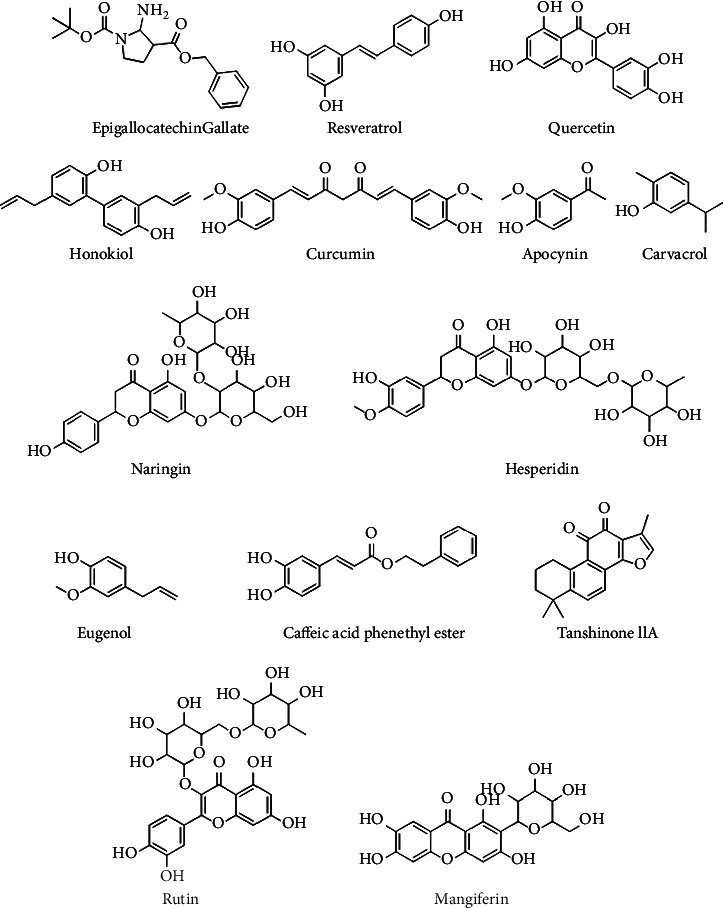
Chemical configurations of some efficient chemical complexes as opposed to spinal cord injury.
Table 1.
Various preclinical investigations have investigated the effect of polyphenols in combating OS and in the after of SCI.
| Polyphenol | Dose/concentration | Study model | Pharmacological mode of actions | References |
|---|---|---|---|---|
| Epigallocatechin gallate | 50 mg/kg (i.p), instantly and one h after SCI | Female SD rats | Diminished Bax and MDA; improved Bcl-2 | [65] |
| 30 mg/kg (i.p.); 7 days after SCI | Female BALB/c mice | Decreased TNF-α and RhoA | [67] | |
| 10, 20 mg/kg (i.t) | Female SD rats | Decreased Bax; increased Bcl-2 and BDNF | [156] | |
| 25 mg/kg (p.o), 1 and 6 h later to SCI | Male adult CD1 mice | Decreased Bax, TNF-α, MPO, MDA, NF-κB, iNOS, PARP; increased Bcl-2 | [157] | |
|
| ||||
| Resveratrol | 1 and 10 mg/kg (p.o); 30 min earlier to SCI | Wistar male rats | Decreased NO and MDA | [159] |
| 400 mg/kg (p.o.); 10 days after SCI | SD male rats | Decreased MDA and IL-6 | ||
| 50, 100 mg/kg (i.p.) | SD male and female rats | Decreased MDA; improved Na+, K+-ATPase activities | [160] | |
| 200 mg/kg (i.p.); until three days after SCI | SD rats | Decreased MDA, MPO, IL-1β, IL-10, and TNF-α; increased SOD | [79] | |
| 50, 100, 200 mg/kg (i.v.); until seven days after SCI | Female mice | Decreased p38MAPK; NF-κB | [158] | |
| 100 mg/kg (i.p.) | Long Evans female rats | Decreased MDA, NO, and TBARS | [161] | |
| 200 mg/kg (i.p.); directly after SCI | Wistar male rats | Enhanced SOD, GPx, and CAT | [162] | |
| 100 mg/kg (i.p), directly after SCI | Male SD rats | Diminished TNF-α, IL-1β, IL-10, and mTOR; enhanced AMPK, LC3, and Beclin-1 | [81] | |
| 200 mg/kg (i.p), Immediately after SCI | Male C57BL/6 mice | Decreased Bax; increased Bcl-2, LC3, and Beclin-1 | [83] | |
|
| ||||
| Quercetin | 10,100mg/kg (i.p), first 3 days after SCI | Wistar male rats | Decreased MDA and NO | [103] |
| 100 mg/kg (i.p.) for three days following SCI | Male SD rats | Decreased ROS, IL-1β, IL-18, and TNF-α | [108] | |
| 20 mg/kg (i.p.), twice per day for seven days following SCI | Wistar albino rats | Decreased MDA, IL-6, TNF-α, and caspase-3 | [105] | |
| Up until ten days following SCI, 7.5 mg/kg (i.p.), two times per day | Female SD rats | Decreased TNF-α, iNOS, NF-κB, and IL 12; enhanced IL-4 IL-10, and TGF-β | [107] | |
|
| ||||
| Honokiol | 20 mg/kg (i.p.) | Female SD rats | Decreased MPO, iNOS, COX-2, IL-1β, IL-6, and TNF-α | [126] |
| 50, 100 mg/kg (i.p.), until three days following SCI | Female SD mice | Decreased MDA, ROS, and TNF-α | [163] | |
|
| ||||
| Curcumin | 100 mg/kg (i.p), 15 min following SCI | Male SD rats, | Decreased IL6, IL1β, TNF-α, NF-κB, and TLR4 | [164] |
| 200 mg/kg (i.p), 1week before SCI | Male Wistar albino rats | Degraded caspase-3, IL1β, TNF-α, MDA, SOD, and GSH | [145] | |
| 60 mg/kg (i.t), directly after SCI, until three weeks, once weekly | Wistar rats | Decreased IL4, IL1β, IL12, and TNF-α, | [143] | |
| 200 mg/kg (i.m), until eight weeks after SCI | Male SD rats | Decreased caspase-3, Bax, and Bcl-2 | [140] | |
| 60 mg/kg (i.m), 30 min after SCI, until three weeks | Male SD rats | Decreased mTOR, p62, and Akt | [165] | |
|
| ||||
| Naringin | 50, 100 mg/kg (p.o.), three days before SCI until seven days after SCI | Male SD rats | Diminished TNF-α, IL8, IL-1β, and IL-6 | [166] |
| 20 mg/kg (i.p.), directly and one h after SCI | Female SD rats | Reduced MDA and Bax; enhanced Bcl-2 and GSH | [167] | |
| 50, 100 mg/kg (i.p), 1week before SCI | Female SD rats | Decreased TNF-α, IL-1β, IL-6, NF-κB, MPO, MDA, and SOD; increased GSH, and CAT | [168] | |
| 20, 40 mg/kg (p.o), until six weeks after SCI | Female SD rats | Decreased caspase-3 and Bax; increased Bcl-2 and BDNF | [151] | |
|
| ||||
| Apocynin | 0.1 mg/kg (i.t) | Male SD rats, | Decreased ROS | [169] |
| 100 mg/kg (i.p) | Male SD rats | Decreased Caspase-1, ROS, NF-κB, JNK, and p38 | [170] | |
| 5 mg/kg (i.p), 1 and 6 h after SCI | Male CD1 mice | Decreased NADPH oxidase, JNK, p38, FasL, MPO, and Bcl-2 | [171] | |
| 5 mg/kg (i.p), 1 and 6 h after SCI until 1week | C57BL/6 female mice | Decreased ROS | [172] | |
|
| ||||
| Carvacrol | 25,75 and 150 mg/kg (i.p) | Male SD rats | Diminished TNF-α, IL-1β, MPO, and NF-κB | [173] |
|
| ||||
| Hesperidin | 100 mg/kg; 7 days before SCI until seven days after SCI | Female SD rats | Decreased IL-1β, NF-κB, and PARP; increased SOD, HO-1, and p-p38 | [174] |
|
| ||||
| Rutin | 30 mg/kg (i.p.) | Rats | Diminished MDA; IL-6; TNF-α; and NF-κB; increased SOD; GSH; CAT | [175] |
| 30 mg/kg (i.p.), until 3 days | Male SD rats | Decreased TNF-α; MDA; ROS; TGF-β1; and Smad2 | [176] | |
|
| ||||
| Mangiferin | 20, 40 mg/kg (i.p.), until 30 days after SCI | Male SD rats | Decreased MDA, NF-κB; increased SOD, GPx, and CAT | [177] |
| 10, 25, 50 mg/kg (i.p.) | SD rats | Decreased MDA, NF-κB, TNF-α, and caspase-9; increased CAT, SOD, and GSH | [178] | |
| 0.2 mg/kg (i.p.), 1 h after SCI | Male SD rats | Decreased iNOS, p38MAPK, MDA, and SOD | [92] | |
| 0.25 μmol/kg (i.p.), 1 h after SCI | Wistar male rats | Decreased MPO | [102] | |
|
| ||||
| Caffeic acid phenethyl ester | 10 μL; 1 μg/kg (i.t.), 1 h after SCI | Wistar female mice | Decreased MDA, SOD, and TOA; increased TAC | [179] |
| 10 μg/kg (i.p.), 30 min after SCI | Wistar female rats | Increased IL-1β, and TNF-α | [180] | |
|
| ||||
| Tanshinone IIA | 50 mg/kg (i.p) 1h before SCI (20 mg/kg) until 7 days after SCI | Male SD rats | Decreased TNF-α, NF-κB, MAPK, and JNK | [181] |
|
| ||||
| Eugenol | 25, 50 mg/kg (p.o), until seven weeks after SCI | Female SD rats | Decreased, NF-κB, and iNOS; increased SOD, and CAT | [182] |
In vitro experiments revealed that PC12 cells to 0-2000 mol/L of EGCG hindered ROS generation [63]. Dosages of EGCG (10, 25, or 50 mg/kg, i.p.) drastically diminished NADPH/neuronal nitric oxide synthase (nNOS) representation following nerve damage in mice [53] and inhibited neurodegeneration by activating the cyclic adenosine monophosphate (cAMP) for 18 days with 25-75 mg/kg dosage scale of myeloperoxidase (MPO) function, inducible TNF-α, interleukin 1 beta (IL-1β), poly-ADP ribose polymerase (PARP), nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) representation were all reduced in the rat spinal cord after a 50 mg/kg dose of EGCG [60, 64].
Khalatbary et al. also swiftly exhibited a 50 mg/kg i.p. injection of EGCG and 1 hour after SCI lowered malonaldehyde (MDA) [65]. In a rat spinal cord organic culture, EGCG at a five-molar level for 48 hours suppressed OS and preserved motor neurons, according to in vitro experiments [66]. Thermal hyperalgesia was minimized in mice after administering 30 mg/kg of EGCG for a week following SCI, inhibiting the expression of RhoA and TNF-α [67].
5.2. Resveratrol
Resveratrol (3,4′,5-trihydroxystilbene) (Figure 5) is a natural phytoalexin identified in Veratrum grandiflorum, grape, and peanut that protects counter to stress damage fungal growth [68, 69]. Resveratrol is a potent antioxidant because it scavenges free radicals, protects against ROS-stimulated DNA damage [70], and reduces the generation of H2O2. Resveratrol significantly suppressed oxidized glutathione reductase [63], GSH function, TNF-α, and IL-1β production [64]. Additionally, resveratrol promoted autophagy by stimulating the nuclear factor erythroid 2–related factor 2 (Nrf2) gene and prevented programmed cell death by increased expression of the sirtuin 1 (SIRT1) gene [71, 72].
According to some studies, resveratrol is a SIRT1 activator that may prevent OS, inflammation, and apoptotic neurons, according to some studies [73]. The SIRT1/Akt1 pathway was developed by resveratrol, resulting in cell survival [74]. Suppressing the TLR-4/MyD88/NF-κB enhanced mitochondrial function/biogenesis [75].
By surpassing the NF-κB signaling pathway, the resveratrol might reduce the SCI health consequence severity [76]. Resveratrol (Table 1) (100 mg/kg, i.p.) induced the activity of p-AMPK, Bcl-2, and SIRT1, while lowering the transcription of p62, caspase-3, caspase-9, and Bax, which following SCI. Resveratrol was also reported to protect neurons by downregulating via the SIRT1/AMPK signaling pathway [77, 78].
Apoptosis-related genes were revealed to be helpful in the SCI rat model by Liu et al. [79]. Resveratrol exhibited anti-apoptotic impacts after SCI, according to Zhang et al., by reducing associated p53, caspase-3, and cytochrome C [80]. Additionally, resveratrol suppressed neuroinflammation following SCI by triggering autophagy by the AMPK/mTOR pathway [81]. Resveratrol significantly benefited neuronal autophagic flux to minimize programmed cell death and stimulate operational repair in rats to post to SCI [82].
A further study demonstrated that resveratrol (200 mg/kg) diminished programmed cell death, OS, and inflammation [30]. In mice, a particular quantity of resveratrol improved autophagic proteins while reducing apoptotic ones [83]. Senturk et al. reported that resveratrol (Table 1) (10 mg/kg) exhibited anti-inflammatory characteristics after SCI [84]. Polydatin (20, 40 mg/kg), a glucoside of resveratrol [85], via the Nrf2/heme oxygenase-1 (HO-1) pathway, suppressed OS and protected apoptosis post-SCI [86].
5.3. Quercetin
Flavonoids such as Quercetin (Figure 5) are observed in several fruits, vegetables, and grains. It exhibits anti-inflammatory, anti-carcinogenic, antioxidant, and antiviral activities, among other pharmacological attributes. Quercetin has also been demonstrated to enhance neuronal dysregulation and mental/physical malfunction by inhibiting lipid peroxidation and capillary penetrability and encouraging mitochondrial biogenesis [87–90]. Quercetin's phenolic hydroxyl groups can effectively scavenge. OH, superoxide anions, and LPO [91]. Quercetin can also connect to conversion metals and inhibit oxidation and decrease, forming metal chelates that can be used to neutralize transition metals, notably copper and iron [92]. Quercetin's neuroprotective properties have been widely exhibited in several in vivo studies. After brain damage considerably reduced GSH levels and MPO function [93]. In traumatic brain damage [94], quercetin boosted the activities of SOD, GPx, and AT, lowered the increased MMP-9 level [95], and regulated the tropomyosin receptor kinase B (TrkB) and brain-derived neurotrophic factor (BDNF) [96].
Quercetin (Table 1) (30 mg/kg) also reduced OS, spinal cytokine secretion, and glial cell facilitation of GFAP [97]. Additional studies revealed that a ten-day i.p. quercetin management at a 20 mg/kg/day dosage scale could mitigate monosodium Glu-induced neurotoxicity by lowering p38MAPK, decreasing OS, and boosting GFAP transcription [98]. According to Azevedo et al. [89], quercetin (25, 50, and 100 mg/kg) mitigated OS-induced degeneration by lowering LPO, which was in agreement with Liu et al. [99, 100].
Following SCI, a 7-day i.p. processing of 20 mg/kg quercetin inhibits the p38MAPK/iNOS signaling pathway and synchronizes secondary OS by blocking the BDNF and JAK2/STAT3 signaling pathways [101]. Quercetin administration at a frequency of 0.25 mol/kg diminished MPO expression, according to Schültke et al. [102]. In addition, a particular dose of quercetin provided during three days of SCI enhanced overall antioxidant levels while lowering NO and MDA levels [103]. Quercetin raised overall antioxidant potential and paraoxonase function in rats following SCI [104].
A further research paper discovered that delivering 20 mg/kg of quercetin could safeguard against SCI-stimulated OS by behaving as an antioxidant and anti-inflammatory [105]. Wang et al. observed that quercetin (50 mol/kg) attenuated proinflammatory cytokines while elevating anti-inflammatory cytokines relevant to oxidative mechanisms. The treatment significantly attenuated the cystic cavity size while enhancing macrophage polarization, neuronal function, and axonal survival [106]. Based on in vivo and in vitro investigations, quercetin (7.5 mg/kg) suppressed oligodendrocyte necroptosis after SCI by modulating the STAT1 and NF-κB pathways [107]. Jiang et al. discovered that 100 mg/kg of quercetin lowered ROS construction, IL-1, TNF-α, and IL-18 in female rats following SCI [108]. Therefore, quercetin appears to be a favorable treatment for reducing OS after neurodegeneration and SCI.
5.4. Honokiol
Magnolia grandiflora has a pleiotropic lignan called honokiol (Figure 5) [109]. Antioxidant [110], anti-inflammatory [111], analgesic [112], depressive [113], antitumorigenic [114], and neuroprotective [115] actions are among its therapeutic benefits. Honokiol has been shown to reduce OS factors in tissue diversity, involving the heart [116], liver [117], kidney [118], and brain [119]. Honokiol reduced ROS generation in microglial cells via the ERK/NADPH oxidase pathway [120]. To exhibit neuroprotective effects, it also triggered Nrf2 [121], suppressed xanthine oxidase (XO), and regulated the PI3K/Akt pathway [122]. Furthermore, honokiol protected mitochondrial respiratory chain enzymes by targeting PKC, MAPKs, and NF-κB [123–125]. 20 mg/kg of honokiol decreased the generation of proinflammatory cytokines and prevented neutrophil permeation and microglial stimulation in a rat version of SCI, all of which are linked to oxidative factors [126]. In ischemic brains, 10 g/kg of honokiol reduced neutrophil infiltration and ROS production while maintaining Na+/K+-ATPase function and mitochondrial biogenesis against OS [113]. Honokiol also conserved mitochondrial respiratory chain enzyme [125]. In a rat model of SCI, 20 mg/kg of honokiol lowered the manufacture of proinflammatory cytokines, blocked neutrophil penetration, and prevented microglial activation, all associated with oxidative factors [126]. 10 g/kg of honokiol (Table 1) reduced neutrophil infiltration and ROS generation in ischemic brains while maintaining Na+/K+-ATPase activity and mitochondrial biogenesis [113].
5.5. Curcumin
Curcumin (Figure 5) is an organic polyphenol substance isolated from the Curcuma longa rhizome [127, 128]. In many studies, curcumin has antioxidant, anti-inflammatory, and anticancer estates, which have antioxidant, anti-inflammatory, and anticancer properties. Curcumin exerts anti-inflammatory actions via upregulating the PPAR- linked with the NF-κB pathway [129, 130]. Curcumin inhibited the stimulation of NF-κB, lowered the production of COX-2, IL-1, IL-6, IL-8, and TNF-α [131], and boosted the SOD activity [132]. Curcumin's anti-inflammatory impact after SCI has been linked to suppression of NF-κB, IL-1β, IL-6, and TNF-α activity, as well as an enhancement in Nrf2 [133] and stimulation of the TLR4/NF-κB signaling route [134].
Curcumin generated antioxidative preservation via Nrf2 routes and a reduction in ROS as a consequence of NF-κB stimulation [135]. In treating SCI, curcumin also affects the mTOR signaling pathway [136]. Curcumin, a more potent antioxidant that targets antioxidant enzymes such as GPx and SOD than vitamin E, has been reduced by methoxy and phenolic groups [137]. Curcumin elevated the CDGSH iron sulfur domain 2 (CISD2) as a durability gene due to its activities in Ca2+ metabolism after SCI. CISD2 improved BCL-2/Beclin-1 binding. It is guarded against programmed cell death and mitochondrial dysfunction. At the ER stress, CISD2 reduced a rise in excitotoxic Ca2+ [138].
Curcumin reduced neuron death and inhibited neuronal death following SCI, according to Lin et al. [139]. In the long-term treatment of SCI, curcumin outperformed methylprednisolone by lowering Bax and caspase-3 while increasing Bcl-2 [140]. Following curcumin therapy in humans or mice, tetrahydrocurcumin is among the most common curcumin metabolites isolated from the liver cytoplasm and small intestine [141]. In SCI patients, tetrahydrocurcumin (80 mg/kg/day) has been reported to lower OS and death [142]. Curcumin decreased inflammatory cytokines with pro-apoptotic effects in rats after SCI [143].
Curcumin entirely inhibited TGF-β following SCI. They also discovered that curcumin inhibits NF-κB, a protein implicated in the apoptotic and inflammatory mechanisms [144]. Curcumin's anti-apoptotic action was also exhibited in the spinal cord damage rat model, later being given intravenously. Curcumin was also found to decline caspase-3 [145], enhance Bcl-2 [146], and have anti-inflammatory antioxidant estates [147]. In a rabbit model of SCI, curcumin was discovered to block apoptotic (caspase-3) [147].
5.6. Naringin
Naringin (Figure 5) is considered a flavanone glycoside attained from citrus fruits. Naringinase hydrolyzes it to yield naringenin, which can effortlessly intersect the blood-brain barrier [148]. The inflammatory and OS reactions in adults' brains were controlled by naringin therapy. Naringin also has neuroprotective estates by stimulating neurotrophic factors and constraining apoptosis [149, 150]. Naringin can be an apoptotic inhibitor because the inflammatory factors and apoptotic mediators are linked. Following SCI, naringin (Table 1) (20, 40 mg/kg, p.o.) raised BDNF and vascular endothelial growth factor (VEGF) levels while inhibiting brain apoptosis [151]. BDNF reduced apoptosis and MAPK pathways via interacting with TrkB [152, 153], although the β-catenin/GSK-3β signaling route has been found to promote remyelination following SCI [154]. Naringenin, a naringin aglycone analog, has shown promising neuroprotective benefits and may be used in SCI in the future. Naringenin diminished the expression of IL-6, TNF-α, and CXCL10 mRNA in the spinal cord, which is an essential factor in apoptosis [155].
5.7. Apocynin
Apocynin (Figure 5), also known as acetovanillone, is an organic polyphenolic substance extracted from the rhizomes of Apocynum androsaemifolium [183]. Apocynin is a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor that suppresses p47phox's serine phosphorylation and prevents it from binding to gp91phox, delaying NADPH oxidase activity [184]. H2O2 and myeloperoxidase (MPO) stimulate apocynin, resulting in the formation of an apocynin radical. NADPH oxidase is inhibited by thiol-oxidizing compounds [185], a significant source of ROS in the cell [186]. This method has significantly altered redox-sensitive signaling pathways in neuroinflammation in different NDDs, particularly SCI. Sun and colleagues have found that apocynin (50 mg/kg) (Table 1) reduced SCI-induced neurodegenerative in rats by diminishing inflammatory cytokine production, improving glutathione (GSH)/SOD activity, and decreasing MPO and malondialdehyde levels (MDA). Apocynin (5 mg/kg) inhibited apoptosis after SCI by lowering FasL stimulation and phospho-JNK, P38, inflammatory cytokines (IL-1, TNF-α), and NF-κB representation levels [171]. Corresponding to research by Liu et al., apocynin can aid histology results and forelimb motor control restoration following SCI. Furthermore, Zhang and coworkers demonstrated the prospective neuroprotective estates of apocynin by decreasing neuroinflammation in spinal cord injured rats by suppressing the growth of NADPH oxidase-mediated ROS [172]. In an SCI chronic animal experiment, ROS and lipid peroxidation were similarly reduced by apocynin, implying an indirect control of apoptosis [169].
5.8. Carvacrol
Carvacrol (Figure 5) is a monoterpenoid phenolic product of cymene and has been demonstrated to have anxiolytic [187], depressive [188], antibacterial, antioxidant [189], anticancer, antimutagenic [190], anti-inflammatory [191], and antihepatotoxic properties [192]. Carvacrol strengthened the regulations of Nrf2 and ERK1 in PC12 cells that had been suppressed by cadmium [193]. Cells following exposure to iron ions and in cells exposed to H2O2 exhibited anti-carcinogenic characteristics via HO-1 [194, 195]. The Fenton reaction combines an excess of iron ions with oxygen, causing oxidative damage such as mitochondrial dysfunction and LPO [19]. Carvacrol also has anti-inflammatory and proinflammatory cytokine modulating properties [196]. After administering (25, 75, and 150 mg/kg), it inhibited OS factors like MDA, GSH, and NO [173]. However, more investigations are required to identify the neuroprotective properties of carvacrol following SCI via oxidative mechanisms.
5.9. Hesperidin
Hesperidin (Figure 5) is an anti-inflammatory, antioxidant, anticancer, and anti-apoptotic flavanoglycone obtained from citrus fruits [197, 198]. Hesperidin regulated Nrf2/ARE/HO 1 and TGF1/Smad3 signaling, which decreased OS and inflammation [199]. Hesperidin modulation of the ERK/MAPK pathway is implicated in the production of HO-1 and Nrf2 in an in vitro investigation based on OS [200]. In vitro, hesperidin triggered Nrf2/ARE/HO-1 and upregulated the Keap1-Nrf2/HO-1 pathway, enhancing the action of antioxidant enzymes in kidney tissue [201]. As a result of stimulating the Nrf2/HO-1/ARE and PPAR mechanisms, it reduced OS and inflammation [201, 202].
5.10. Rutin
The flavonol glycoside rutin, commonly identified as vitamin P, is derived from buckwheat [203]. Rutin (Figure 5) has a number of pharmacological properties, such as cytoprotection, antioxidant [204], anticancer [205], vasoprotection [206], neuroprotective effects [207], and anti-inflammation [163]. Rutin lowered OS by increasing CAT function, decreasing LPO and protein carbonyl content, and modulating the MAPK [208] and iNOS/ Nrf2 signaling pathways. In ischemic neuronal apoptosis, rutin suppressed LPO and p53 expression, enhanced antioxidant defense enzymes, and lowered ROS generation [209]. In mice, it alleviated diabetic neuropathy by lowering OS via HO-1 and Nrf2 [210]. Rutin boosted the transcription of BDNF, CREB, and ERK1 genes in the hippocampus at 100 mg/kg [211] and shielded PC12 cells against sodium nitroprusside stimulation by regulating the PI3K/Akt/mTOR and ERK1/2 pathways [212]. Oral medication with 10 mg/kg rutin for three weeks reduced OS [213].
A further study noticed that three-day rutin (Table 1) (50 and 100 mg/kg) substantially reduced ROS, MDA, IL-1, IL-18, and TNF-α [163]. Rutin protected cells from OS and apoptosis caused by H2O2 in vitro studies by directing the Bax/Bcl-2 ratio and the NF-κB/p65 signaling route, managing ROS, reducing LPO, and maintaining the intracellular antioxidant enzyme activities [214]. Rutin also safeguarded neurons from oxidative DNA damage and degeneration resulting from a lack of food [215]. Furthermore, 30 mg/kg rutin in the SCI animal paired with mild hypothermia for three days after SCI decreased inflammatory factors by blocking the TGF-β/Smad route [215].
5.11. Mangiferin
Mangiferin (Figure 5) is a bioactive xanthonoid extracted from various mango components. It is a potent antioxidant [216] with a variety of health benefits, notably immunomodulatory [217], antiviral [218], anti-inflammatory [219], antidiabetic [220], anticancer [221], and analgesic [222] activities. Mangiferin inhibits LPO and DNA damage by neutralizing free radicals and generating mangiferin-iron complexes [216, 223]. In an in vivo study, mice were recovered from cadmium chloride contamination by administering 50 mol/L of mangiferin for 4 hours, which reduced LPO rates and increased GSH, CAT, GST, and SOD activity [224]. Mangiferin increased Nrf2 levels, altered NQO1 expression, and increased ROS levels in vitro research [225]. Interestingly, 20 and 100 mg/kg of mangiferin triggered the Nrf2/HO-1 pathway in a dose-dependent approach in a brain injury model [177]. Mangiferin (Table 1) (20 and 100 mg/kg) for 30 days after SCI significantly decreased MDA at the same time as significantly boosted SOD, CAT, and GPx [178]. Mangiferin's neuroprotective properties in concentrations of 10, 25, and 50 mg/kg 30 days following SCI were connected with diminished spinal cord edema, reduction of OS, and inflammatory condition [226].
5.12. Caffeic Acid Phenethyl Ester
Honeybee propolis contains phenethyl caffeate [227]. Because of the associated hydroxyl groups in the catechol ring, it has antioxidant [228], anti-inflammatory [229], antibacterial [230], anticancer, and cytotoxic effects [231]. The phenethyl ester of caffeic acid inhibits NF-κB [232] and protein tyrosine kinase [233]. Hypoxic-ischemic brain injury models inhibit lipoxygenase activity [234] and limit calcium-induced cytochrome c release [235]. Following ischemia-reperfusion injury, caffeic acid phenethyl ester suppressed superoxide anion generation and XO [236] and decreased MPO and Na+/K+ ATPase capacities [237]. Caffeine's phenethyl ester increased HO-1 synthesis by activating Nrf2 and the extracellular signal-regulated kinases (ERK) signaling route [238]. It binds to Keap1, allowing Nrf2 to better connect to ARE [239]. MDA, LPO, and total oxidant action were reduced after SCI with an intrathecal infusion of 1 g/kg caffeic acid phenethyl ester. After SCI, it boosted antioxidative mediators [240], even as it decreased IL-6 levels in tissue and serum [241]. In a similar vein, Ak et al. found that caffeic acid phenethyl ester (10 g/kg) infusions lowered TNF-α and IL-1β levels after SCI [179].
After SCI, 10 mol/kg of this phytochemical enhanced motor function and decreased lesion size by lowering IL-1β, NOS, and COX-2 expression [180]. Caffeic acid phenethyl ester, 10 mol/kg (Table 1), was given before surgery to minimize ischemic damage in the spinal cord and to enhance microcirculation by blocking endothelial cell lysis by activated leukocyte proteases [242]. It also inhibited ROS and iNOS catalytic performance at a 50 mol/mL dosage, which had neuroinflammatory effects [243].
5.13. Tanshinone IIA
Tanshinone IIA (Figure 5) is extracted from the roots of Salvia miltiorrhiza. Tanshinone IIA has been found to have anti-apoptotic and anti-inflammatory properties in investigations [244]. Tanshinone IIA's antioxidant development is associated with efficient communication among DNA and lipid peroxidation product avoidance, DNA conservation by inhibiting NADPH oxidase, lipid peroxidation, and lipid-free radical clearance [245, 246]. Tanshinone IIA also inhibited the onset of neuroinflammation in neurodegenerative pathologies by preventing the production [247]. MAPKs are also critical signaling mediators that control cell development and death [248]. Tanshinone IIA (20, 50 mg/kg) (Table 1) has been demonstrated to suppress inflammation and apoptosis during SCI by decreasing NF-κB, MAPK, IL-1β, TNF-α, IL-6, iNOS, and caspase-3 boosting Bcl-2 [181]. Other investigations [249, 250] determined the spinal levels of inflammatory factors after tanshinone IIA treatment. These inflammatory factors also interact with apoptotic factors, as aforementioned. Tanshinone IIA has been shown to have the ability to improve neuronal autophagic factors and pathways (PI3K/Akt/mTOR) [251].
5.14. Eugenol
Eugenol (Figure 5), often known as clove oil (4-allyl-2-methoxy phenol), is an organic chemical derived from the Syzygium aromaticum (clove) plant [252]. Eugenol has antitumor [253], anti-microbial [254], anti-inflammatory [255], and antioxidant properties. It has been proven that proinflammatory cytokines, inflammation enzymes, and antioxidative enzymes reduce inflammation [256]. Eugenol has been shown to have therapeutic efficacy by lowering TRPV1 and sodium channels [257], connecting with Ca2+ channels [258], and boosting autophagy via the AMPK/mTOR pathway [259]. Eugenol lowered OS, inflammatory markers, and caspase-3 [182]. In neuroprotective effects, Eugenol increased Bcl-2 but decreased Bax [238] and TNF-α [239]. It has also been demonstrated to stimulate neuronal autophagy by the Akt/AMPK route [259].
6. Clinical Studies
Polyphenols are potential secondary metabolites with a comprehensive scale of favorable health outcomes. The US Food and Drug Administration (FDA) has acknowledged curcuminoids as relatively reliable and highly allowed effective forms in clinical studies, even at concentrations of up to 12,000 mg/day [260]. In controlled clinical research, curcumin's impacts on inflammatory and stress markers in 100 osteoarthritis patients of both genders have been investigated [261]. In a prospective randomized open-end blinded examination (PROBE) of 80 individuals with knee osteoarthritis, researchers discovered that consuming 30 mg of curcumin three times a day (p.o.) for four weeks decreased COX-2 concentrations [262]. Another RDBPC analysis [263] shows the anti-inflammatory efficacy of oral curcumin (400mg/3 times a day, p.o.) in type 2 diabetic cases and a substantial decrease in MDA, IL-6, and TNF-α levels.
In one hundred individuals with SCI, curcumin was significantly connected to decrease osteoporosis development and bone metabolism markers after six months [264]. According to randomized, parallel-group outcomes controlled clinical research on 20 participants, the InflanNox tablet (curcumin 1200 mg/day) has additional anti-inflammatory and antioxidant characteristics, lowers IL-1β, and improves depression and anxiety in SCI patients [265]. In 50 individuals with multiple sclerosis, administration of nanocurcumin (80 mg/day) was linked to a considerable increase in TGF-β and IL-10 expression [266]. Nanocurcumin was governed in a randomized of 40 diabetes people. In this investigation, nanocurcumin was discovered to be an antioxidant that may minimize OS and free radicals [267].
Polyphenol supplements (200mL/day) reportedly regulated plasma homocysteine concentrations in 48 Alzheimer's patients in an eight-month multiple center RDBC experiment [268]. In a multicenter, double-blind clinical investigation, thirty-four diabetic patients with neuropathy (aged 21 to 72) were given a topical preparation including quercetin to reduce OS [269]. Verlaet et al. showed antioxidant properties in a randomized controlled experiment examining the treatment properties of the herbal, polyphenol-rich extract [270]. Furthermore, another study found that meals high in polyphenols could increase cognitive reserve [271]. Another polyphenol-rich extract has shown promising antioxidative consequences in healthful people and those suffering from NDDs [272–274].
7. Conclusion and Future Perspectives
The complicated pathophysiological mechanisms in SCI seem to be orchestrated by OS to influence other interrelated pathways, such as neuroinflammation. Thus, an interaction between OS and neuroinflammatory/apoptotic pathways is complex.
In this line, Nrf2/Keap1/ARE, SOD, CAT, GSH, MDA, HO-1, and XO have significantly reduced the associated pathways/mediators contributing to neuroprotection in NDDs and SCI. Because of the polyphenol's shortcomings, researchers must apply novel drug delivery strategies in clinical studies, such as nanoformulations. Nanoformulations of polyphenols are proposed to overcome such restrictions due to the management indicated above and the advantageous effect of nanoparticles in boosting spinal cord medication distribution. It will enable the chemical's favorable impacts on SCI and other NDDs. To address SCI difficulties, metal nanoparticles (iron oxide, gold, silver, and so on), liposomes, and inorganics have all been utilized to create nanoparticles [275].
Equivalent recommendations will aid in raising understanding of the complexities of dysregulated signal transduction pathways after the SCI and the significance of discovering new and more effective multitarget alternative natural intermediaries with more excellent safety and efficacy among the scientific community. The exact molecular pathogenesis and signaling pathways associated with NDDs and the secondary phase of SCI must be revealed in further research studies. The mediators represent promising options to prevent associated pathogenicity in an oxidative way. Polyphenols are suggested to be the primary focus in this line of work as alternatives to interventions with fewer complications and greater efficacy.
Polyphenols/phenolic compounds are secondary metabolites with a broad scale of biological activity and health improvements exploited in modern medication to generate novel drugs [276]. Clinical studies are currently evaluating the therapeutic effect of polyphenols in the treatment of NDDs; however, clinical research to investigate the promise of polyphenols in treating following SCI consequences is lacking [277]. Therefore, well-designed clinical trials will aid in revealing polyphenols' therapeutic promise in addressing sensory-motor dysfunction after SCI and pave the way to address any recommendations for the future of their administration. The role of OS in modifying the inflammatory and apoptotic pathways in NDDs, with a particular focus on SCI, was investigated in this work. As potential multitarget neuroprotective treatments, we also emphasized the need to synthesize polyphenols and phenolic compounds that proinflammatory cytokines, extrinsic axonal related pathways, and other pathways involved with OS. Co-administering polyphenols/phenolic chemicals may also help treat SCI side effects. These research projects will explore potential pharmacological targets for avoiding, controlling, and treating NDDs and SCI.
Acknowledgments
The authors would like to express their gratitude to King Khalid University, Saudi Arabia, for providing administrative and technical support. This publication was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University, Al- Kharj, Saudi Arabia as well as the authors are sincerely grateful to Egyptian Russian University, Badr, Egypt.
Data Availability
All data used to establish the conclusions of this study are integrated into the article.
Conflicts of Interest
The authors proclaim that they have no conflicts of interest.
References
- 1.Islam F., Khadija J. F., Harun-Or-Rashid M., et al. Bioactive compounds and their derivatives: an insight into prospective phytotherapeutic approach against Alzheimer’s disease. Oxidative Medicine and Cellular Longevity . 2022;2022 doi: 10.1155/2022/5100904.5100904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam F., Nafady M. H., Islam M., et al. Resveratrol and neuroprotection: an insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Molecular Neurobiology . 2022;59:4384–4404. doi: 10.1007/s12035-022-02859-7. [DOI] [PubMed] [Google Scholar]
- 3.Chopra H., Bibi S., Singh I., et al. Nanomedicines in the management of Alzheimer’s disease: current view and future prospects. Frontiers in Aging Neuroscience . 2022;14:p. 726. doi: 10.3389/fnagi.2022.879114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomedicine & Pharmacotherapy . 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Abbaszadeh F., Fakhri S., Khan H. Targeting apoptosis and autophagy following spinal cord injury: therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacological Research . 2020;160, article 105069 doi: 10.1016/j.phrs.2020.105069. [DOI] [PubMed] [Google Scholar]
- 6.Sezer N., Akkuş S., Uğurlu F. G. Chronic complications of spinal cord injury. Chronic Complications of Spinal Cord Injury . 2015;6(1):24–33. doi: 10.5312/wjo.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiote M., Abe K. Amyotrophic lateral sclerosis, Nippon Rinsho. Japanese Journal of Clinical Medicine . 2001;59(8):490–494. [PubMed] [Google Scholar]
- 8.Tran A. P., Warren P. M., Silver J. The biology of regeneration failure and success after spinal cord injury. Physiological Reviews . 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausmann O. N. Post-traumatic inflammation following spinal cord injury. Spinal Cord . 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 10.Jia Z., Zhu H., Li J., Wang X., Misra H., Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord . 2012;50:264–274. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- 11.Naseri R., Farzaei F., Fakhri S., et al. Polyphenols for diabetes associated neuropathy: pharmacological targets and clinical perspective. DARU Journal of Pharmaceutical Sciences . 2019;27:781–798. doi: 10.1007/s40199-019-00289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassanzadeh K., Rahimmi A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: could targeting these pathways write a good ending? Journal of Cellular Physiology . 2018;234:23–32. doi: 10.1002/jcp.26865. [DOI] [PubMed] [Google Scholar]
- 13.Yepes-Nuñez J. J., Urrútia G., Romero-García M., Alonso-Fernández S. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Revista Espanola de Cardiologia . 2021;74(9):790–799. doi: 10.1016/j.recesp.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Fehlings M. G., Tator C. H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Experimental Neurology . 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 15.McDonald J. W., Sadowsky C. Spinal-cord injury. Lancet . 2002;359(9304):417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 16.Ackery A., Tator C., Krassioukov A. A global perspective on spinal cord injury epidemiology. Journal of Neurotrauma . 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 17.Yip P. K., Malaspina A. Spinal cord trauma and the molecular point of no return. Molecular Neurodegeneration . 2012;7:1–10. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norenberg M. D., Smith J., Marcillo A. The pathology of human spinal cord injury: defining the problems. Journal of Neurotrauma . 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 19.Cramer S. C., Lastra L., Lacourse M. G., Cohen M. J. Brain motor system function after chronic, complete spinal cord injury. Brain . 2005;128:2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- 20.Yiu G., He Z. Glial inhibition of CNS axon regeneration. Nature Reviews Neuroscience . 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulndreaj A., Chio J. C. T., Ahuja C. S., Fehlings M. G. Modulating the immune response in spinal cord injury. Expert review of Neurotherapeutics . 2016;16:1127–1129. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- 22.Cooney S. J., Zhao Y., Byrnes K. R. Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radical Research . 2014;48:929–939. doi: 10.3109/10715762.2014.927578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamme I., Petit E., Divoux D., Gerbi A., Maixent J. M., Nouvelot A. Modulation of mouse cerebral Na+, K+-ATPase activity by oxygen free radicals. Neuroreport . 1995;7:333–337. doi: 10.1097/00001756-199512000-00080. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig P. E., Patil A. A., Chamczuk A. J., Agrawal D. K. Hormonal therapy in traumatic spinal cord injury. American Journal of Translational Research . 2017;9:3881–3895. [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M., Wu W., Li H., et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. The Journal of Spinal Cord Medicine . 2015;38:745–753. doi: 10.1179/2045772314Y.0000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Wang H., Tao Y., Zhang S., Wang J., Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience . 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Gallo V., Bertolotto A., Levi G. The proteoglycan chondroitin sulfate is present in a subpopulation of cultured astrocytes and in their precursors. Developmental Biology . 1987;123:282–285. doi: 10.1016/0012-1606(87)90450-7. [DOI] [PubMed] [Google Scholar]
- 28.Bracken M. B., Holford T. R. Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. Journal of Neurosurgery: Spine . 2002;96:259–266. doi: 10.3171/spi.2002.96.3.0259. [DOI] [PubMed] [Google Scholar]
- 29.Silva N. A., Sousa N., Reis R. L., Salgado A. J. From basics to clinical: a comprehensive review on spinal cord injury. Progress in Neurobiology . 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Sengelaub D. R., Xu X. M. Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury. Neural Regeneration Research . 2018;13:971–976. doi: 10.4103/1673-5374.233434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedreag O. H., Rogobete A. F., Sărăndan M., et al. Stresul oxidativ şi terapia antioxidantă în leziunile medulare traumatice. Romanian Journal of Anaesthesia and Intensive Care . 2014;21:123–129. [PMC free article] [PubMed] [Google Scholar]
- 32.Khattab M. G., Sayed Z. S., Altaf R. A., et al. The prophylactic roles of dietary antioxidants for medical radiology workers: A mini-review. Natural Resources for Human Health . 2022;2022:1–17. doi: 10.53365/nrfhh/146248. [DOI] [Google Scholar]
- 33.Islam F., Akter A., Mimi A. A., et al. Neuropharmacological effects of Chassalia curviflora (Rubiaceae) leaves in Swiss albino mice model. Archives of Razi Institute . 2022;77:881–890. doi: 10.22092/ARI.2021.356880.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrocco I., Altieri F., Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Archives of Razi Institute . 2017;2017, article 6501046 doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campuzano O., del Mar Castillo-Ruiz M., Acarin L., Gonzalez B., Castellano B. Decreased myeloperoxidase expressing cells in the aged rat brain after excitotoxic damage. Experimental Gerontology . 2011;46(9):723–730. doi: 10.1016/j.exger.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Mitra S., Anjum J., Muni M., et al. Exploring the journey of emodin as a potential neuroprotective agent: novel therapeutic insights with molecular mechanism of action. Biomedicine & Pharmacotherapy . 2022;149, article 112877 doi: 10.1016/j.biopha.2022.112877. [DOI] [PubMed] [Google Scholar]
- 37.Peluso I., Morabito G., Urban L., Ioannone F., Serafi M. Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) . 2012;12:351–360. doi: 10.2174/187153012803832602. [DOI] [PubMed] [Google Scholar]
- 38.Islam F., Shohag S., Uddin M. J., et al. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials (Basel) . 2022;15:p. 2160. doi: 10.3390/ma15062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch A. C., Palmer C., Lynch A. C., et al. Nutritional and immune status following spinal cord injury: a case controlled study. Spinal Cord . 2002;40:627–630. doi: 10.1038/sj.sc.3101382. [DOI] [PubMed] [Google Scholar]
- 40.Farkas G. J., Pitot M. A., Berg A. S., Gater D. R. Correction: Nutritional status in chronic spinal cord injury: a systematic review and meta-analysis. Spinal Cord . 2019;57(1):3–17. doi: 10.1038/s41393-018-0218-4. [DOI] [PubMed] [Google Scholar]
- 41.Kijima K., Kubota K., Hara M., et al. The acute phase serum zinc concentration is a reliable biomarker for predicting the functional outcome after spinal cord injury. EBioMedicine . 2019;41:659–669. doi: 10.1016/j.ebiom.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller R. A., Sperl A., Seelig J., et al. Zinc concentration dynamics indicate neurological impairment odds after traumatic spinal cord injury. Antioxidants . 2020;41:659–669. doi: 10.3390/antiox9050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D., Tian H., Li X., et al. Zinc promotes functional recovery after spinal cord injury by activating Nrf2/HO-1 defense pathway and inhibiting inflammation of NLRP3 in nerve cells. Life Sciences . 2020;245, article 117351 doi: 10.1016/j.lfs.2020.117351. [DOI] [PubMed] [Google Scholar]
- 44.Hall E. D., Wang J. A., Bosken J. M., Singh I. N. Lipid peroxidation in brain or spinal cord mitochondria after injury. Journal of Bioenergetics and Biomembranes . 2016;48:169–174. doi: 10.1007/s10863-015-9600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao S., Lin Y., Du Y., et al. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. Journal of Materials Chemistry B . 2019;7:2648–2656. doi: 10.1039/c8tb02520g. [DOI] [PubMed] [Google Scholar]
- 46.Seelig J., Heller R. A., Hackler J., et al. Selenium and copper status - potential signposts for neurological remission after traumatic spinal cord injury. Journal of Trace Elements in Medicine and Biology . 2020;57, article 126415 doi: 10.1016/j.jtemb.2019.126415. [DOI] [PubMed] [Google Scholar]
- 47.Sperl A., Heller R. A., Biglari B., et al. The role of magnesium in the secondary phase after traumatic spinal cord injury. A prospective clinical observer study. Antioxidants . 2019;8(11):p. 509. doi: 10.3390/antiox8110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meeran M. F. N., Goyal S. N., Suchal K., Sharma C., Patil C. R., Ojha S. K. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise. Frontiers in Pharmacology . 2018;9:p. 892. doi: 10.3389/fphar.2018.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang W., Li M., He F., et al. Neuroprotective effect of asiatic acid against spinal cord injury in rats. Life Sciences . 2016;157:45–51. doi: 10.1016/j.lfs.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences . 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschopp J., Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nature Reviews Immunology . 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 52.Khalatbary A. R. Natural polyphenols and spinal cord injury. Iranian Biomedical Journal . 2014;18:120–129. doi: 10.6091/ibj.1278.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam M. R., Islam F., Nafady M. H., et al. Natural small molecules in breast cancer treatment: understandings from a therapeutic viewpoint. Molecules . 2022;27(7):p. 2165. doi: 10.3390/molecules27072165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decker E. A. Phenolics: prooxidants or antioxidants? Nutrition Reviews . 1997;55:396–398. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 55.Kasote D. M., Katyare S. S., Hegde M. V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences . 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaveri N. T. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sciences . 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Pervin M., Unno K., Ohishi T., Tanabe H., Miyoshi N., Nakamura Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules . 2018;23:p. 1297. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y., Cui J. (-)-Epigallocatechin-3-gallate provides neuroprotection via AMPK activation against traumatic brain injury in a mouse model. Naunyn-Schmiedeberg's Archives of Pharmacology . 2020;393:2209–2220. doi: 10.1007/s00210-020-01841-1. [DOI] [PubMed] [Google Scholar]
- 59.Machova Urdzikova L., Ruzicka J., Karova K., et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology . 2017;126:213–223. doi: 10.1016/j.neuropharm.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Wei I. H., Tu H. C., Huang C. C., Tsai M. H., Tseng C. Y., Shieh J. Y. (-)-Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. BMC Neuroscience . 2011;12:1–11. doi: 10.1186/1471-2202-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salah N., Miller N. J., Paganga G., Tijburg L., Paul Bolwell G., Riceevans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Archives of Biochemistry and Biophysics . 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J., Fang S., Yuan Y., et al. Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress. Neural Regeneration Research . 2014;9:1379–1385. doi: 10.4103/1673-5374.137591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye Q., Ye L., Xu X., et al. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complementary and Alternative Medicine . 2012;12 doi: 10.1186/1472-6882-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalatbary A. R., Ahmadvand H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iranian Biomedical Journal . 2011;15:31–37. [PMC free article] [PubMed] [Google Scholar]
- 65.Khalatbary A. R., Tiraihi T., Boroujeni M. B., Ahmadvand H., Tavafi M., Tamjidipoor A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Research . 2010;1306:168–175. doi: 10.1016/j.brainres.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 66.Che F., Wang G., Yu J., et al. Effects of epigallocatechin-3-gallate on iron metabolismin spinal cord motor neurons. Molecular Medicine Reports . 2017;16:3010–3014. doi: 10.3892/mmr.2017.6919. [DOI] [PubMed] [Google Scholar]
- 67.Álvarez-Pérez B., Homs J., Bosch-Mola M., et al. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. European Journal of Pain . 2016;20:341–352. doi: 10.1002/ejp.722. [DOI] [PubMed] [Google Scholar]
- 68.Chopra H., Bibi S., Islam F., et al. Emerging trends in the delivery of resveratrol by nanostructures: applications of nanotechnology in life sciences. Journal of Nanomaterials . 2022;20223083728 [Google Scholar]
- 69.Pervaiz S., Holme A. L. Resveratrol: its biologic targets and functional activity. Antioxidants Redox Signal . 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 70.Leonard S. S., Xia C., Jiang B. H., et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochemical And Biophysical Research Communications . 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 71.Bastianetto S., Ménard C., Quirion R. Neuroprotective action of resveratrol. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2015;1852(6):1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Kesherwani V., Atif F., Yousuf S., Agrawal S. K. Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience . 2013;241:80–88. doi: 10.1016/j.neuroscience.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Borra M. T., Smith B. C., Denu J. M. Mechanism of human SIRT1 activation by resveratrol. Journal of Biological Chemistry . 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J., Feng X., Wu J., et al. Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by β-amyloid through activation of SIRT1/Akt1 pathway. BioFactors . 2014;40:258–267. doi: 10.1002/biof.1149. [DOI] [PubMed] [Google Scholar]
- 75.Salehi B., Mishra A. P., Nigam M., et al. Resveratrol: a double-edged sword in health benefits. Biomedicines . 2018;6(3):p. 91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu L., Botchway B. O. A., Zhang S., Zhou J., Liu X. Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Frontiers in Neuroscience . 2018;12:p. 690. doi: 10.3389/fnins.2018.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao H., Chen S., Gao K., et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience . 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 78.Yan P., Bai L., Lu W., Gao Y., Bi Y., Lv G. Regulation of autophagy by AMP-activated protein kinase/sirtuin 1 pathway reduces spinal cord neurons damage. Iranian Journal of Basic Medical Sciences . 2017;20:1029–1036. doi: 10.22038/IJBMS.2017.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C., Shi Z., Fan L., Zhang C., Wang K., Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Research . 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 80.Zhang G., Liu Y., Xu L., Sha C., Zhang H., Xu W. Resveratrol alleviates lipopolysaccharide-induced inflammation in PC-12 cells and in rat model. BMC Biotechnol . 2019;19:1–9. doi: 10.1186/s12896-019-0502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng H. Y., Shao D. C., Li H., et al. Resveratrol improves neurological outcome and neuroinflammation following spinal cord injury through enhancing autophagy involving the AMPK/mTOR pathway. Molecular Medicine Reports . 2018;18:2237–2244. doi: 10.3892/mmr.2018.9194. [DOI] [PubMed] [Google Scholar]
- 82.Wang P., Jiang L., Zhou N., et al. Resveratrol ameliorates autophagic flux to promote functional recovery in rats after spinal cord injury. Oncotarget . 2018;9:8427–8440. doi: 10.18632/oncotarget.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu J., Han H., Cao P., et al. Resveratrol improves neuron protection and functional recovery through enhancement of autophagy after spinal cord injury in mice. American Journal of Translational Research . 2017;9:4607–4616. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong K., Li Y., Tang Y., et al. Cytokine profile and glial activation following brachial plexus roots avulsion injury in mice. Journal of Neuroimmunology . 2021;353, article 577517 doi: 10.1016/j.jneuroim.2021.577517. [DOI] [PubMed] [Google Scholar]
- 85.Du Q. H., Peng C., Zhang H. Polydatin: a review of pharmacology and pharmacokinetics. Pharmaceutical Biology . 2013;51:1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 86.Lv R., Du L., Zhang L., Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life sciences . 2019;217:119–127. doi: 10.1016/j.lfs.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 87.Rahman M. M., Islam F., Anwar Parvez M. A. K., Ashraf G. M., Ullah M. F., Ahmed M. Citrus limon L.(lemon) seed extract shows neuro-modulatory activity in an in vivo thiopental-sodium sleep model by reducing the sleep onset and enhancing the sleep duration. Journal of Integrative Neuroscience . 2022;21:1–9. doi: 10.31083/j.jin2101042. [DOI] [PubMed] [Google Scholar]
- 88.Rahman M., Islam F., Afsana Mim S., et al. Multifunctional therapeutic approach of nanomedicines against inflammation in cancer and aging. Journal of Nanomaterials . 2022;20224217529 [Google Scholar]
- 89.Davis J. M., Murphy E. A., Carmichael M. D. Effects of the dietary flavonoid quercetin upon performance and health. Current Sports Medicine Reports . 2009;8:206–213. doi: 10.1249/JSR.0b013e3181ae8959. [DOI] [PubMed] [Google Scholar]
- 90.Aguirre L., Arias N., Macarulla M. T., Gracia A., Portillo M. P. Beneficial effects of quercetin on obesity and diabetes. The Open Nutraceuticals Journal . 2011;4:189–198. doi: 10.2174/1876396001104010189. [DOI] [Google Scholar]
- 91.Spencer J. P. E., Kuhnle G. G. C., Williams R. J., Rice-Evans C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochemical Journal . 2003;372:173–181. doi: 10.1042/BJ20021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song Y., Liu J., Zhang F., Zhang J., Shi T., Zeng Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sciences . 2013;92:1215–1221. doi: 10.1016/j.lfs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Schültke E., Kamencic H., Zhao M., et al. Neuroprotection following fluid percussion brain trauma: a pilot study using quercetin. Journal of Neurotrauma . 2005;22:1475–1484. doi: 10.1089/neu.2005.22.1475. [DOI] [PubMed] [Google Scholar]
- 94.Yang T., Kong B., Gu J. W., et al. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cellular and Molecular Neurobiology . 2014;34:797–804. doi: 10.1007/s10571-014-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J. K., Kwak H. J., Piao M. S., Jang J. W., Kim S. H., Kim H. S. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochirurgica . 2011;153:1321–1329. doi: 10.1007/s00701-010-0889-x. [DOI] [PubMed] [Google Scholar]
- 96.Yao R. Q., Qi D. S., Yu H. L., Liu J., Yang L. H., Wu X. X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochemical Research . 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 97.Borghi S. M., Pinho-Ribeiro F. A., Fattori V., et al. Quercetin inhibits peripheral and spinal cord nociceptive mechanisms to reduce intense acute swimming-induced muscle pain in mice. PLoS One . 2016;11, article e0162267 doi: 10.1371/journal.pone.0162267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Firgany A. E. D. L., Sarhan N. R. Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via p38 MAPK inhibition. Acta Histochem . 2020;122, article 151554 doi: 10.1016/j.acthis.2020.151554. [DOI] [PubMed] [Google Scholar]
- 99.Azevedo M. I., Pereira A. F., Nogueira R. B., et al. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Molecular Pain . 2013;9:1744–8069. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J. B., Tang T. S., Yang H. L. Antioxidation of quercetin against spinal cord injury in rats. Chinese Journal of Traumatology . 2006;9:303–307. [PubMed] [Google Scholar]
- 101.Wang Y., Li W., Wang M., et al. Quercetin reduces neural tissue damage and promotes astrocyte activation after spinal cord injury in rats. Journal of Cellular Biochemistry . 2018;119:2298–2306. doi: 10.1002/jcb.26392. [DOI] [PubMed] [Google Scholar]
- 102.Schültke E., Griebel R. W., Juurlink B. H. J. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord . 2010;48:857–861. doi: 10.1038/sc.2010.45. [DOI] [PubMed] [Google Scholar]
- 103.Ocal O., Borcek A. O., Pasaoglu O., Gundogdu A. C., Kaplanoglu G. T., Baykaner M. K. Can quercetin be an option for treatment of spinal cord injury? An experimental study. Turkish Neurosurgery . 2019;29:247–253. doi: 10.5137/1019-5149.JTN.23799-18.1. [DOI] [PubMed] [Google Scholar]
- 104.Çiftçi U., Delen E., Vural M., et al. Deneysel spinal kord travması sonrası resveratrol ve kuersetin’in etkinliğinin araştırılması. Ulus. Travma ve Acil Cerrahi Derg . 2016;22:423–431. doi: 10.5505/tjtes.2016.44575. [DOI] [PubMed] [Google Scholar]
- 105.Çevik Ö., Erşahin M., Şener T. E., et al. Beneficial effects of quercetin on rat urinary bladder after spinal cord injury. Journal of Surgical Research . 2013;183:695–703. doi: 10.1016/j.jss.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 106.Wang X., Wang Y. Y., Zhang L. L., Li G. T., Zhang H. T. Combinatory effect of mesenchymal stromal cells transplantation and quercetin after spinal cord injury in rat. European Review for Medical and Pharmacological Sciences . 2018;22:2876–2887. doi: 10.26355/eurrev_201805_14990. [DOI] [PubMed] [Google Scholar]
- 107.Fan H., Bin Tang H., Shan L. Q., et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. Journal of Neuroinflammation . 2019;16:1–15. doi: 10.1186/s12974-019-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang W., Huang Y., Han N., et al. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord . 2016;54:592–596. doi: 10.1038/sc.2015.227. [DOI] [PubMed] [Google Scholar]
- 109.Alonso-Castro A. J., Zapata-Bustos R., Domínguez F., García-Carrancá A., Salazar-Olivo L. A. Magnolia dealbata Zucc and its active principles honokiol and magnolol stimulate glucose uptake in murine and human adipocytes using the insulin-signaling pathway. Phytomedicine . 2011;18:926–933. doi: 10.1016/j.phymed.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Han X., Pang Y., Liu S., et al. Antidiarrhea and antioxidant activities of honokiol extract from magnoliae officinalis cortex in mice. Tropical Journal of Pharmaceutical Research . 2014;13:1643–1651. doi: 10.4314/tjpr.v13i10.11. [DOI] [Google Scholar]
- 111.Kim B. H., Cho J. Y. Anti-inflammatory effect of honokiol is mediated by PI3K/Akt pathway suppression. Acta Pharmacologica Sinica . 2008;29:113–122. doi: 10.1111/j.1745-7254.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 112.Yang Y., Jin S. J., Wang H. L., et al. Effects of aloperine on acute and inflammatory pain models in mice. Scandinavian Journal of Pain . 2015;8:28–34. doi: 10.1016/j.sjpain.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Xu Q., Yi L. T., Pan Y., et al. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Progress in Neuro-Psychopharmacology and Biological Psychiatry . 2008;32:715–725. doi: 10.1016/j.pnpbp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Prasad R., Katiyar S. K. Honokiol, an active compound of Magnolia plant, inhibits growth, and progression of cancers of different organs. Anti-inflammatory Nutraceuticals and Chronic Diseases . 2016;928:245–265. doi: 10.1007/978-3-319-41334-1_11. [DOI] [PubMed] [Google Scholar]
- 115.Chen C. M., Liu S. H., Lin-Shiau S. Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic & Clinical Pharmacology & Toxicology . 2007;101:108–116. doi: 10.1111/j.1742-7843.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y., Zhang Z. Z., Wu Y., Zhan J., He X. H., Wang Y. L. Honokiol protects rat hearts against myocardial ischemia reperfusion injury by reducing oxidative stress and infammation. Experimental and Therapeutic Medicine . 2013;5:315–319. doi: 10.3892/etm.2012.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin H. Q., Je Y. T., Kim Y. C., et al. Magnolia officinalis reverses alcoholic fatty liver by inhibiting the maturation of sterol regulatory element-binding protein-1c. Journal of Pharmacological Sciences . 2009;109:486–495. doi: 10.1254/jphs.08182FP. [DOI] [PubMed] [Google Scholar]
- 118.Yu Y., Li M., Su N., et al. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Molecular Medicine Reports . 2016;13(2):1353–1360. doi: 10.3892/mmr.2015.4660. [DOI] [PubMed] [Google Scholar]
- 119.Hou Y., Peng S., Li X., Yao J., Xu J., Fang J. Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. Acs Chemical Neuroscience . 2018;9:3108–3116. doi: 10.1021/acschemneuro.8b00290. [DOI] [PubMed] [Google Scholar]
- 120.Chuang D. Y., Chan M. H., Zong Y., et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. Journal of Neuroinflammation . 2013;10:1–14. doi: 10.1186/1742-2094-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajgopal A., Missler S. R., Scholten J. D. Magnolia officinalis (Hou Po) bark extract stimulates the Nrf2-pathway in hepatocytes and protects against oxidative stress. Journal of Ethnopharmacology . 2016;193:657–662. doi: 10.1016/j.jep.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 122.Chang W. S., Chang Y. H., Lu F. J., Chiang H. C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Research . 1994;14:501–506. [PubMed] [Google Scholar]
- 123.Akter A., Islam F., Bepary S., et al. CNS depressant activities of Averrhoa carambola leaves extract in thiopental-sodium model of Swiss albino mice: implication for neuro-modulatory properties. Biologia (Bratisl) . 2022;77:1–10. [Google Scholar]
- 124.Islam F., Mitra S., Nafady M. H., et al. Neuropharmacological and antidiabetic potential of Lannea coromandelica (Houtt.) merr. leaves extract: an experimental analysis. Evidence-Based Complementary and Alternative Medicine . 2022;2022 doi: 10.1155/2022/6144733.6144733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang J. P., Hsu E. F., Raung S. L., et al. Inhibition by magnolol of formylmethionyl-leucyl-phenyl alanine-induced respiratory burst in rat neutrophils. Journal of Pharmacy and Pharmacology . 2010;51:285–294. doi: 10.1211/0022357991772466. [DOI] [PubMed] [Google Scholar]
- 126.Liu J., Zhang C., Liu Z., Zhang J., Xiang Z., Sun T. Honokiol downregulates kruppel-like factor 4 expression, attenuates inflammation, and reduces histopathology after spinal cord injury in rats. Spine . 1976;40(2015):363–368. doi: 10.1097/BRS.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 127.Islam F., Khadija J. F., Islam R., et al. Investigating polyphenol nanoformulations for therapeutic targets against diabetes mellitus. Evidence-Based Complementary and Alternative Medicine . 2022;2022 doi: 10.1155/2022/5649156.5649156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nawaz A., Khan G. M., Hussain A., Ahmad A., Khan A. Curcumin: a natural product of biological. Gomal University Journal of Research . 2011;27:7–14. [Google Scholar]
- 129.Tagde P., Tagde P., Islam F., et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules . 2021;26:p. 7109. doi: 10.3390/molecules26237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jacob A., Wu R., Zhou M., Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Research . 2007;2007 doi: 10.1155/2007/89369.89369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jurenka J. S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research, Altern. Alternative Medicine Review . 2009;14:141–153. [PubMed] [Google Scholar]
- 132.Şahin Kavakli H., Koca C., Alici Ö. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg . 2011;17:14–18. doi: 10.5505/tjtes.2011.31391. [DOI] [PubMed] [Google Scholar]
- 133.Jin W., Wang J., Zhu T., et al. Anti-inflammatory effects of curcumin in experimental spinal cord injury in rats. Inflammation Research . 2014;63:381–387. doi: 10.1007/s00011-014-0710-z. [DOI] [PubMed] [Google Scholar]
- 134.Ni H., Jin W., Zhu T., et al. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. The Journal of Spinal Cord Medicine . 2015;38:199–206. doi: 10.1179/2045772313Y.0000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daverey A., Agrawal S. K. Curcumin protects against white matter injury through NF-κB and Nrf2 cross talk. Journal of Neurotrauma . 2020;37:1255–1265. doi: 10.1089/neu.2019.6749. [DOI] [PubMed] [Google Scholar]
- 136.Lin J., Huo X., Liu X. “mTOR signaling pathway”: a potential target of curcumin in the treatment of spinal cord injury. BioMed Research International . 2017;2017 doi: 10.1155/2017/1634801.1634801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Motterlini R., Foresti R., Bassi R., Green C. J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radical Biology and Medicine . 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 138.Lin C. C., Lin M. S. New insight into curcumin-based therapy in spinal cord injuries: CISD2 regulation. Neural Regeneration Research . 2016;11:222–223. doi: 10.4103/1673-5374.177718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin M. S., Lee Y. H., Chiu W. T., Hung K. S. Curcumin provides neuroprotection after spinal cord injury. Journal of Surgical Research . 2011;166:280–289. doi: 10.1016/j.jss.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Liu X., Zhang Y., Yang Y., et al. Therapeutic effect of curcumin and methylprednisolone in the rat spinal cord injury. The Anatomical Record . 2018;301:686–696. doi: 10.1002/ar.23729. [DOI] [PubMed] [Google Scholar]
- 141.Sangartit W., Kukongviriyapan U., Donpunha W., et al. Tetrahydrocurcumin protects against cadmium-induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS One . 2014;9(12, article e114908) doi: 10.1371/journal.pone.0114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xi J., Luo X., Wang Y., et al. Tetrahydrocurcumin protects against spinal cord injury and inhibits the oxidative stress response by regulating FOXO4 in model rats. Experimental and Therapeutic Medicine . 2019;18(5):3681–3687. doi: 10.3892/etm.2019.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ruzicka J., Urdzikova L. M., Kloudova A., et al. Anti-inflammatory compound curcumin and mesenchymal stem cells in the treatment of spinal cord injury in rats. Acta Neurobiologiae Experimentalis . 2018;78:358–374. [PubMed] [Google Scholar]
- 144.Yuan J., Botchway B. O. A., Zhang Y., Tan X., Wang X., Liu X. Curcumin can improve spinal cord injury by inhibiting TGF-β-SOX9 signaling pathway. Cellular and Molecular Neurobiology . 2019;39(5):569–575. doi: 10.1007/s10571-019-00671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gokce E. C., Kahveci R., Gokce A., et al. Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia-reperfusion injury in rats. Journal of Stroke and Cerebrovascular Diseases . 2016;25:1196–1207. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 146.Hao Q., Wang H. W., Yu Q., et al. Effects of curcumin on the recovery of hind limb function after spinal cord injury in rats and its mechamism. Chinese Journal of Applied Physiology . 2017;33:441–444. doi: 10.12047/j.cjap.5548.2017.106. [DOI] [PubMed] [Google Scholar]
- 147.Kurt G., Yildirim Z., Cemil B., Celtikci E., Kaplanoglu G. T. Effects of curcumin on acute spinal cord ischemia-reperfusion injury in rabbits. Journal of Neurosurgery: Spine . 2014;20:464–470. doi: 10.3171/2013.12.SPINE1312. [DOI] [PubMed] [Google Scholar]
- 148.Ho P. C., Saville D. J., Coville P. F., Wanwimolruk S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharmaceutica Acta Helvetiae . 2000;74:379–385. doi: 10.1016/S0031-6865(99)00062-X. [DOI] [PubMed] [Google Scholar]
- 149.Rahman M., Majumder S., Akter F., Islam F., Shahriar M., Alam J. Pre-clinical investigation of analgesic, anti-diarrheal and CNS depressant effect of Pterocarpus indicus in Swiss albino mice. Jordan Journal of Pharmaceutical Sciences . 2021;14(1) [Google Scholar]
- 150.Kim S. R. Naringin as a beneficial natural product against degeneration of the nigrostriatal dopaminergic projection in the adult brain. Neural Regeneration Research . 2017;12:1375–1376. doi: 10.4103/1673-5374.213694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rong W., Wang J., Liu X., et al. Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochemical Research . 2012;37:1615–1623. doi: 10.1007/s11064-012-0756-7. [DOI] [PubMed] [Google Scholar]
- 152.Mitra S., Lami M. S., Uddin T. M., et al. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomedicine & Pharmacotherapy . 2022;150, article 112932 doi: 10.1016/j.biopha.2022.112932. [DOI] [PubMed] [Google Scholar]
- 153.Benarroch E. E. Brain-derived neurotrophic factor. Neurology . 2015;84:1693–1704. doi: 10.1212/WNL.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 154.Rong W., Pan Y. W., Cai X., et al. The mechanism of Naringin-enhanced remyelination after spinal cord injury. Neural Regeneration Research . 2017;12:470–477. doi: 10.4103/1673-5374.202923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J., Qi Y., Niu X., Tang H., Meydani S. N., Wu D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. The Journal of Nutritional Biochemistry . 2018;54:130–139. doi: 10.1016/j.jnutbio.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Tian W., Han X. G., Liu Y. J., et al. Correction to: Intrathecal epigallocatechin gallate treatment improves functional recovery after spinal cord injury by upregulating the expression of BDNF and GDNF. 2013;38(4):772–779. doi: 10.1007/s11064-013-0976-5. [DOI] [PubMed] [Google Scholar]
- 157.Paterniti I., Genovese T., Crisafulli C., et al. Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma, Naunyn. Schmiedebergs. Naunyn-Schmiedeberg's Archives of Pharmacology . 2009;380:179–192. doi: 10.1007/s00210-009-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang N., Wei G., Ye J., et al. Effect of curcumin on acute spinal cord injury in mice via inhibition of inflammation and TAK1 pathway. Pharmacological Reports . 2017;69:1001–1006. doi: 10.1016/j.pharep.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 159.Kiziltepe U., Turan N. N. D., Han U., Ulus A. T., Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. Journal of Vascular Surgery . 2004;40:138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 160.Yang Y. B., Piao Y. J. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacologica Sinica . 2003;24:703–710+727. [PubMed] [Google Scholar]
- 161.Alvarado-Sanchez B. G., Salgado-Ceballos H., Torres-Castillo S., et al. Electroacupuncture and curcumin promote oxidative balance and motor function recovery in rats following traumatic spinal cord injury. Neurochemical Research . 2019;44:498–506. doi: 10.1007/s11064-018-02704-1. [DOI] [PubMed] [Google Scholar]
- 162.Cemil B., Topuz K., Demircan M. N., et al. Curcumin improves early functional results after experimental spinal cord injury. Acta Neurochirurgica . 2010;152:1583–1590. doi: 10.1007/s00701-010-0702-x. [DOI] [PubMed] [Google Scholar]
- 163.Wu J., Maoqiang L., Fan H., et al. Rutin attenuates neuroinflammation in spinal cord injury rats. Journal of Surgical Research . 2016;203:331–337. doi: 10.1016/j.jss.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 164.Ni H., Jin W., Yuan B., et al. Curcumin inhibits the increase of labile zinc and the expression of inflammatory cytokines after traumatic spinal cord injury in rats. Journal of Surgical Research . 2014;187:646–652. doi: 10.1016/j.jss.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 165.Li W., Yao S., Li H., Meng Z., Sun X. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. The Journal of Spinal Cord Medicine . 2021;44:37–45. doi: 10.1080/10790268.2019.1616147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shi L. B., Tang P. F., Zhang W., Zhao Y. P., Zhang L. C., Zhang H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene . 2016;592:128–133. doi: 10.1016/j.gene.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 167.Khalatbary A. R., Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurological Research . 2012;34:44–51. doi: 10.1179/1743132811Y.0000000058. [DOI] [PubMed] [Google Scholar]
- 168.Cui Y., Zhao W., Wang X., Chen M., Zhang L., Liu Z. Protective effects of naringin in a rat model of spinal cord ischemia–reperfusion injury. Tropical Journal of Pharmaceutical Research . 2017;16:649–656. doi: 10.4314/tjpr.v16i3.21. [DOI] [Google Scholar]
- 169.Hassler S. N., Johnson K. M., Hulsebosch C. E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. Journal of Neurochemistry . 2014;131:413–417. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Z., Yao X., Jiang W., et al. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Journal of Neuroinflammation . 2020;17:1–21. doi: 10.1186/s12974-020-01751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Impellizzeri D., Mazzon E., Esposito E., Paterniti I., Bramanti P., Cuzzocrea S. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radical Research . 2011;45:221–236. doi: 10.3109/10715762.2010.526604. [DOI] [PubMed] [Google Scholar]
- 172.Zhang B., Bailey W. M., McVicar A. L., Stewart A. N., Veldhorst A. K., Gensel J. C. Reducing age-dependent monocyte-derived macrophage activation contributes to the therapeutic efficacy of NADPH oxidase inhibition in spinal cord injury. Brain, Behavior, and Immunity . 2019;76:139–150. doi: 10.1016/j.bbi.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alvarenga E. M., Souza L. K. M., Araújo T. S. L., et al. Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation. Chemico-Biological Interactions . 2016;260:129–140. doi: 10.1016/j.cbi.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 174.Heo S. D., Kim J., Choi Y., Ekanayake P., Ahn M., Shin T. Hesperidin improves motor disability in rat spinal cord injury through anti-inflammatory and antioxidant mechanism via Nrf-2/HO-1 pathway. Neuroscience Letters . 2020;715 doi: 10.1016/j.neulet.2019.134619. [DOI] [PubMed] [Google Scholar]
- 175.Song H. L., Zhang X., Wang W. Z., et al. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural Regeneration Research . 2018;13:128–134. doi: 10.4103/1673-5374.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yao S., Wang L., Chen Q., Lu T., Pu X., Luo C. The effect of mild hypothermia plus rutin on the treatment of spinal cord injury and inflammatory factors by repressing tgf-β/smad pathway. Acta Cirúrgica Brasileira . 2021;36 doi: 10.1590/acb360307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luo Y., Fu C., Wang Z., Zhang Z., Wang H., Liu Y. Mangiferin attenuates contusive spinal cord injury in rats through the regulation of oxidative stress, inflammation and the Bcl-2 and Bax pathway. Molecular Medicine Reports . 2015;12:7132–7138. doi: 10.3892/mmr.2015.4274. [DOI] [PubMed] [Google Scholar]
- 178.Xu L., Liang J., Jin T., Zhou F. Neuroprotective effects of mangiferin on acute spinal cord injury in rats and its mechanism. Chinese Journal of Reparative and Reconstructive Surgery . 2016;30:1019–1025. doi: 10.7507/1002-1892.20160205. [DOI] [PubMed] [Google Scholar]
- 179.Hakan A. K., Gülşen İ., Karaaslan T., et al. The effects of caffeic acid phenethyl ester on inflammatory cytokines after acute spinal cord injury. Ulusal Travma Ve Acil Cerrahi Dergisi-Turkish Journal Of Trauma & Emergency Surgery . 2015;21:96–101. doi: 10.5505/tjtes.2015.33848. [DOI] [PubMed] [Google Scholar]
- 180.Kasai M., Fukumitsu H., Soumiya H., Furukawa S. Caffeic acid phenethyl ester reduces spinal cord injury-evoked locomotor dysfunction. Biomedical Research . 2011;32:1–7. doi: 10.2220/biomedres.32.1. [DOI] [PubMed] [Google Scholar]
- 181.Yin X., Yin Y., Le Cao F., et al. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One . 2012;7, article e38381 doi: 10.1371/journal.pone.0038381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ma L., Mu Y., Zhang Z., Sun Q. Eugenol promotes functional recovery and alleviates inflammation, oxidative stress, and neural apoptosis in a rat model of spinal cord injury. Restorative Neurology and Neuroscience . 2018;36:659–668. doi: 10.3233/RNN-180826. [DOI] [PubMed] [Google Scholar]
- 183.Simonyi A., Serfozo P., Lehmidi T. M., et al. The neuroprotective effects of apocynin. Frontiers in bioscience (Elite edition) . 2012;4:2183–2193. doi: 10.2741/535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muijsers R. B. R., Van Den Worm E., Folkerts G., et al. Apocynin inhibits peroxynitrite formation by murine macrophages. British Journal of Pharmacology . 2000;130:932–936. doi: 10.1038/sj.bjp.0703401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Johnson D. K., Schillinger K. J., Kwait D. M., et al. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium . 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 186.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell . 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 187.Melo F. H. C., Venâncio E. T., De Sousa D. P., et al. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: involvement with GABAergic transmission. Fundamental & Clinical Pharmacology . 2010;24:437–443. doi: 10.1111/j.1472-8206.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 188.Melo F. H. C., Moura B. A., de Sousa D. P., et al. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: involvement of dopaminergic system. Fundamental & Clinical Pharmacology . 2011;25:362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 189.Chen F., Shi Z., Neoh K. G., Kang E. T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnology and Bioengineering . 2009;104:30–39. doi: 10.1002/bit.22363. [DOI] [PubMed] [Google Scholar]
- 190.Kumar N., Yadav A., Gulati S., Kanupriya N., Aggarwal R. G. Antigenotoxic potential of curcumin and carvacrol against malathion-induced DNA damage in cultured human peripheral blood and its relation to GSTM1 and GSTT1 polymorphism. Biomarkers and Genomic Medicine . 2015;7:98–104. doi: 10.1016/j.bgm.2015.02.002. [DOI] [Google Scholar]
- 191.Guimarães A. G., Xavier M. A., De Santana M. T., et al. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn-Schmiedeberg's Archives of Pharmacology . 2012;385:253–263. doi: 10.1007/s00210-011-0715-x. [DOI] [PubMed] [Google Scholar]
- 192.Can Baser K. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Current Pharmaceutical Design . 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 193.Banik S., Akter M., Corpus Bondad S. E., Saito T., Hosokawa T., Kurasaki M. Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food and Chemical Toxicology . 2019;134, article 110835 doi: 10.1016/j.fct.2019.110835. [DOI] [PubMed] [Google Scholar]
- 194.Chenet A. L., Duarte A. R., de Almeida F. J. S., Andrade C. M. B., de Oliveira M. R. Carvacrol depends on heme oxygenase-1 (HO-1) to exert antioxidant, anti-inflammatory, and mitochondria-related protection in the human neuroblastoma SH-SY5Y cells line exposed to hydrogen peroxide. Neurochemical Research . 2019;44:884–896. doi: 10.1007/s11064-019-02724-5. [DOI] [PubMed] [Google Scholar]
- 195.Cui Z. W., Xie Z. X., Wang B. F., et al. Carvacrol protects neuroblastoma SH-SY5Y cells against Fe2+-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacologica Sinica . 2015;36:1426–1436. doi: 10.1038/aps.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mahmoodi M., Amiri H., Ayoobi F., et al. Carvacrol ameliorates experimental autoimmune encephalomyelitis through modulating pro- and anti-inflammatory cytokines. Life Sciences . 2019;219:257–263. doi: 10.1016/j.lfs.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 197.Tareq A. M., Farhad S., Uddin A. N., et al. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon . 2020;6(6, article e04061) doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siddiqi A., Saidullah B., Sultana S. Anti-carcinogenic effect of hesperidin against renal cell carcinoma by targeting COX-2/PGE2 pathway in Wistar rats. Environmental Toxicology . 2018;33:1069–1077. doi: 10.1002/tox.22626. [DOI] [PubMed] [Google Scholar]
- 199.Mahmoud A. M., Mohammed H. M., Khadrawy S. M., Galaly S. R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chemico-Biological Interactions . 2017;277:146–158. doi: 10.1016/j.cbi.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 200.Chen M., Gu H., Ye Y., et al. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food and Chemical Toxicology . 2010;48:2980–2987. doi: 10.1016/j.fct.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 201.Zhu C., Dong Y., Liu H., Ren H., Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomedicine & Pharmacotherapy . 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 202.Mitra S., Islam F., Das R., et al. Pharmacological potential of Avicennia alba leaf extract: an experimental analysis focusing on antidiabetic, anti-inflammatory, analgesic, and antidiarrheal activity. BioMed Research International . 2022;2022 doi: 10.1155/2022/7624189.7624189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ganeshpurkar A., Saluja A. The pharmacological potential of catechin. Indian Journal of Biochemistry and Biophysics . 2020;57:505–511. [Google Scholar]
- 204.Potapovich A. I., Kostyuk V. A. Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochemistry . 2003;68:514–519. doi: 10.1023/a:1023947424341. [DOI] [PubMed] [Google Scholar]
- 205.Nouri Z., Fakhri S., Nouri K., Wallace C. E., Farzaei M. H., Bishayee A. Targeting multiple signaling pathways in cancer: the rutin therapeutic approach. Cancers (Basel) . 2020;12:1–34. doi: 10.3390/cancers12082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mellou F., Loutrari H., Stamatis H., Roussos C., Kolisis F. N. Enzymatic esterification of flavonoids with unsaturated fatty acids: effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochemistry . 2006;41:2029–2034. doi: 10.1016/j.procbio.2006.05.002. [DOI] [Google Scholar]
- 207.Javed H., Khan M. M., Ahmad A., et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience . 2012;210:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 208.Rahman M. M., Rahaman M. S., Islam M. R., et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules . 2021;27(1):p. 233. doi: 10.3390/molecules27010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gęgotek A., Ambrożewicz E., Jastrząb A., Jarocka-Karpowicz I., Skrzydlewska E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Archives of Dermatological Research . 2019;311:203–219. doi: 10.1007/s00403-019-01898-w. [DOI] [PubMed] [Google Scholar]
- 210.Tian R., Yang W., Xue Q., et al. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. European Journal of Pharmacology . 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 211.Moghbelinejad S., Nassiri-Asl M., Naserpour Farivar T., et al. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicology Letters . 2014;224:108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 212.Wang R., Sun Y., Huang H., Wang L., Chen J., Shen W. Rutin, a natural flavonoid protects PC12 cells against sodium nitroprusside-induced neurotoxicity through activating PI3K/Akt/mTOR and ERK1/2 pathway. Neurochemical Research . 2015;40:1945–1953. doi: 10.1007/s11064-015-1690-2. [DOI] [PubMed] [Google Scholar]
- 213.Al-Harbi N. O., Imam F., Al-Harbi M. M., et al. Rutin inhibits carfilzomib-induced oxidative stress and inflammation via the NOS-mediated NF-κB signaling pathway. Inflammopharmacology . 2019;27:817–827. doi: 10.1007/s10787-018-0550-5. [DOI] [PubMed] [Google Scholar]
- 214.Zhou Y. F., Guo B., Ye M. J., Liao R. F., Li S. L. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Current Eye Research . 2016;41:933–942. doi: 10.3109/02713683.2015.1082186. [DOI] [PubMed] [Google Scholar]
- 215.Nassiri-Asl M., Ghorbani A., Salehisar S., Asadpour E., Sadeghnia H. R. Effect of rutin on oxidative DNA damage in PC12 neurons cultured in nutrients deprivation condition. Iranian Journal of Basic Medical Sciences . 2020;23:390–395. doi: 10.22038/IJBMS.2020.31832.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Matkowski A., Kus P., Goralska E., Wozniak D. Mangiferin–a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Reviews in Medicinal Chemistry . 2013;13:439–455. doi: 10.2174/1389557511313030011. [DOI] [PubMed] [Google Scholar]
- 217.Núñez Selles A. J., Daglia M., Rastrelli L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors . 2016;42:475–491. doi: 10.1002/biof.1299. [DOI] [PubMed] [Google Scholar]
- 218.Heng M., Lu Z. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chinese Medical Journal . 1990;103:160–165. [PubMed] [Google Scholar]
- 219.Saha S., Sadhukhan P., Sil P. C. Mangiferin: a xanthonoid with multipotent anti-inflammatory potential. BioFactors . 2016;42:459–474. doi: 10.1002/biof.1292. [DOI] [PubMed] [Google Scholar]
- 220.Dutta T., Paul A., Majumder M., Sultan R. A., Emran T. B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochemistry and Biophysics Reports . 2020;21, article 100715 doi: 10.1016/j.bbrep.2019.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Khurana R. K., Kaur R., Lohan S., Singh K. K., Singh B. Mangiferin: a promising anticancer bioactive. Pharmaceutical Patent Analyst . 2016;5:169–181. doi: 10.4155/ppa-2016-0003. [DOI] [PubMed] [Google Scholar]
- 222.Dar A., Faizi S., Naqvi S., et al. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biological and Pharmaceutical Bulletin . 2005;28:596–600. doi: 10.1248/bpb.28.596. [DOI] [PubMed] [Google Scholar]
- 223.Kasi Viswanadh E., Nageshwar Rao B., Satish Rao B. S. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Human & Experimental Toxicology . 2010;29:409–418. doi: 10.1177/0960327110361752. [DOI] [PubMed] [Google Scholar]
- 224.Zhang B. P., Zhao J., Li S. S., et al. Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro: a novel Nrf2 activator without a “dark side”. Acta Pharmacologica Sinica . 2014;35:257–266. doi: 10.1038/aps.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Saha S., Sadhukhan P., Sinha K., Agarwal N., Sil P. C. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochemistry and Biophysics Reports . 2016;5:313–327. doi: 10.1016/j.bbrep.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kabir M. S. H., Hossain M. M., Kabir M. I., et al. Phytochemical screening, antioxidant, thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. Journal of Young Pharmacists . 2016;8(4):391–397. doi: 10.5530/jyp.2016.4.15. [DOI] [Google Scholar]
- 227.Murtaza G., Karim S., Akram M. R., et al. Caffeic acid phenethyl ester and therapeutic potentials. BioMed Research International . 2014;2014 doi: 10.1155/2014/145342.145342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sud'Ina G. F., Mirzoeva O. K., Pushkareva M. A., Korshunova G., Sumbatyan N. V., Varfolomeev S. D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Letters . 1993;329:21–24. doi: 10.1016/0014-5793(93)80184-V. [DOI] [PubMed] [Google Scholar]
- 229.Armutcu F., Akyol S., Ustunsoy S., Turan F. F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (review) Experimental and Therapeutic Medicine . 2015;9:1582–1588. doi: 10.3892/etm.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Meyuhas S., Assali M., Huleihil M., Huleihel M. Antimicrobial activities of caffeic acid phenethyl ester. Journal of Molecular Biochemistry . 2015;4:21–31. [Google Scholar]
- 231.Ozturk G., Ginis Z., Akyol S., Erden G., Gurel A., Akyol O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. European Review for Medical and Pharmacological Sciences . 2012;16:2064–2068. [PubMed] [Google Scholar]
- 232.Natarajan K., Singh S., Burke T. R., Grunberger D., Aggarwal B. B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proceedings of the National Academy of Sciences; 1996; pp. 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kimura Y., Okuda H., Okuda T., Hatano T., Arichi S. Studies on the activities of tannins and related compounds, X. Effects of caffeetannins and related compounds on arachidonate metabolism in human polymorphonuclear leukocytes. Journal of Natural Products . 1987;50:392–399. doi: 10.1021/np50051a009. [DOI] [PubMed] [Google Scholar]
- 234.Laranjinha J., Vieira O., Madeira V., Almeida L. Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: consumption vs regeneration. Archives Of Biochemistry and Biophysics . 1995;323:373–381. doi: 10.1006/abbi.1995.0057. [DOI] [PubMed] [Google Scholar]
- 235.Wei X., Zhao L., Ma Z., et al. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain . 2004;127:2629–2635. doi: 10.1093/brain/awh316. [DOI] [PubMed] [Google Scholar]
- 236.Al Mahmud Z., Emran T. B., Qais N., Bachar S. C., Sarker M., Uddin M. M. N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb) Journal of Basic and Clinical Physiology and Pharmacology . 2016;27(1):63–70. doi: 10.1515/jbcpp-2015-0056. [DOI] [PubMed] [Google Scholar]
- 237.Özeren M., Sucu N., Tamer L., et al. Caffeic acid phenethyl ester (CAPE) supplemented St. Thomas’ hospital cardioplegic solution improves the antioxidant defense system of rat myocardium during ischemia-reperfusion injury. Pharmacological Research . 2005;52:258–263. doi: 10.1016/j.phrs.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 238.Kim J. K., Jang H. D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. International journal of molecular sciences . 2014;15:12149–12165. doi: 10.3390/ijms150712149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kim H., Kim W., Yum S., et al. Caffeic acid phenethyl ester activation of Nrf2 pathway is enhanced under oxidative state: structural analysis and potential as a pathologically targeted therapeutic agent in treatment of colonic inflammation. Free Radical Biology and Medicine . 2013;65:552–562. doi: 10.1016/j.freeradbiomed.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 240.Gocmez C., Celik F., Kamasak K., et al. Effects of intrathecal caffeic acid phenethyl ester and methylprednisolone on oxidant/antioxidant status in traumatic spinal cord injuries. Journal of Neurological Surgery Part A: Central European Neurosurgery . 2015;76:20–24. doi: 10.1055/s-0034-1371513. [DOI] [PubMed] [Google Scholar]
- 241.Akgun B., Ozturk S., Artas G., Erol F. S. Effects of intrathecal Caffeic Acid Phenethyl Ester (CAPE) on IL-6 and TNF-α levels and local inflammatory responses in spinal cord injuries. Turkish Neurosurgery . 2018;28:625–629. doi: 10.5137/1019-5149.JTN.20011-17.1. [DOI] [PubMed] [Google Scholar]
- 242.Ilhan A., Koltuksuz U., Ozen S., Uz E., Ciralik H., Akyol O. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. European Journal of Cardio-Thoracic Surgery . 1999;16:458–463. doi: 10.1016/S1010-7940(99)00246-8. [DOI] [PubMed] [Google Scholar]
- 243.Yiğit U., Kırzıoğlu F. Y., Özmen Ö., Uğuz A. C. Protective effects of caffeic acid phenethyl ester on the heart in experimental periodontitis against oxidative stress in rats. Dental and Medical Problems . 2021;58:335–341. doi: 10.17219/dmp/132388. [DOI] [PubMed] [Google Scholar]
- 244.Jiang Z., Gao W., Huang L. Tanshinones, critical pharmacological components in salvia miltiorrhiza. Frontiers in Pharmacology . 2019;10:p. 202. doi: 10.3389/fphar.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou S., Chen W., Su H., Zheng X. Protective properties of tanshinone I against oxidative DNA damage and cytotoxicity. Food and Chemical Toxicology . 2013;62:407–412. doi: 10.1016/j.fct.2013.08.084. [DOI] [PubMed] [Google Scholar]
- 246.Huang L., Zhu J., Zheng M., Zou R., Zhou Y., Zhu M. Tanshinone IIA protects against subclinical lipopolysaccharide induced cardiac fibrosis in mice through inhibition of NADPH oxidase. International Immunopharmacology . 2018;60:59–63. doi: 10.1016/j.intimp.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 247.Jiang P., Li C., Xiang Z., Jiao B. Tanshinone IIA reduces the risk of Alzheimer’s disease by inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation in the temporal lobes of rat models of Alzheimer’s disease. Molecular Medicine Reports . 2014;10:689–694. doi: 10.3892/mmr.2014.2254. [DOI] [PubMed] [Google Scholar]
- 248.Il Jang S., Jin Kim H., Kim Y. J., Il Jeong S., You Y. O. Tanshinone IIA inhibits LPS-induced NF-κB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. European Journal of Pharmacology . 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 249.Ma Y. Q., Chen Y. R., Leng Y. F., Wu Z. W. Tanshinone IIA downregulates HMGB1 and TLR4 expression in a spinal nerve ligation model of neuropathic pain. Evidence-Based Complementary and Alternative Medicine . 2014;2014 doi: 10.1155/2014/639563.639563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sun S., Yin Y., Yin X., et al. Anti-nociceptive effects of Tanshinone IIA (TIIA) in a rat model of complete Freund’s adjuvant (CFA)-induced inflammatory pain. Brain Research Bulletin . 2012;88:581–588. doi: 10.1016/j.brainresbull.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 251.Zhu Y., Tang Q., Wang G., Han R. Tanshinone IIA protects hippocampal neuronal cells from reactive oxygen species through changes in autophagy and activation of phosphatidylinositol 3-kinase, protein kinas B, and mechanistic target of rapamycin pathways. Current Neurovascular Research . 2017;14:132–140. doi: 10.2174/1567202614666170306105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Markowitz K., Moynihan M., Liu M., Kim S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surgery, Oral Medicine, Oral Pathology . 1992;73:729–737. doi: 10.1016/0030-4220(92)90020-Q. [DOI] [PubMed] [Google Scholar]
- 253.Fangjun L., Zhijia Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thoracic Cancer . 2018;9(1):25–29. doi: 10.1111/1759-7714.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ghosh V., Mukherjee A., Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids and Surfaces B: Biointerfaces . 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 255.Kaur G., Athar M., Sarwar Alam M. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center . 2010;49(3):290–301. doi: 10.1002/mc.20601. [DOI] [PubMed] [Google Scholar]
- 256.Barboza J. N., da Silva Maia C., Bezerra Filho R. O., Silva J. V. R., Medeiros J. V. R., de Sousa D. P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxidative Medicine and Cellular Longevity . 2018;2018 doi: 10.1155/2018/3957262.3957262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang B. H., Piao Z. G., Kim Y. B., et al. Activation of vanilloid receptor 1 (VR1) by Eugenol. Journal of Dental Research . 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]
- 258.Seo H., Li H. Y., Perez-Reyes E., Lee J. H. Effects of eugenol on T-type Ca2+ channel isoforms. Journal of Pharmacology and Experimental Therapeutics . 2013;347:310–317. doi: 10.1124/jpet.113.207936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sun X., Wang D., Zhang T., et al. Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Frontiers in Pharmacology . 2020;11:p. 84. doi: 10.3389/fphar.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Forouzanfar F., Majeed M., Jamialahmadi T., Sahebkar A. Curcumin: a review of its effects on epilepsy. Advances in Experimental Medicine and Biology . 2021;1291:363–373. doi: 10.1007/978-3-030-56153-6_21. [DOI] [PubMed] [Google Scholar]
- 261.Belcaro G., Cesarone M. R., Dugall M., et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Alternative Medicine Review . 2010;15:337–344. [PubMed] [Google Scholar]
- 262.Kertia N., Asdie A. H., Rochmah W. Ability of curcuminoid compared to diclofenac sodium in reducing the secretion of cycloxygenase-2 enzyme by synovial fluid’s monocytes of patients with osteoarthritis. Acta Medica Indonesiana . 2012;44:105–113. [PubMed] [Google Scholar]
- 263.Satoskar R. R., Shah S. J., Shenoy S. G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation . 1986;24:651–654. [PubMed] [Google Scholar]
- 264.Hatefi M., Ahmadi M. R. H., Rahmani A., Dastjerdi M. M., Asadollahi K. Effects of curcumin on bone loss and biochemical markers of bone turnover in patients with spinal cord injury. World Neurosurgery . 2018;114:e785–e791. doi: 10.1016/j.wneu.2018.03.081. [DOI] [PubMed] [Google Scholar]
- 265.Allison D. J., Josse A. R., Gabriel D. A., Klentrou P., Ditor D. S. Targeting inflammation to influence cognitive function following spinal cord injury: a randomized clinical trial. Spinal Cord . 2017;55:26–32. doi: 10.1038/sc.2016.96. [DOI] [PubMed] [Google Scholar]
- 266.Dolati S., Babaloo Z., Ayromlou H., et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. Journal of Neuroimmunology . 2019;327:15–21. doi: 10.1016/j.jneuroim.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 267.Asadi S., Gholami M. S., Siassi F., Qorbani M., Khamoshian K., Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: a randomized double-blind placebo- controlled clinical trial. Complementary Therapies in Medicine . 2019;43:253–260. doi: 10.1016/j.ctim.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 268.Morillas-Ruiz J. M., Rubio-Perez J. M., Albaladejo M. D., Zafrilla P., Parra S., Vidal-Guevara M. L. Effect of an antioxidant drink on homocysteine levels in Alzheimer’s patients. Journal of the Neurological Sciences . 2010;299:175–178. doi: 10.1016/j.jns.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 269.Valensi P., Le Devehat C., Richard J. L., et al. A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy: a preliminary report. Journal of Diabetes and its Complications . 2005;19:247–253. doi: 10.1016/j.jdiacomp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 270.Verlaet A. A. J., Ceulemans B., Verhelst H., et al. Effect of Pycnogenol® on attention-deficit hyperactivity disorder (ADHD): study protocol for a randomised controlled trial. Trials . 2017;18:1–9. doi: 10.1186/s13063-017-1879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Clark D. O., Xu H., Moser L., et al. MIND food and speed of processing training in older adults with low education, the MINDSpeed Alzheimer’s disease prevention pilot trial. Contemporary Clinical Trials . 2019;84 doi: 10.1016/j.cct.2019.105814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ferianec V., Fülöp M., Ježovičová M., et al. The oak–wood extract robuvit® improves recovery and oxidative stress after hysterectomy: a randomized, double-blind, placebo-controlled pilot study. Nutrients . 2020;12:1–13. doi: 10.3390/nu12040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.You Y. X., Shahar S., Rajab N. F., et al. Effects of 12 weeks Cosmos caudatus supplement among older adults with mild cognitive impairment: a randomized, double-blind and placebo-controlled trial. Nutrients . 2021;13:1–16. doi: 10.3390/nu13020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Di Pierro D., Ciaccio C., Sbardella D., et al. Effects of oral administration of common antioxidant supplements on the energy metabolism of red blood cells. Attenuation of oxidative stress-induced changes in Rett syndrome erythrocytes by CoQ10. Molecular and Cellular Biochemistry . 2020;463:101–113. doi: 10.1007/s11010-019-03633-5. [DOI] [PubMed] [Google Scholar]
- 275.Zuidema J. M., Gilbert R. J., Osterhout D. J. Nanoparticle technologies in the spinal cord. Cells Tissues Organs . 2016;202:102–115. doi: 10.1159/000446647. [DOI] [PubMed] [Google Scholar]
- 276.Peng Y., Chen L., Ye S., et al. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian Journal of Pharmaceutical Sciences . 2020;15:220–236. doi: 10.1016/j.ajps.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang H. D., Shi Y. M., Li L., Guo J. D., Zhang Y. P., Hou S. X. Treatment with resveratrol attenuates sublesional bone loss in spinal cord-injured rats. British Journal of Pharmacology . 2013;170:796–806. doi: 10.1111/bph.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to establish the conclusions of this study are integrated into the article.


