Dear Editor,
After the COVID-19 pandemic, the WHO declared the monkeypox outbreak a public health emergency of international concern. Monkeypox is a rare infectious disease caused by the monkeypox virus (MPXV), and can occur in both humans and non-human primates [1]. The disease typically presents with flu-like symptoms such as fever, swollen lymph nodes, and rashes. Within several days, clusters of fluid-filled blisters typically appear all over the body, including the face, and then these blisters break open and crust over [2]. MPXV has recently re-emerged and is spreading at an alarming rate globally. As of August 11, 2022, approximately 80 countries reported 34,057 confirmed cases, with 12 mortalities (Monkeypox; Our World in Data, https://ourworldindata.org/monkeypox).
In the 1980s, contact tracing of monkeypox cases revealed an overall attack rate of 7.2% and 0.9% for contacts unvaccinated versus those vaccinated, respectively [3]. As the infectivity rate of MPXV has the potential to induce a pandemic, efforts should be directed to its containment. Early intervention measures can minimize the branching process for spread of the infection. Primary prevention with vaccination can be critical for control of MPX [4]. The benefits of ring vaccination are double-fold: it can break the chain of virus transmission and, bring to a halt the course of a severe disease [5].
Two vaccines licensed for smallpox by the Food and Drug Administration (FDA) authority demonstrated efficacy for cross-protection against the MPXV: the ACAM2000 vaccine manufactured by Sanofi Pasteur Biologics Co., and the JYNNEOS vaccine manufactured by Bavarian Nordic A/S and marketed as Jynneos in the United States and as Imvanex in Europe. They are given as two subcutaneous injections (four weeks apart) and the vaccination offers complete protection two weeks after inoculation of the second dose (CDC, https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html).
Ring vaccination i.e., vaccinating close contacts of confirmed cases, can be successful if infectious cases are promptly diagnosed. Because of the inherently stochastic nature of epidemic outbreaks, both the size and duration of contained outbreaks are highly variable. Earlier ring vaccination strategy had been utilized for eradication of the Ebola virus. Vaccinating immediate contacts of an infected individual can contain the MPX infection spread. The strategy has been implemented by the United States of America (USA) and several European countries. Recently, India's Union Ministry of Health and Family Welfare agreed to adopt the ring vaccination strategy to contain the infectious disease. In light of current outbreaks, ring vaccination has been attempted in several countries [[6], [7], [8]]. For this method to be effective, individuals should be vaccinated within 1–4 days of exposure (UK Health Security Agency. https://www.gov.uk/guidance/monkeypox-outbreak-vaccination-strategy).
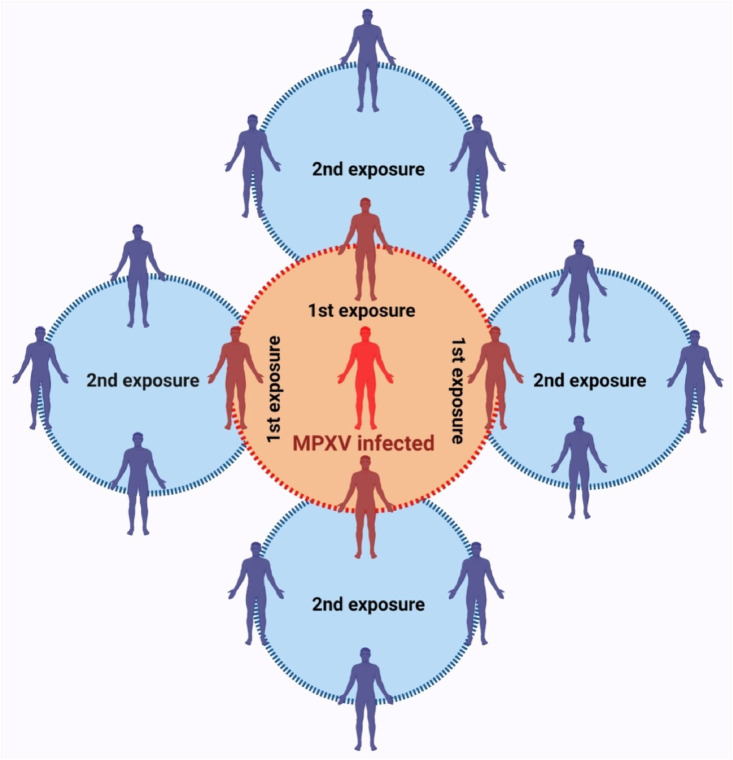
Specific to the MPX outbreak and due to the nature of the disease where there is a slow transmission and a long incubation period, ring vaccination offers a promising solution [5]. Furthermore, ease of implementation, efficient use of available resources (limited vaccines and staff), lower odds of spreading, limited number of vaccines required to complete the ring, and greater efficacy for isolated communities with smaller population sizes are all advantageous to health authorities (Fig. 1 ). In an observational analysis of consecutive high-risk close contacts to confirmed cases and those who were vaccinated using the IMVANEX vaccine, a third-generation smallpox vaccine, researchers at Université Paris Cité found the vaccine to be well tolerated and it did not cause any adverse effects. Of the 276 vaccinated individuals, 12 subjects had a confirmed breakthrough infection, while another 12 close contacts developed symptoms 5 days post-vaccination. Another two had infections, 22 and 25 days later, respectively. The authors concluded that although the vaccine was able to lower the risk of infection, it was not able to completely eradicate the disease [8].
Fig. 1.
Schematic diagram of a ring vaccination strategy. The first ring (red) consists of close contacts of confirmed monkeypox virus (MPXV)‐infected individuals, or those exposed to an MPXV‐infected person in the proximate environment, such as the passengers in the same compartment. The second ring (blue) consists of close contacts of, or those exposed to, people from the first ring. People in these two rings should be vaccinated with priority. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Precautions ought to be taken with the ring vaccination approach. Firstly, this approach relies on rigorous testing and contact tracing, which is challenging since MPX can potentially spread through sexual contact among men who have sex with other men (MSM), and therefore in societies where stigma exists, and may prove difficult to infiltrate. MSM usually feel embarrassed and discriminated against due to religious, societal, and legal reasons, especially in countries where such behavior is prohibited. Thus, tracing these cases and their sexual contacts can potentially be difficult and might jeopardize the ring vaccination strategy [7]. Concurrently, public awareness campaigns, specialized care centers for sexually transmitted cases, and social support services are needed for these societies. Secondly, the immediate implementation of ring vaccination could be hindered for logistic, manufacturing, and financial reasons in affected countries [9]. Thirdly, since available vaccines can sometimes result in rare but serious adverse events, convincing the public to be vaccinated can be difficult. Therefore, country-wide awareness campaigns will be required to educate individuals to facilitate and quicken the process of implementing ring vaccination. Finally, besides case isolation, contact tracing, and ring vaccination, there is an urgent need to develop new vaccines that specifically target MPXV to effectively contain this disease, and genomic surveillance for MPXV in human and animals. International initiatives to structure development and equitable distribution of vaccines are of paramount importance [9].
Ethical approval
This article does not require any human/animal subject to acquire such approval.
Sources of funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Om Prakash Choudhary: Conceptualization, Data Curation, Supervision, Writing-Original Draft, Writing-review & editing. Priyanka: Conceptualization, Writing-Original Draft, Writing-review & editing. Mathumalar Loganathan Fahrni: Writing-Original Draft, Writing-review & editing. AbdulRahman A. Saied: Writing-Original Draft, Writing-review & editing. Hitesh Chopra: Writing-Original Draft, Writing-review & editing. All authors critically reviewed and approved the final version of the manuscript.
Research registration Unique Identifying Number (UIN)
-
1.
Name of the registry: Not applicable.
-
2.
Unique Identifying number or registration ID: Not applicable.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Om Prakash Choudhary, Assistant Professor (Senior Scale), Department of Veterinary Anatomy and Histology, College of Veterinary Sciences and Animal Husbandry, Central Agricultural University (I), Selesih, Aizawl-796015, Mizoram, India. Tel: +91–9928099090; Email:dr.om.choudhary@gmail.com.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Data statement
The data in this correspondence article is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Declaration of competing interest
All authors report no conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijsu.2022.106873.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dhawan M., Priyanka, Choudhary O.P. Emergence of monkeypox: risk assessment and containment measures. Trav. Med. Infect. Dis. 2022;49:102392. doi: 10.1016/j.tmaid.2022.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A., Priyanka Fahrni ML., Choudhary O.P. Monkeypox outbreak: new zoonotic alert after the COVID-19 pandemic. Int. J. Surg. 2022;104 doi: 10.1016/j.ijsu.2022.106812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jezek Z., Marennikova S.S., Mutumbo M., Nakano J.H., Paluku K.M., Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J. Infect. Dis. 1986;154(4):551–555. doi: 10.1016/j.ijsu.2022.106774. [DOI] [PubMed] [Google Scholar]
- 4.Fahrni M.L., Priyanka, Sharma A., Choudhary O.P. Monkeypox: prioritizing public health through early intervention and treatment. Int. J. Surg. 2022;104 doi: 10.1016/j.ijsu.2022.106774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sah R., Abdelaal A., Asija A., Basnyat S., Sedhai Y.R., Ghimire S., Sah S., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Monkeypox virus containment: the application of ring vaccination and possible challenges. J. Trav. Med. 2022 doi: 10.1093/jtm/taac085. taac085. [DOI] [PubMed] [Google Scholar]
- 6.Kozlov M. Monkeypox vaccination begins- can the global outbreaks be contained? Nature. 2022;606:444–445. doi: 10.1038/d41586-022-01587-1. [DOI] [PubMed] [Google Scholar]
- 7.Kretzschmar M., den Hof S.V., Wallinga J., Wijngaarden J.V. Ring vaccination and smallpox control. Emerg. Infect. Dis. 2004;10(5):832. doi: 10.3201/eid1005.030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thy M., Peiffer-Smadja N., Mailhe M., Kramer L., Ferré V.M., Houhou-Fidouh N., Tarhini H., Bertin C., Beaumont A.L., Garé M., Pluart D.L., Perrineau S., Rahi M., Laurène Deconinck L., Phung B., Mollo B., Cortier M., Cresta M., Vaux V., Joly V., Lariven S., Somarriba C., Lescure F., Charpentier C., Yazdanpanah Y., Ghosn J. Breakthrough infections after post-exposure vaccination against monkeypox. medRxiv. 2022 doi: 10.1101/2022.08.03.22278233. 08.03.22278233. [DOI] [PubMed] [Google Scholar]
- 9.Khairi L.N.H.M., Fahrni M.L., Lazzarino A.I. The race for global equitable access to COVID‐19 vaccines. Vaccines. 2022;10:1306. doi: 10.3390/vaccines10081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.