Abstract
Objectives
Diagnostic delays are a major source of morbidity and mortality. Despite the adverse outcomes associated with diagnostic delays, few studies have examined the incidence and factors that influence diagnostic delays for different infectious diseases. The objective of this study was to understand the relative frequency of diagnostic delays for six infectious diseases commonly seen by infectious diseases (ID) consultants and to examine contributing factors for these delays.
Methods
A 25-item survey to examine diagnostic delays in six infectious diseases was sent to all infectious diseases physicians in the Emerging Infections Network (EIN) who provide care to adult patients. Diseases included (1) tuberculosis, (2) non-tuberculous mycobacterial infections, (3) syphilis, (4) epidural abscess, (5) infective endocarditis, and (6) endemic fungal infections (e.g., histoplasmosis, blastomycosis).
Results
A total of 533 of 1,323 (40%) EIN members responded to the survey. Respondents perceived the diagnosis not being considered initially and the appropriate test not being ordered as the two most important contributors to diagnostic delays. Unusual clinical presentations and not consulting ID physicians early enough were also reported as a contributing factor to delays. Responses recorded in open-text fields also indicated errors related to testing as a likely cause of delays; specifically, test-related errors included ordering the wrong laboratory test, laboratory delays (specialized labs not available at the facility), and lab processing delays.
Conclusions
Diagnostic delays commonly occur for the infectious diseases we considered. The contributing factors we identified are potential targets for future interventions to decrease diagnostic delays.
Keywords: consultation, diagnostic delays, infectious diseases
Introduction
Diagnostic delays are a major source of morbidity and mortality [1], [2], [3], [4] and are underappreciated as a cause of patient injury [2, 5]. Diagnostic delays occur for a wide spectrum of diseases, but they are especially important to consider for infectious diseases. At the individual level, even short delays in diagnosis and treatment can result in increased morbidity for some infectious diseases (e.g., endocarditis, epidural abscesses) [6], [7], [8]. From a public-health perspective, delays in the diagnosis of communicable disease (e.g., tuberculosis, syphilis) can generate additional cases [9]. In addition, infectious diseases, unlike many other diseases, can result from specific environmental exposures (e.g., exposure to vector-borne disease from soil exposure or from travel to an endemic area) or specific behaviors or practices (e.g., intravenous drug use). The failure to consider these important risk factors in the diagnostic process may dramatically increase delays leading to worse outcomes [10].
Many infectious diseases are difficult to diagnose due to variable presentations and the limitations of diagnostic testing. Many signs and symptoms (e.g., fevers and chills) are nonspecific markers of inflammation that are shared across many different infections regardless of the pathogen. Accordingly, many different infections may initially appear with a similar clinical presentation. For example, potentially life-threating bacterial infections may be initially indistinguishable from common self-limited viral infections. Furthermore, when common pathogens are not revealed by common tests like blood cultures/urine cultures or PCR assays, providers must invoke less common diagnoses in the differential diagnosis to order the appropriate second line microbiologic testing (e.g., zoonosis serology). However, these specific diseases must be considered on the differential diagnosis in order for the appropriate test to be ordered.
Diagnostic delays are difficult to quantify, and the incidence of such delays is unknown for many infectious diseases. Traditional approaches to identify diagnostic delays are resource intensive (e.g., chart review) [6, 11], [12], [13] or of limited scope (e.g., autopsy or malpractice claims) [11, 12, 14]. An alternative approach for identifying and investigating diagnostic delays is to query clinicians about their personal observations pertaining to diagnostic delays. The objective of this study was to understand the relative frequency of diagnostic delays for six infectious diseases commonly seen by infectious diseases (ID) consultants and to examine contributing factors for these delays.
Materials and methods
Survey description
A 25-item survey was developed to examine diagnostic delays for six infectious diseases. The six diseases were chosen from a larger list of diseases that could be diagnosed either by serologic or microbiologic testing and occur in a sufficient volume for the majority of members to see commonly in their clinical practice. We excluded common infectious diseases for which infectious disease consultations may not be routinely requested (e.g., pneumonia).
The survey questions were refined based on iterative revisions and pilot testing by multiple members of the Emerging Infections Network (EIN). For each disease, we selected four questions and one open-ended question to limit the total length of the survey. These four questions related to different aspects of observed diagnostic delays. The survey is available at: https://ein.idsociety.org/surveys/survey/116/.
Survey questions addressed diagnostic delays that respondents had encountered in their practice pertaining to (1) tuberculosis, (2) non-tuberculous mycobacteria, (3) syphilis, (4) epidural abscess, (5) infective endocarditis, and (6) endemic fungal infections (e.g., histoplasmosis, blastomycosis). For each disease category, there was an “opt out” option for responding physicians who believed they saw too few cases in that specific category to provide an objective assessment.
The same four questions were asked for each infectious disease category, including perceived frequency of diagnostic delays, assessment of timing of ID consultation, the impact of late ID consultation requests, and the most common contributor to observed diagnostic delays. A diagnostic delay was defined as “a case where sufficient data were available to make the correct diagnosis at an earlier point in the course of a patient’s illness” [15]. Relative frequency of diagnostic delays was categorized as: rarely (1–2 cases ever); infrequently (1 case every few years); occasionally (a few cases each year); commonly (several cases each month). Timing of ID consultations was categorized as: too early, at the right time, or too late. The impact of late ID consultation requests was categorized as: no impact (no effect at all), minor impact (patient dissatisfaction or minor delay), moderate impact (prolonged hospitalization/short-term morbidity), or major impact (permanent disability/life-threatening event). Finally, the options for diagnostic-delay contributors included: diagnosis not considered initially, specific tests (e.g., cultures, serologies) not ordered, missing data (e.g., travel history, immune status), unusual presentation, or none of the above. The final question of the survey was an open-ended query where physicians were instructed to submit the most frequently seen diagnostic delays in their practice.
Survey administration and analysis
The Centers for Disease Control and prevention established the EIN as a sentinel network of ID physicians in North America [16, 17]. The EIN maintains a member database that includes professional characteristics such as adult or pediatric practice, years in ID practice, geographic location, hospital type, and size. This survey was distributed via email to all EIN members who provide care to adult patients. The survey was open from October 16 to November 9, 2019. After the initial survey distribution, two email reminders were sent to non-respondents at weekly intervals. The response denominator includes EIN members who have ever responded to an EIN survey (which is the customary reporting practice in previous EIN survey reports). Differences in frequencies were analyzed for statistical significance using chi-square tests, Student’s t-tests, and Mann-Whitney U tests. A p-value of less than 0.05 was statistically significant. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Characteristics of respondents
Overall, 533 of 1,323 (40%) EIN members who have responded to EIN surveys in the past responded to the survey. Practice characteristics for respondents and non-respondents are shown in Table 1. The survey response rate was significantly higher from physicians who had been practicing for more than 25 years and from physicians affiliated with the Department of Veterans Affairs.
Table 1:
Characteristics of respondents’ ID practices.
| Responders n=533 n (row %) |
Non respondents n=790 n (row %) |
|
|---|---|---|
| U.S. Census Bureau Division | ||
| New England | 43 (43) | 56 (57) |
| Mid Atlantic | 79 (41) | 113 (59) |
| East North Central | 72 (39) | 113 (61) |
| West North Central | 72 (51) | 69 (59) |
| South Atlantic | 93 (38) | 150 (62) |
| East South Central | 20 (35) | 37 (65) |
| West South Central | 39 (41) | 56 (59) |
| Mountain | 21 (36) | 38 (64) |
| Pacific | 89 (37) | 150 (63) |
| Canada and Puerto Rico | 5 (38) | 8 (62) |
| Years since infectious disease fellowship | ||
| <5 | 88 (31) | 197 (69) |
| 5–14 | 168 (33) | 339 (67) |
| 15–24 | 88 (41) | 125 (59) |
| ≥25a | 189 (59) | 129 (51) |
| Employment | ||
| Hospital/clinic | 191 (41) | 275 (59) |
| Private/group practice | 129 (38) | 211 (62) |
| University/medical school | 172 (38) | 276 (62) |
| Veterans Affairs and militarya | 41 (59) | 28 (41) |
| Primary hospital type | ||
| Community | 131 (35) | 240 (65) |
| Veterans Affairs/Department of Defensea | 44 (58) | 32(42) |
| Non-university teaching | 129 (41) | 184 (59) |
| University | 199 (41) | 288 (59) |
| City/county | 30 (39) | 46 (61) |
| Primary hospital bed size | ||
| <200 | 58 (45) | 71 (55) |
| 200–350 | 113 (39) | 178 (61) |
| 351–450 | 84 (39) | 132 (61) |
| 451–600 | 106 (41) | 151 (59) |
| >600 | 171 (40) | 257 (60) |
aRespondents were significantly more likely than non-respondents to have ≥25 years of ID experience (p<0.0001), to be employed by the federal government (Veterans Affairs or military) (p=0.001), and to work in a Veterans Affairs or Department of Defense hospital (p=0.008).
Frequency of diagnostic delays
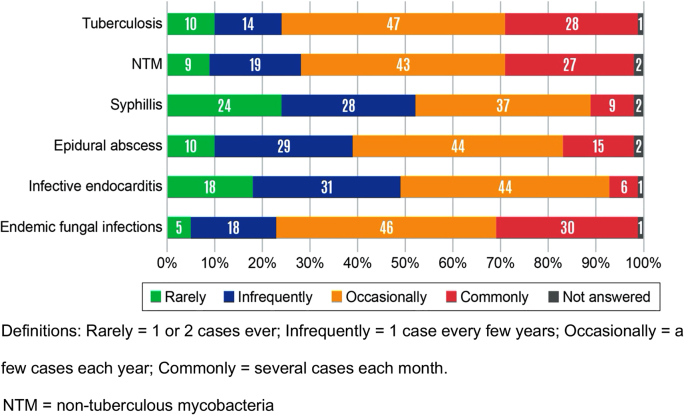
The most frequently encountered diagnosis in the respondents’ practices where they reported diagnostic delay were infective endocarditis (n=394, 74%) and epidural abscess (n=371, 70%) (Table 2). Forty-nine percent of the respondents opted out of answering diagnostic delay questions about endemic fungal infections in their practice. Diagnostic delays were most frequently reported as common for endemic fungal infections (30% of respondents), tuberculosis (28%), and nontuberculous mycobacterial infections (27%) (Figure 1). Most respondents reported that diagnostic delays for syphilis were rare.
Table 2:
‘Opt out’ of answering based on frequency with which specified infection is seen by the reporting ID physician.
| Infection | Do not see enough to answer, n (row %) |
Questions on this infection answered, n (row %) |
|---|---|---|
| Infective endocarditis | 139 (26) | 394 (74) |
| Epidural abscess | 162 (30) | 371 (70) |
| Syphilis | 198 (37) | 335 (63) |
| Nontuberculous mycobacterial infections | 204 (38) | 329 (62) |
| Tuberculosis | 212 (40) | 321 (60) |
| Endemic fungal infections (e.g., histoplasmosis, blastomycosis) | 259 (49) | 274 (51) |
Figure 1:
Relative frequency of diagnostic delays as reported by infectious diseases (ID) physicians.
Timing of ID consultation requests
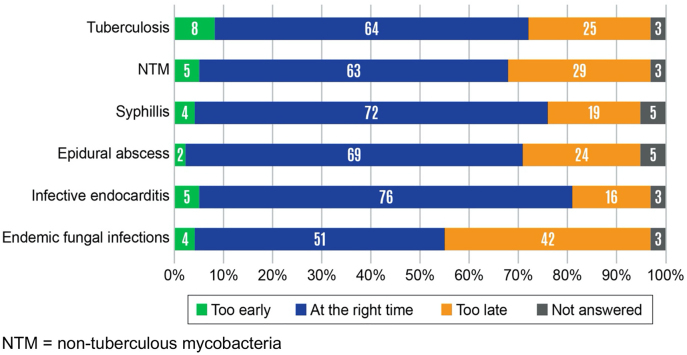
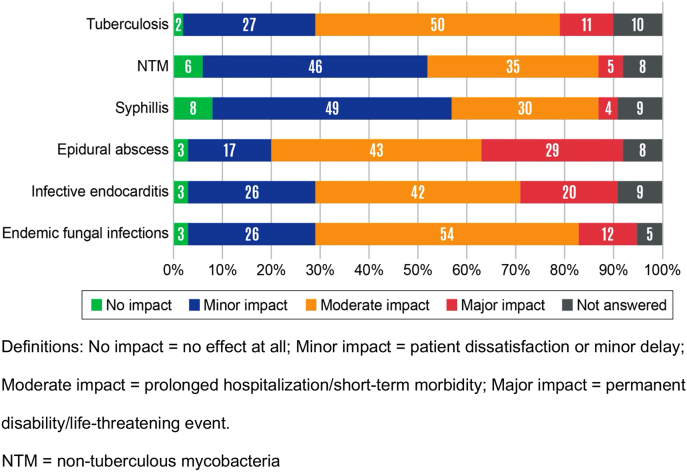
ID physicians believed that consultation requests were too late most often for endemic fungal infections (42% of respondents), followed by consultation requests for non-tuberculous mycobacteria (29%) (Figure 2). Consultation requests for endocarditis were believed to occur at the right time by 76% of respondents. When ID physicians were consulted too late, they believed that the delay typically resulted in a major impact for epidural abscesses (29% of respondents) and infective endocarditis (20%) (Figure 3). Late ID consultations for syphilis were believed to have either no impact or minor impact by 57% of respondents.
Figure 2:
Timing of infectious diseases (ID) consultation requests as reported by ID physicians.
Figure 3:
Impact of late infectious diseases (ID) consultation requests.
Contributing factors for diagnostic delays
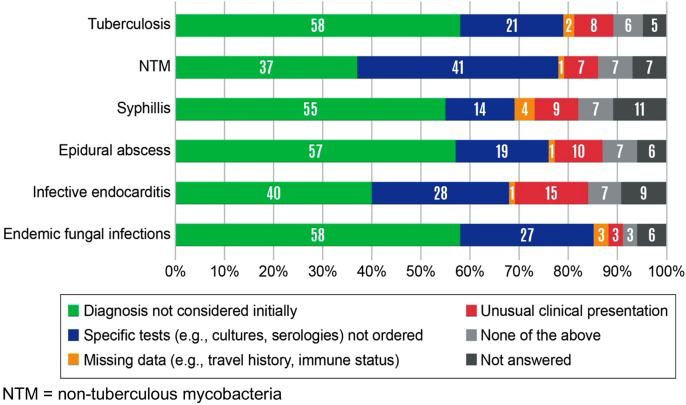
Across all six infectious diseases, the two most important contributors to diagnostic delays (as selected from pre-specified options in the penultimate survey question) were: the diagnosis not being considered initially (37–58%) and the appropriate test not being ordered (14–41%) (Figure 4). Missing data (e.g., travel history or immune status) and unusual clinical presentations were also reported as contributors to diagnostic delays.
Figure 4:
Common contributing factors for diagnostic delays.
Themes from the open-ended comments field
One-hundred ninety-four respondents also offered responses to the free text field asking about the diagnostic delays seen most frequently in individual practices. Some respondents reported specific diseases while others reflected on factors contributing to delays. The most common diseases mentioned were tuberculosis (n=25 respondents), fungal infections (n=18), HIV (n=15), endocarditis (n=12), syphilis (n=10), and epidural abscesses (n=8). In addition, 24 respondents commented on delays caused by send-out laboratory tests or reference laboratory tests not done onsite at their institution, and 12 respondents provided comments about delays in getting ordered tests performed, e.g., no testing done on weekends or delaying a biopsy so that it can be done as an outpatient rather than during a hospitalization.
Discussion
This is the largest survey of infectious disease physicians, to our knowledge, to assess their experience with diagnostic delay and perceived causes of these delays in six infections commonly seen by ID consultants. Among the infections we investigated, diagnostic delays were most frequently reported for infective endocarditis and epidural abscesses. In contrast, reports of diagnostic delays for syphilis were uncommon. Among physicians who reported seeing a sufficient volume of patients to comment, delays were commonly reported for endemic fungal infections (e.g., histoplasmosis, blastomycosis), tuberculosis, and non-tuberculous mycobacterial infections. Overall, respondents cited diagnostic-test-related failures as the leading cause of diagnostic delays for the diseases considered. Other reported contributing factors to diagnostic delays included missing data (e.g., travel history, immune status) and unusual clinical presentations.
Diagnostic delays in endocarditis, epidural abscesses, tuberculosis, and histoplasmosis have been previously reported. Studies of malpractice claims frequently highlight both endocarditis and epidural abscesses [3, 14, 18, 19]. This is not surprising given the high morbidity, mortality, and potential for lasting disability associated with both of these infections. For both infective endocarditis and epidural abscess, the prompt administration of appropriate antibiotics is critical, and urgent or emergency surgery may be needed. For tuberculosis, reports in several different countries with varying levels of disease activity report diagnostic delays [20], [21], [22]. Also, diagnostic delays have been previously described for histoplasmosis in case reports [23, 24] and in studies using insurance claims [25]. However, to the best of our knowledge, no large population-based studies exist for nontuberculous mycobacterial infections. Given that both endemic fungal infections and nontuberculous mycobacterial infections are associated with newer immunosuppressive therapies (i.e., biologics) [26, 27], as these infections become more common, understanding their diagnostic delays may be increasingly important. Finally, because syphilis can mimic several other diseases and syndromes, we were somewhat surprised that respondents did not report observing frequent diagnostic delays. This could be due to improved screening efforts, especially in people living with HIV, alternatively, it may be secondary to the widespread availability of diagnostic screening and testing algorithms as well as the rapidity of testing (results, which are often available at the point of care or within hours).
Collectively, our results from both structured questions and free-text responses suggest that the most important contributor to diagnostic delays for the infectious diseases we considered was diagnostic-testing failures. The testing failures cited by respondents occur during multiple stages of the testing process. In addition to the failure to order the correct test, respondents reported problems with specimen processing, test performance, interpretation, and follow-up. These findings are especially concerning because, in our prior work, we found that an increasing number of hospitals are using off-site laboratories [28]. Another potential testing-related failure reported was other physicians declining to perform appropriate testing, including biopsies for definitive diagnosis or acting on potential disincentive to do a procedure/testing while the patient was an inpatient. In such cases, the diagnosis was suspected but could not be adequately verified. Accordingly, for some diseases, a team-based multidisciplinary collaborative approach may be needed to help decrease diagnostic delays. The value of ID consultation has been demonstrated for many complex infectious diseases (e.g., Staphylococcus aureus bacteremia and endocarditis) [29, 30]. Enhanced communication and teamwork is often necessary when biopsies or surgical interventions are needed to make a diagnosis. Responses from our query also suggest that improving the communication during transitions of care from inpatient to outpatient teams is another important area for improving the diagnostic process.
While not as common as testing-related failures, diagnostic failures associated with clinician assessment were also reported. Such cognitive errors include not considering a diagnosis ( hypothesis generation) and not giving approprate weight to a condition in the differential diagnosis [5, 31]. In the open response section of the survey, multiple cognitive factors were mentioned: diagnosis not considered by the treating physician, failure to seek help from a specialist early on, limited history (lack of social history) or physical examination by the treating provider, treating with antibiotics without a clear diagnosis, overreliance on test results (e.g., “negative echo means no endocarditis”), and premature closure (diagnosis without appropriate verification). Use of antimicrobial drugs without a clear diagnosis has been associated with an increased risk for delayed and masked or missed diagnoses of infectious diseases and non-infectious diseases [32]. This problem may also contribute to the emergence of antimicrobial resistance.
Our results suggest that infective endocarditis and epidural abscess are two high-priority diseases that would benefit from more in-depth investigations and targeted interventions. While both diseases can be caused by similar bacteria and share some diagnostic approaches (e.g., the need for blood cultures and imaging), disease-specific questions need to be addressed in future studies to design interventions to decrease diagnostic errors.
Responses to our survey support the consideration of multidisciplinary care teams to help address the potential need for and timing of surgery. For tuberculosis, diagnostic tests exist, but there is some delay (e.g., with skin testing, access to point-of-care testing for IGRA). Furthermore, these tests do not differentiate between latent and active tuberculosis, necessitating multiple visits and interpreting lab results in the context of clinical presentation. The most common signs and symptoms for tuberculosis overlap with other respiratory diseases, complicating efforts to make a timely diagnosis. Histoplasmosis shares many of the same clinical features as tuberculosis and thus many of the same diagnostic challenges. Both diseases can be re-activated and exposures may be remote from disease presentation. Gathering relevant exposure and risk factor information (a delay factor that was noted by many respondents) are important for tuberculosis and histoplasmosis. Given the several potential testing-related failures noted by members, tuberculosis and histoplasmosis are two diseases that would benefit from more generally available rapid diagnostics that do not require off-site testing. Finally, given the lack of existing literature regarding diagnostic delays for non-tuberculous mycobacterial infections, in comparison to the other infections we considered, there is clearly a need for more investigation.
Our study has several limitations. First, as with all voluntary surveys, selection bias could yield results that are not generalizable to all ID specialists. Second, our results may be subject to a response bias and survey answers may not accurately reflect clinical practice. We did not independently review medical records of reported cases and relied exclusively on respondents’ recall. Third, a sizable minority of respondents did not see a sufficient volume of patients with a particular infection to enable them to comment on the frequency of diagnostic delays (they “opted out” of questions concerning those specific infections). However, our overall response rate is similar to previous EIN surveys [17]. Fourth, although we considered multiple infectious diseases, our results to not include estimates of the duration of delays for different infectious diseases nor do they provide population-based estimates of the incidence of diagnostic delays for different infections across multiple healthcare settings. Finally, we are unable to capture the clinical and public health consequences of diagnostic delays. All of these factors will be important for future investigations to further inform and prioritize interventions to decrease diagnostic delays.
Despite our limitations, our results highlight several different infectious diseases for which diagnostic delays commonly occur. Delays occur for multiple reasons including not initially considering the diagnosis, limitations of testing, unusual clinical presentations, and failure to consult ID physicians earlier in the diagnostic process. These results highlight potential directions for future interventions to decrease diagnostic delays.
Footnotes
Research funding: This publication was supported by a cooperative agreement (U50 CK000477) funded by the Centers for Disease Control and Prevention, a grant (5R01HS027375) funded by the Agency for Healthcare Research and Quality, and a grant (UL1TR002537) funded by the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Gurpreet Dhaliwal MD is a member of the board of directors for the Society to Improve Diagnosis in Medicine. All authors report no conflicts of interest relevant to this article.
Informed consent: Not applicable.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
- 1.Graber M. Diagnostic errors in medicine: a case of neglect. Jt Comm J Qual Patient Saf. 2005;31:106–13. doi: 10.1016/s1553-7250(05)31015-4. [DOI] [PubMed] [Google Scholar]
- 2.Newman-Toker DE, Pronovost PJ. Diagnostic errors--the next frontier for patient safety. JAMA. 2009;301:1060–2. doi: 10.1001/jama.2009.249. [DOI] [PubMed] [Google Scholar]
- 3.Newman-Toker DE, Schaffer AC, Yu-Moe CW, Nassery N, Saber Tehrani AS, Clemens GD, et al. Serious misdiagnosis-related harms in malpractice claims: the "Big Three" - vascular events, infections, and cancers. Diagnosis. 2019;6:227–40. doi: 10.1515/dx-2019-0019. [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences E, Medicine . Improving diagnosis in health care. Washington DC, USA: National Academies Press; 2015. [PubMed] [Google Scholar]
- 5.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493–9. doi: 10.1001/archinte.165.13.1493. [DOI] [PubMed] [Google Scholar]
- 6.Epaulard O, Roch N, Potton L, Pavese P, Brion JP, Stahl JP. Infective endocarditis-related stroke: diagnostic delay and prognostic factors. Scand J Infect Dis. 2009;41:558–62. doi: 10.1080/00365540902984701. [DOI] [PubMed] [Google Scholar]
- 7.Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285–91. doi: 10.1016/j.jemermed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169:1881–7. doi: 10.1001/archinternmed.2009.333. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Chen W, Yang Y, Chu P, Liu X, Zhao M, et al. Effect of diagnostic and treatment delay on the risk of tuberculosis transmission in Shenzhen, China: an observational cohort study, 1993-2010. PLoS One. 2013;8:e67516. doi: 10.1371/journal.pone.0067516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiff GD, Kim S, Abrams R, Cosby K, Lambert B, Elstein AS, et al. In: Advances in patient safety: from research to implementation. Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Rockville (MD): Agency for Healthcare Research and Quality; 2005. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. [PubMed] [Google Scholar]
- 11.Donovan FM, Wightman P, Zong Y, Gabe L, Majeed A, Ynosencio T, et al. Delays in coccidioidomycosis diagnosis and associated healthcare utilization, Tucson, Arizona, USA. Emerg Infect Dis. 2019;25:1745–7. doi: 10.3201/eid2509.190023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhise V, Meyer AND, Singh H, Wei L, Russo E, Al-Mutairi A, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130:975–81. doi: 10.1016/j.amjmed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 13.PHOA LL, Teleman MD, WANG YT, Chee CB. Characteristics of patients with delayed diagnosis of infectious pulmonary tuberculosis. Respirology. 2005;10:196–200. doi: 10.1111/j.1440-1843.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RL, Bartholomew LA, Dovey SM, Fryer GE, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13:121–6. doi: 10.1136/qshc.2003.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh H. Helping organizations with defining diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40:102. doi: 10.1016/s1553-7250(14)40012-6. [DOI] [PubMed] [Google Scholar]
- 16.The emerging infections network: a new venture for the Infectious Diseases Society of America. Executive committee of the Infectious Diseases Society of America Emerging Infections Network. Clin Infect Dis. 1997;25:34–6. [PubMed] [Google Scholar]
- 17.Pillai SK, Beekmann SE, Santibanez S, Polgreen PM. The Infectious Diseases Society of America emerging infections network: bridging the gap between clinical infectious diseases and public health. Clin Infect Dis. 2014;58:991–6. doi: 10.1093/cid/cit932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145:488–96. doi: 10.7326/0003-4819-145-7-200610030-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kachalia A, Gandhi TK, Puopolo AL, Yoon C, Thomas EJ, Griffey R, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49:196–205. doi: 10.1016/j.annemergmed.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 20.Guderian LJ, Miller WC, Seña AC, Stout JE. Increased prevalence of advanced tuberculosis in rural low tuberculosis caseload counties in North Carolina. Int J Tuberc Lung Dis. 2011;15:1455–60. doi: 10.5588/ijtld.11.0103. i. [DOI] [PubMed] [Google Scholar]
- 21.Miller AC, Polgreen LA, Cavanaugh JE, Hornick DB, Polgreen PM. Missed opportunities to diagnose tuberculosis are common among hospitalized patients and patients seen in emergency departments. Open Forum Infect Dis. 2015;2:ofv171. doi: 10.1093/ofid/ofv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace RM, Kammerer JS, Iademarco MF, Althomsons SP, Winston CA, Navin TR. Increasing proportions of advanced pulmonary tuberculosis reported in the United States: are delays in diagnosis on the rise? Am J Respir Crit Care Med. 2009;180:1016–22. doi: 10.1164/rccm.200901-0059oc. [DOI] [PubMed] [Google Scholar]
- 23.Brickner JP, Muenster JE, Pancoast TH, Tosh AK. An adolescent with asthma presenting with worsening cough. Int J Adolesc Med Health. 2015;27:459–61. doi: 10.1515/ijamh-2014-0046. [DOI] [PubMed] [Google Scholar]
- 24.Ruegg G, Zimmerli S, Trachsel M, Berezowska S, Engelbrecht S, Martin Y, et al. Pulmonary histoplasmosis mimicking metastatic lung cancer: a case report. Diagnostics (Basel) 2021;11:328. doi: 10.3390/diagnostics11020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedict K, Beer KD, Jackson BR. Histoplasmosis-related healthcare use, diagnosis, and treatment in a commercially insured population, United States. Clin Infect Dis. 2020;70:1003–10. doi: 10.1093/cid/ciz324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep. 2016;18:29. doi: 10.1007/s11926-016-0572-1. [DOI] [PubMed] [Google Scholar]
- 27.Brode SK, Jamieson FB, Ng R, Campitelli MA, Kwong JC, Paterson JM, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax. 2015;70:677–82. doi: 10.1136/thoraxjnl-2014-206470. [DOI] [PubMed] [Google Scholar]
- 28.Pentella M, Weinstein MP, Beekmann SE, Polgreen PM, Ellison RT. Impact of changes in clinical microbiology laboratory location and ownership on the practice of infectious diseases. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01508-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015;60:1451–61. doi: 10.1093/cid/civ120. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt S, McQuillen DP, Nahass R, Martinelli L, Rubin M, Schwebke K, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis. 2014;58:22–8. doi: 10.1093/cid/cit610. [DOI] [PubMed] [Google Scholar]
- 31.Kassirer JP, Kopelman RI. Cognitive errors in diagnosis: instantiation, classification, and consequences. Am J Med. 1989;86:433–41. doi: 10.1016/0002-9343(89)90342-2. [DOI] [PubMed] [Google Scholar]
- 32.Liu YC, Huang WK, Huang TS, Kunin CM. Inappropriate use of antibiotics and the risk for delayed admission and masked diagnosis of infectious diseases: a lesson from Taiwan. Arch Intern Med. 2001;161:2366–70. doi: 10.1001/archinte.161.19.2366. [DOI] [PubMed] [Google Scholar]