Abstract
We herein report two patients with hepatocellular carcinoma (HCC) who exhibited intraabdominal bleeding caused by tumor rupture soon after lenvatinib initiation. A hypervascular nodule was present in the lateral segment manifesting extrahepatic protrusion in an 81-year-old-man and in the caudate lobe, which was completely occupied by the tumor, in an 83-year-old-man. Both patients were given lenvatinib, and epigastralgia occurred suddenly three and five days later. Computed tomography revealed high-attenuation areas suggesting bleeding around the left and caudate lobes. Considering the strong antiangiogenic effects by lenvatinib, transcatheter arterial embolization should be performed before lenvatinib initiation in patients with subcapsular HCC.
Keywords: hepatocellular carcinoma, lenvatinib, rupture
Introduction
Lenvatinib is a multikinase inhibitor that acts on intracellular signals derived from vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor (PDGF) receptors, receptor for stem cell factor (c-KIT), and the rearranged during transfection (RET) proto-oncogene (1). The REFLECT trial, a global randomized phase 3 trial, revealed the non-inferiority of lenvatinib relative to sorafenib in terms of the overall survival rate (OSR) in patients with unresectable hepatocellular carcinoma (HCC) who had received either of these molecular-targeted agents as a first-line treatment (2). The trial also showed that the objective response rate (ORR) was higher among patients receiving lenvatinib than among those receiving sorafenib when the therapeutic efficacies were evaluated based on a masked independent contrast-enhanced computed tomography (CT) image review performed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (40.6% vs. 12.4%) (3). Excellent early therapeutic efficacies were also obtained in patients receiving lenvatinib in clinical practice (4,5); the ORR based on contrast-enhanced CT images obtained between 4 and 8 weeks after the initiation of lenvatinib was 48.1% according to mRECIST in patients with unresectable HCC at our institute (5). In addition, early tumor shrinkage and/or necrotic lesions in tumors were reportedly seen frequently after lenvatinib initiation (5-8). These observations suggest that HCC rupture may occur in patients with hypervascular HCC if the HCC nodules are located adjacent to the liver surface.
We herein report two patients with unresectable HCC who exhibited intraabdominal bleeding as a result of HCC rupture soon after the initiation of lenvatinib chemotherapy.
Case Reports
Case 1
An 81-year-old man with cirrhosis caused by alcohol intake was diagnosed with Barcelona Clinic Liver Cancer (BCLC) stage C HCC. CT revealed a large hypervascular tumor with a maximal diameter of 86 mm in the lateral segments of the liver accompanied by metastasis to the intraabdominal lymph nodes. As shown in Fig. 1, the tumor protruded beyond the surface of the liver parenchyma.
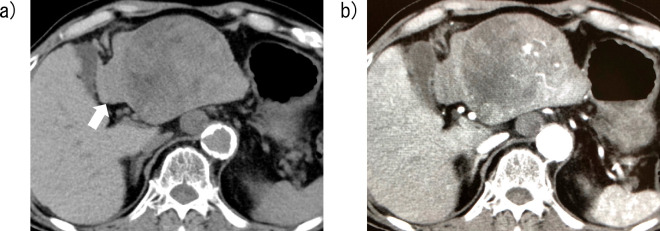
Figure 1.
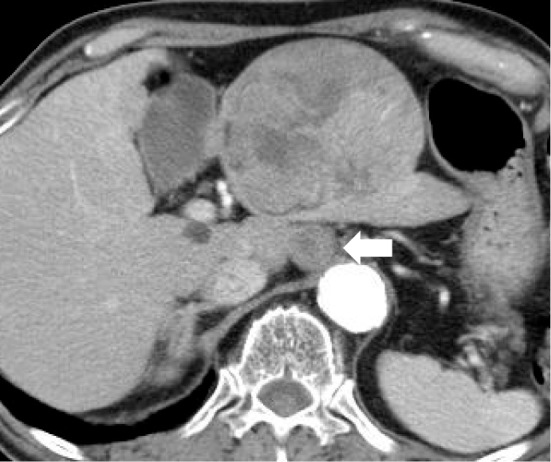
Contrast-enhanced computed tomography image of Case 1 obtained four weeks before chemotherapy with lenvatinib. A large hypervascular tumor with a maximal diameter of 86 mm and extrahepatic protrusion is visible in the lateral segments of the liver. Intraabdominal lymph node metastases (arrow) are also visible.
Blood examinations showed a hemoglobin concentration of 12.5 g/dL, serum levels of albumin and total bilirubin of 4.3 g/dL and 0.6 mg/dL, respectively, and a prothrombin time of 59% relative to the control value, which corresponded to an international normalization ratio (INR) of 1.36. The serum α-fetoprotein (AFP) and des-γ-carboxyprothrombin levels were 66.5 ng/mL and 247,445 mAU/mL, respectively. The extent of liver damage according to the Child-Pugh score was class A (5), and the modified albumin-bilirubin (mALBI) was classified as grade 1 (Table).
Table.
Blood Examination Results of Cirrhotic Patients with Hepatocellular Carcinoma at Baseline of Lenvatinib Administration.
| Case 1 | Case 2 | ||||
|---|---|---|---|---|---|
| WBC | 5,280 | 4,100 | /mm3 | ||
| RBC | 3.78×104 | 4.13×104 | /mm3 | ||
| Hemoglobin | 12.5 | 13.4 | g/dL | ||
| Hematocrit | 37.0 | 39.5 | % | ||
| Platelets | 16.9×104 | 14.8×104 | /mm3 | ||
| AST | 68 | 92 | U/L | ||
| ALT | 20 | 53 | U/L | ||
| LDH | 260 | 191 | U/L | ||
| GGT | 37 | 34 | U/L | ||
| ALP | 247 | 432 | U/L | ||
| Total bilirubin | 0.6 | 0.7 | mg/dL | ||
| BUN | 19.1 | 17.0 | mg/dL | ||
| Creatinine | 0.86 | 0.82 | mg/dL | ||
| Na | 143 | 143 | mEq/L | ||
| K | 3.7 | 4.2 | mEq/L | ||
| Cl | 105 | 109 | mEq/L | ||
| CRP | 0.28 | 0.14 | mg/dL | ||
| Ammonia | 38 | 56 | μg/dL | ||
| TP | 7.2 | 7.5 | g/dL | ||
| Alb | 4.3 | 3.5 | g/dL | ||
| PT (%) | 59 | 87 | % | ||
| INR | 1.36 | 1.08 | |||
| AFP | 66.5 | 19.1 | ng/mL | ||
| DCP | 247,445 | 103 | mAU/mL | ||
| HBs-antigen | <0.04 | <0.04 | IU/mL | ||
| Anti-HCV | 0.04 | 13.60 | S/CO |
RBC: red blood cells, AST: aspartate transaminase, ALT: alanine transaminase, LDH: lactate dehydrogenase, GGT: γ-glutamyl transpeptidase ALP: alkaline phosphatase, BUN: blood urea nitrogen, CRP: C-reactive protein, TP: total protein, PT: prothrombin time, INR: international normalized ratio, AFP: α-fetoprotein, DCP: des-γ-carboxyprothrombin, HB: hepatitis B, HCV: hepatitis C virus
He had been given medication for diabetes mellitus, hypertension and hyperlipidemia and administered apixaban to prevent blood clot formation because of atrial fibrillation. He was also being treated with lenvatinib at a daily dose of 8 mg as chemotherapy for HCC based on his body weight of 56.6 kg.
Three days later, he experienced sudden-onset epigastralgia. Blood examinations revealed decreased hemoglobin levels of 11.9 g/dL. CT demonstrated high-attenuation areas around the left lobe of the liver, indicating intraabdominal bleeding as a result of HCC rupture (Fig. 2). Lenvatinib administration was discontinued, and transcatheter arterial embolization (TAE) for ruptured HCC was performed seven days after the discontinuation. Although hepatic arteriography revealed no extravasation of the contrast medium from ruptured HCC, lateral subsegmental artery embolization was done with porous gelatin particles. Chemotherapy with lenvatinib was initiated again six weeks after the TAE procedure following the confirmation of increased hemoglobin levels. CT images obtained 7 weeks later revealed decreased diameters of the lesions that were enhanced in the arterial phase, so the therapeutic efficacy of lenvatinib was diagnosed as partial response (PR) according to the mRECIST.
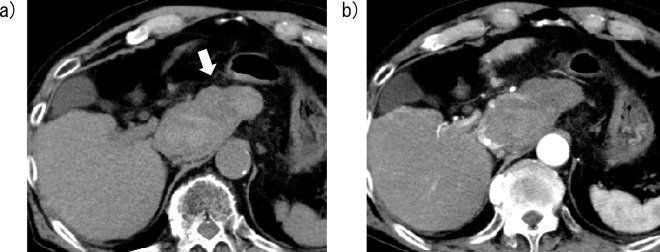
Figure 2.
Computed tomography (CT) images of Case 1 obtained three days after lenvatinib initiation. a) A nonenhanced CT image shows a high-attenuation area around the tumor, indicating a hematoma caused by hepatocellular carcinoma rupture (arrow). b) A contrast-enhanced CT image shows the pooling of contrast medium in the tumor.
Case 2
An 83-year-old man with cirrhosis caused by hepatitis C virus infection was diagnosed as having HCC 6 years previously. He received radiofrequency ablation therapy twice followed by transcatheter arterial chemoembolization (TACE) for a total of 11 times, but the therapeutic efficacies were insufficient following the 10th and 11th TACE procedures. Consequently, he was diagnosed with HCC that was refractory to TACE. CT demonstrated a hypervascular tumor with a maximal diameter of 31 mm in the caudate lobe, which was completely occupied by the tumor (Fig. 3a). Small contrast-enhanced lesions, indicating the intrahepatic metastasis of HCC from the HCC in the caudate lobe, were also seen in the left and right lobes of the liver (Fig. 3b, c). The serum levels of albumin and total bilirubin were 3.5 g/dL and 0.7 mg/dL, respectively, and the prothrombin time was 87% relative to the control values, corresponding to an INR of 1.09. The extent of liver damage according to the Child-Pugh score was class A (6), and mALBI was classified as grade 2a. The serum AFP and des-γ-carboxyprothrombin levels were 19.1 ng/mL and 103 mAU/mL, respectively (Table).
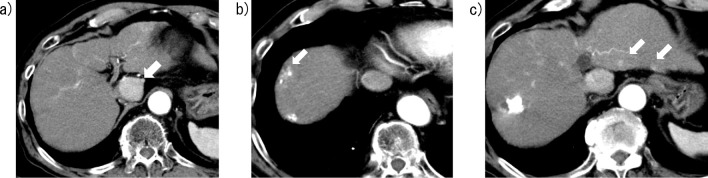
Figure 3.
Contrast-enhanced computed tomography images of Case 2 obtained seven weeks before chemotherapy with lenvatinib. a) A hypervascular tumor with a maximal diameter of 31 mm is visible in the caudate lobe, which is almost completely occupied by the tumor with almost no normal liver parenchyma present around the tumor. b, c) Small, contrast-enhanced lesions indicating intrahepatic metastases are visible in both the left and right lobes (arrows).
He had been given medication for hypertension. He received lenvatinib at a daily dose of 12 mg based on his body weight of 64.5 kg, and epigastralgia occurred suddenly 5 days after the initiation of treatment. CT revealed high-attenuation areas around the caudate lobe, and the attenuation values in the HCC nodule were lower than those observed at baseline, indicating intraabdominal bleeding as a result of the rupture of the necrotic HCC (Fig. 4). Chemotherapy with lenvatinib, however, was continued, since the extent of the reduction in hemoglobin levels was minimal. CT performed four weeks after lenvatinib initiation revealed that the contrast-enhanced areas were absent in the caudate lobe, with no notable increase in hematoma volume, so the therapeutic efficacy of lenvatinib for the ruptured tumor was deemed a complete response (CR) according to the mRECIST. However, contrast-enhanced lesions developed around a high-attenuation area, indicating lipiodol deposition after the prior TACE procedures, in the anterior-superior segment of the liver (Fig. 5).
Figure 4.
Computed tomography (CT) images of Case 2 obtained five days after lenvatinib initiation. a) A nonenhanced CT image shows a high-attenuation area around the caudate lobe, indicating a hematoma caused by hepatocellular carcinoma rupture (arrow). b) A contrast-enhanced CT image shows a necrotic area in the HCC nodule in the caudate lobe.
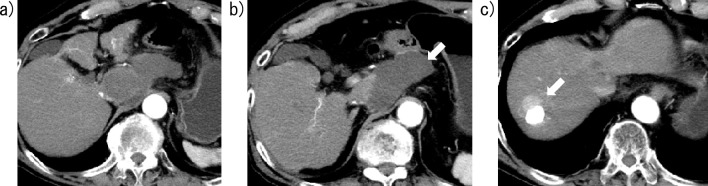
Figure 5.
Contrast-enhanced computed tomography images of Case 2 obtained four weeks after lenvatinib initiation. a) No enhanced areas are visible in the caudate lobe. b) The hematoma has not increased in volume compared with the results obtained five days after lenvatinib initiation (arrow). c) Hypervascular lesions (arrow) are visible around a high attenuation area, indicating lipiodol deposition after the prior TACE procedures, in the anterior-superior segment.
Discussion
Intraabdominal bleeding following spontaneous tumor rupture has been shown to occur in 2.3% to 26% of patients with HCC (9). HCC rupture is a potentially life-threatening event and responsible for the death of 2.3% of HCC patients in Japan (10). The mechanisms involved in spontaneous HCC rupture have not been elucidated, although several hypotheses have been proposed, such as the vascular injury hypothesis, the venous congestion hypothesis, and the small room hypothesis (9,11). HCC rupture has been observed relatively frequently in patients with large HCC nodules and/or HCC nodules with extrahepatic protrusion (9,12). The locations of tumors have also been associated with HCC rupture; in a case control study, Li et al. reported that HCC rupture occurred more frequently for HCC nodules located in segments II, III, and VI than for those located in other segments (13). Hypervascular HCC located in the caudate lobe as well as in segments II, III, and VI may be at risk for rupture during tumor growth, since the normal liver parenchyma surrounding HCC in the caudate lobe is thinner than that around HCC in other segments. Furthermore, tumor necrosis and/or vascular injuries following TACE procedures have been shown to act as triggers for HCC rupture (14,15).
Rombolà et al. reported a patient who experienced HCC rupture during chemotherapy with sorafenib (16). Sorafenib is an inhibitor of intracellular signals derived from VEGF receptors that inhibits the regeneration of damaged vascular endothelial cells, potentially leading to hemorrhaging. Similar to sorafenib, lenvatinib is also a multikinase inhibitor, but the antiangiogenic effects of lenvatinib have been shown to be more powerful than those produced by sorafenib according to the REFLECT trial (2), and the ORRs as evaluated according to mRECIST at between 4 and 8 weeks after lenvatinib initiation were 75.0%, 63.2%, and 21.7% in patients with BCLC stage A, stage B, and stage C HCC, respectively, in our previous study (5). In addition, Kuzuya et al. reported that the ORR at 2 weeks after lenvatinib initiation was 57.5% according to mRECIST, and early tumor shrinkage and/or tumor necrosis were frequently observed in these patients (8). These observations prompted us to be alert for the possibility of HCC rupture in patients receiving lenvatinib.
According to a phase 2 trial for lenvatinib, HCC rupture occurred in 1 patient within 30 days after the discontinuation of chemotherapy with lenvatinib (17). In contrast, in the REFLECT trial, cerebral hemorrhaging occurred in 3 of 462 patients (0.6%) during lenvatinib administration, despite the absence of HCC rupture in these patients (2). In clinical practice, following the approval of lenvatinib in Japan, Ishihara et al. reported a patient with HCC who developed intratumoral hemorrhaging at 12 days after the initiation of lenvatinib (18). In the presently reported study, however, intraabdominal hemorrhaging caused by HCC rupture occurred earlier after the initiation of lenvatinib: Case 1 developed hemorrhaging at 3 days after lenvatinib initiation, and Case 2 developed hemorrhaging at 5 days after lenvatinib initiation. In both patients, the hypervascular HCC nodules were located near the liver surface; indeed, the HCC nodule exhibited extrahepatic protrusion in Case 1, and the nodule was located in the caudate lobe in Case 2. These shapes and locations of hypervascular HCC might have been responsible for the development of intraabdominal bleeding as a result of HCC rupture within seven days after lenvatinib initiation.
In conclusion, careful attention should be paid to HCC nodules located near the liver surface, such as those with extrahepatic protrusion and/or those in the caudate lobe, when chemotherapy using lenvatinib is performed. TAE prior to lenvatinib initiation merits consideration in these patients.
Author's disclosure of potential Conflicts of Interest (COI).
Satoshi Mochida: Honoraria, Abbvie, Gilead Sciences, MSD, Asuka Pharmaceutical, Eisai, Ohtsuka Parmaceutical, Dainihon-Sumitomo Pharma and Torey Medical.
References
- 1.Tohyama O, Matsui J, Kodama KS, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014: 638747, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomized phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30: 52-60, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Hiraoka A, Kumada T, Kariyama K, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res 49: 111-117, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Fuchigami A, Imai Y, Uchida Y, et al. Therapeutic efficacy of lenvatinib for patients with unresectable hepatocellular carcinoma based on the middle-term outcome. PLOS ONE 15: e0231427, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sho T, Suda G, Ogawa K, et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open 4: 54-60, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi A, Moriguchi M, Seko Y, et al. Early tumor shrinkage as a predictive factor for outcomes in hepatocellular carcinoma patients treated with lenvatinib: a multicenter analysis. Cancers 12: 754, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzuya T, Ishigami M, Ito T, et al. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res 50: 374-381, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Mamada Y, Taniai N, et al. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res 46: 13-21, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Izumi N, Kokudo M, et al. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012-2013). Hepatol Res. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 11.Sahu SK, Chawla YK, Dhiman RK, et al. Rupture of hepatocellular carcinoma: a review of literature. J Clin Exp Hepatol 9: 245-256, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki T, Kokudo N, Matsuyama Y, et al. ; Liver Cancer Study Group of Japan. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg 259: 532-542, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Huang L, Liu CF, et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol 27: 9121-9127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JH, Wang LG, Bao HW, et al. Emergency embolization in the treatment of ruptured hepatocellular carcinoma following transcatheter arterial chemoembolization. Hepatogastroenterology 57: 616-619, 2010. [PubMed] [Google Scholar]
- 15.Battula N, Srinivasan P, Madanur M, et al. Ruptured hepatocellular carcinoma following chemoembolization: a western experience. Hepatobiliary Pancreat Dis Int 6: 49-51, 2007. [PubMed] [Google Scholar]
- 16.Rombolà F, Caravetta A, Mollo F, Spinoso A, Peluso L, Guarino R. Sorafenib, risk of bleeding and spontaneous rupture of hepatocellular carcinoma. A clinical case. Acta Medica (Hradec Kralove) 54: 177-179, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol 52: 512-519, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara K. A case of multiple intratumoral hemorrhage and rupture of hepatocellular carcinoma after starting lenvatinib. Case Rep Hepatol 2020: 6659388, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]