Abstract
Metformin-associated lactic acidosis (MALA) is an extremely rare but life-threatening adverse effect of metformin treatment. The lifestyle changes associated with the coronavirus disease 2019 (COVID-19) pandemic may increase the potential risk of MALA development in patients with diabetes. We herein report a 64-year-old Japanese man taking a small dose of metformin who presented with MALA accompanied by hypoglycemia secondary to increased alcohol consumption triggered by lifestyle changes during the pandemic. Physicians should prescribe metformin judiciously to prevent MALA development and pay close attention to lifestyle changes in patients at risk for MALA during the COVID-19 pandemic.
Keywords: metformin, lactic acidosis, hypoglycemia, alcohol, COVID-19
Introduction
Owing to its cost-effectiveness and safety, metformin is the most frequently prescribed antihyperglycemic agent worldwide and is used as initial therapy for patients with type 2 diabetes mellitus (T2DM), unless there are contraindications (1). Metformin-associated lactic acidosis (MALA) is an extremely rare adverse effect of metformin use, with an estimated incidence of less than 5 cases per 100,000 patients yearly but a high mortality rate of 30-50% (2). MALA is generally precipitated by metformin accumulation due to renal insufficiency and dehydration complicated by comorbid conditions leading to the overproduction of lactate and/or reduction in lactate clearance.
The mechanism underlying lactic acidosis due to metformin accumulation is linked to impaired hepatic gluconeogenesis due to inhibition of the mitochondrial respiratory chain complex 1 and compromised lactate clearance due to nicotinamide adenine dinucleotide (NAD+) reduction through the inhibition of mitochondrial glycerol-3-phosphate dehydrogenase (3-6). Absorbed metformin is eliminated unchanged by the kidneys and thus accumulates in patients with acute and chronic kidney disease and dehydration (7). Overproduction of lactate and reduction in lactate clearance are mostly caused by impaired tissue oxygenation resulting from advanced heart failure, shock, or hypovolemia, as well as conditions of hepatic disease and alcoholism (8-11).
The ongoing coronavirus disease 2019 (COVID-19) pandemic has caused a global health emergency and influenced daily living activities, dietary habits, alcohol consumption, and the work style of patients with diabetes (12-14). Although these effects may increase the potential risk of MALA development, there have been no reports of MALA development triggered by lifestyle changes due to the COVID-19 pandemic.
We herein report the first case of a patient with T2DM taking a small dose of metformin who presented with MALA and hypoglycemia secondary to increased alcohol consumption triggered by lifestyle changes during the COVID-19 pandemic.
Case Report
A 64-year-old Japanese man with T2DM, diabetic kidney disease, neuropathy, hyperuricemia, insomnia, and alcoholic liver disease had been treated with vildagliptin (50 mg)/metformin hydrochloride (250 mg) tablet×2/day, pregabalin 75 mg×2/day, tramadol hydrochloride (37.5 mg) and acetaminophen (325 mg) tablet×2/day, febuxostat 20 mg×2/day, and brotizolam 0.25 mg/day by another hospital. His last visit to this hospital had been 3 months before presentation, and his HbA1c, blood urea nitrogen (BUN), creatinine (Cr) levels and estimated glomerular filtration ratio (eGFR) were determined as 6.1%, 21.2 mg/dL, 1.48 mg/dL, and 38.3 mL/min/1.73 m2, respectively.
He had lost his job due to the COVID-19 pandemic 3 weeks before presentation and spent most of his time at home, consuming alcohol during the day. Although the exact amount of alcohol he consumed is unknown, his original excessive alcohol intake (approximately 60 g of ethanol per day) consequently increased significantly. Three days before the presentation, he had an extremely low food intake but continued alcohol consumption while taking his medications. Subsequently, he was found to be in a confused state by his family and was transported to our emergency room (ER).
On arrival to the ER, his height, weight, and body mass index were 162 cm, 56.4 kg, and 21.5 kg/m2, respectively. His consciousness based on the Glasgow Coma Scale (GCS) was as follows: eye response [E], 1 point; verbal response [V], 1 point; and motor response [M], 1 point. Vital sign measurements were as follows: blood pressure, 128/77 mmHg; heart rate, 102 bpm; respiratory rate, 20 breaths/min; oxygen saturation, 98% under 5 L/min of oxygen administration; and body temperature, 34.5°C. His physical examination results were unremarkable except for the smell of alcohol, cold sweat, and dry oral mucosa. Based on a glucometer, he had hypoglycemia (<20 mg/dL).
As shown in Table, his laboratory findings after the administration of 40 mL of 50% dextrose indicated renal insufficiency (BUN: 23.1 mg/dL, Cr: 1.91 mg/dL, eGFR: 29.0 mL/min/1.73 m2, fractional excretion of sodium: 0.79%), electrolyte abnormalities, hepatic insufficiency, and high anion gap (AG) metabolic acidosis (pH: 6.923, bicarbonate: 5.8 mmol/L, corrected AG: 39.0 mEq/L) with hyperlactatemia (15.4 mmol/L) and β-hydroxybutyrate (βOHB) dominant hyperketonemia (acetoacetate: 155 μmol/L, βOHB: 1,549 μmol/L). Head computed tomography (CT) showed no intracranial hemorrhaging or occupational lesions, whereas chest and abdomen CT showed a fatty liver; no urinary tract obstruction, infection, or malignancy was noted. Ultrasound cardiography revealed collapse of the inferior vena cava (exhalation: 8 mm, inhalation: 0 mm), suggesting intravascular dehydration; however, a decreased cardiac function was not observed.
Table.
Laboratory Findings of the Patient.
| Hematology | ||||||||
| White blood cells | 11,600 | /μL | Glucosea | <20 | mg/dL | |||
| Red blood cells | 356×104 | /μL | Glycated hemoglobin A1c | 5.7 | % | |||
| Hemoglobin | 11.9 | g/dL | Lactate | 15.4 | mmol/L | |||
| Hematocrit | 35.6 | % | Acetoacetate | 155 | μmol/L | |||
| Platelet | 155×104 | /μL | 3β-hydroxybutyrate | 1,549 | μmol/L | |||
| Total ketone body | 1,704 | μmol/L | ||||||
| Coagulation | Vitamin B1 | 34 | ng/mL | |||||
| Prothrombin time | 73 | % | ||||||
| APTT | 34.5 | s | Arterial blood gas analysis, under O2 5L/min | |||||
| PH | 6.923 | |||||||
| Chemistry | PO2 | 210 | mmHg | |||||
| Total protein | 5.4 | g/dL | PCO2 | 28.5 | mmHg | |||
| Albumin | 2.6 | g/dL | HCO3- | 5.8 | mmol/L | |||
| Total bilirubin | 0.2 | mg/dL | B.E | -25.8 | mmol/L | |||
| AST | 95 | U/L | ||||||
| ALT | 53 | U/L | ||||||
| ALP | 308 | U/L | Urinalysis | |||||
| γ-GTP | 396 | U/L | SG | 1.015 | ||||
| LDH | 230 | U/L | PH | 6 | ||||
| Creatine kinase | 99 | U/L | Protein | 3+ | ||||
| Urea nitrogen | 23.1 | mg/dL | Glucose | - | ||||
| Creatinine | 1.91 | mg/dL | Blood | ± | ||||
| eGFR | 29 | mL/min/1.73m2 | Ketone | - | ||||
| Sodium | 123 | mEq/L | Na | 30 | mEq/L | |||
| Potassium | 3.3 | mEq/L | K | 17.2 | mEq/L | |||
| Chloride | 83 | mEq/L | Cl | 23 | mEq/L | |||
| Calcium | 7.9 | mg/dL | Cr | 59.1 | mg/dL | |||
| Phosphate | 7.3 | mg/dL | ||||||
| Magnesium | 1.9 | mg/dL | Blood drug concentration | |||||
| C-reactive protein | 0.05 | mg/dL | Metformin | 4.71 | mg/L | |||
aGlucose level was measured by a glucometer.
APTT: activated partial thromboplastin time, AST: aspartate aminotransaminase, ALT: alanine aminotransaminase, ALP: alkaline phosphatase, γ-GTP: gamma glutamyl transpeptidase, LDH: lactate dehydrogenase, eGFR: estimated glomerular filtration ratio, HbA1c: glycated hemoglobin A1c, B.E: base excess
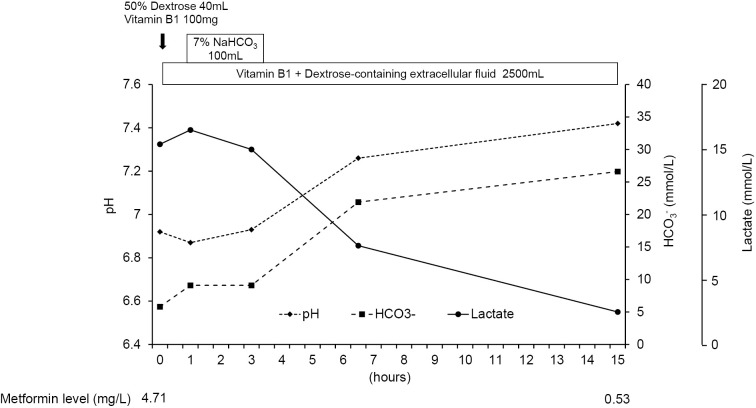
The patient's consciousness soon improved (GCS: E3V4M6) following the administration of 40 mL of 50% dextrose. However, his systolic blood pressure decreased to 70 mmHg, possibly due to dehydration and severe metabolic acidosis. Extracellular fluid resuscitation was initiated, and vitamin B1 and sodium bicarbonate were administered. As a result, his systolic blood pressure recovered to >100 mmHg within an hour without vasopressor administration. Fifteen hours after starting the above treatment, metabolic acidosis and hyperlactatemia improved to almost the standard values without initiating renal replacement therapy (RRT) (Figure).
Figure.
The patient’s clinical course. The patient’s confused state soon improved after the administration of 40 mL of 50% dextrose, and his lactic acidosis was also improved by the administration of sodium bicarbonate and extracellular fluid resuscitation. In addition, his significantly elevated plasma metformin level on arrival (4,71 mg/L) decreased to 0.53 mg/L by 15 hours after starting treatment.
The day after hospitalization, he was started on a low-calorie diabetic diet (960 kcal, 16.6 kcal/kg) with phosphorous and vitamin B1 supplementation to prevent refeeding syndrome. Vildagliptin (50 mg) and metformin hydrochloride (250 mg) tablets were discontinued. Thereafter, we gradually increased the caloric content of his meals. Consequently, the patient improved without developing any serious complications, and his renal function returned to the pre-hospital level (eGFR: 37.5 mL/min/1.73 m2). The patient's glycemic control was well maintained under the administration of linagliptin without restarting metformin, and he was discharged 13 days after hospitalization with strict instructions to stop alcohol consumption. Later, we found that his metformin plasma concentration upon ER arrival (approximately 21 hours after the last administration) had peaked at 4.71 mg/L (therapeutic concentration: 1-2 mg/L) and decreased to 0.53 mg/L within 15 hours of starting treatment (Figure).
Discussion
We encountered a patient with T2DM who had been prescribed a small dose of metformin despite having renal and hepatic insufficiency and excessive alcohol intake. The patient developed high AG metabolic acidosis accompanied by deterioration of the renal function, hypoglycemia, hyperlactatemia, and hyperketonemia secondary to an increase in alcohol consumption during the COVID-19 pandemic. Considering his significantly elevated lactate level (15.4 mmol/L) and mildly elevated total ketone body concentration (1,549 μmol/L), the patient's high AG metabolic acidosis was presumed to be mainly due to hyperlactatemia, although there was some contribution of hyperketonemia. Furthermore, given the plasma metformin level of the patient upon ER arrival (4.71 mg/L) and comorbid conditions leading to reduced lactate clearance, metformin accumulation, as well as hepatic insufficiency and excessive alcohol intake, are suggested to have contributed to the lactic acidosis development.
Notably, an increased alcohol intake is suggested to trigger the development of MALA and hypoglycemia. The diuretic effect of alcohol and extremely low food intake complicated by excessive alcohol intake may have caused dehydration in our patient, resulting in the deterioration of his chronic renal insufficiency, which subsequently accelerated the metformin accumulation. In addition, ethanol metabolism reduces the NAD+/NADH ratio. A decrease in the NAD+/NADH ratio impedes gluconeogenesis and pyruvate conversion to lactate, resulting in increased lactate levels and hypoglycemia, similar to metformin accumulation (9-11). Consequently, excessive alcohol intake is speculated to be involved in lactic acidosis development as well as hypoglycemia with metformin and vildagliptin. Increased alcohol consumption is generally caused by multi-layer factors, such as changes in the social environment and lifestyle and mental illnesses. According to our patient, the cause of his increased alcohol consumption was unemployment and the increased time spent at home during the COVID-19 pandemic.
To our knowledge, this is the first case report of a patient with MALA characterized by hypoglycemia secondary to increased alcohol consumption triggered by lifestyle changes during the pandemic. Recent studies have revealed that the COVID-19 pandemic has influenced the lifestyle of patients with diabetes, including their physical activity and dietary habits. In particular, 10.1-68.5% of patients with diabetes reported an increased alcohol consumption during the pandemic (12-14). This may be attributed to the extended at-home hours and employment issues, such as workplace and working conditions and unemployment (15), consistent with the patient in this report. According to our patient, he did not suffer from mental illness due to unemployment or the COVID-19 pandemic. However, since psychological distress during the COVID-19 pandemic has been associated with increased alcohol consumption (16), we suspect that this may have been a contributing factor. Physicians should therefore pay close attention to lifestyle changes and psychological distress leading to increased alcohol consumption during the ongoing COVID-19 pandemic in patients at risk for MALA.
Metformin is contraindicated in patients predisposed to lactic acidosis. These factors include severe renal insufficiency (eGFR <30 mL/min/1.73 m2), advanced heart failure and pulmonary disease at risk of inducing hypoperfusion/hypoxia, active or progressive liver disease, dehydration, and active alcohol abuse (17). Considering the original renal function of this patient (eGFR, 38.3 mL/min/1.73 m2), the administration of a small dose of metformin (500 mg/day), which had not been administered the first time, may have been acceptable under frequent renal function monitoring. However, given the coexistence of hepatic insufficiency and excessive alcohol intake, the patient was at a high risk for MALA development and may have had contraindications for metformin. Similarly, a significant proportion of patients treated with metformin have been reported to have one or more contraindications (18). Therefore, physicians should prescribe metformin judiciously to prevent MALA development. Furthermore, although details on patient education from the original attending physician were unclear, instructing our patient to stop taking metformin on sick days or when at risk of dehydration was considered quite important.
Supportive care is considered the mainstay treatment for MALA. The use of RRT, which can eliminate accumulated metformin, provide renal support, and correct severe acidemia, was previously successful in treating severe forms of MALA (19). The indications for RRT are severe acidosis with pH <7.0, highly elevated lactate levels (>20 mmol/L), shock, decreased level of consciousness, and failure to improve despite standard supportive treatment (19). In the present case, intravenous hydration and sodium bicarbonate administration without RRT were successful in treating MALA. We attribute this to the patient's mild metformin accumulation and general condition, including his renal function and hemodynamics.
We reported a patient with T2DM who was treated with a small dose of metformin despite having renal and hepatic insufficiency and excessive alcohol intake. The patient developed MALA with hypoglycemia secondary to increased alcohol intake triggered by lifestyle changes during the COVID-19 pandemic. Physicians should prescribe metformin judiciously to prevent the development of MALA and pay close attention to lifestyle changes, including increased alcohol consumption, in patients at risk for developing MALA during the pandemic. Patients should also be educated on MALA and instructed to stop taking metformin when sick or at risk of dehydration.
All procedures were approved by the appropriate institutional review board and complied with the Declaration of Helsinki and its amendments. Written informed consent was obtained from the patient for the publication of this case report.
Author's disclosure of potential Conflicts of Interest (COI).
Tetsuyuki Yasuda: Honoraria, Takeda Pharmaceutical, Novartis Pharmaceuticals, Eli Lilly Japan, Sumitomo Dainippon Pharma and Nippon Boehringer Ingelheim.
References
- 1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes - 2021. Diabetes Care 44: S111-S124, 2021. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 65: 20-29, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607-614, 2000. [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter RW, Hughey CC, Lantier L, et al. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat Med 24: 1395-1406, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494: 256-260, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542-546, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50: 81-98, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Madias NE. Lactic acidosis. Kidney Int 29: 752-774, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Halperin ML, Hammeke M, Josse RG, Jungas RL. Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metabolism 32: 308-315, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Wrenn KD, Slovis CM, Minion GE, Rutkowski R. The syndrome of alcoholic ketoacidosis. Am J Med 91: 119-128, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med 377: 1368-1377, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Yan AF, Sun X, Zheng J, et al. Corrigendum to ‘perceived risk, behavior changes and health-related outcomes during COVID-19 pandemic: findings among adults with and without diabetes in China’. Diabetes Res Clin Pract 177: 108881, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potier L, Hansel B, Larger E, et al. Stay-at-home orders during the COVID-19 pandemic, an opportunity to improve glucose control through behavioral changes in type 1 diabetes. Diabetes Care 44: 839-843, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Maruo Y, Irie Y, Obata Y, et al. Medium-term influence of the coronavirus disease 2019 pandemic on patients with diabetes: a single-center cross-sectional study. Intern Med 61: 303-311, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skotnicka M, Karwowska K, Kłobukowski F, Wasilewska E, Małgorzewicz S. Dietary habits before and during the COVID-19 epidemic in selected European countries. Nutrients 13: 1690, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez LM, Litt DM, Stewart SH. Drinking to cope with the pandemic: the unique associations of COVID-19-related perceived threat and psychological distress to drinking behaviors in American men and women. Addict Behav 110: 106532, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bristol-Myers Squibb Company. Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) prescribing information [Internet]. 2017 Apr [cited 2021 Sep 23]. [35 p]. Available from: http://packagein-serts.bms.com/pipi_glucophage_xr.pdf
- 18. Emslie-Smith AM, Boyle DI, Evans JM, Sullivan F, Morris AD; DARTS/MEMO Collaboration. Contraindications to metformin therapy in patients with type 2 diabetes--a population-based study of adherence to prescribing guidelines. Diabet Med 18: 483-488, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Calello DP, Liu KD, Wiegand TJ, et al. Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med 43: 1716-1730, 2015. [DOI] [PubMed] [Google Scholar]