Abstract
Patients with certain inherited metabolic disorders (IMD) are at high risk for metabolic decompensation with exposure to infections. The COVID-19 pandemic has been particularly challenging for health care providers dealing with IMD patients, in view of its unpredictable consequences in these patients. There is limited data in literature on evaluating the impact and the outcome of COVID-19 infection in these patients. This cross-sectional retrospective study on a large cohort of unvaccinated IMD patients, reviewed the incidence of COVID-19 infection, disease manifestation and outcome during the pandemic between November 2019 and July 2021. In this cohort of 1058 patients, 11.7% (n = 124) were infected with COVID-19. Their median age was 16 years (age range 2–42); 57% (n = 71) were males. Post-exposure positive test was noted in 78% (n = 97) patients, while 19% (n = 24) had symptomatic diagnosis and three patients tested positive during pre-hospital visits screening. Most patients, 68.5% (n = 85) had mild COVID-19 related symptoms such as fever, cough, headache and diarrhea while 13.7% (n = 17) patients had no symptoms. Of twenty-two patients (17.7%) who required hospitalization, 16 were adults with various intoxication and energy metabolism disorders, who developed IMD related complications such as metabolic acidosis, hyperammonemia, acute pancreatitis, hypoglycemia, rhabdomyolysis and thrombosis. Ten patients needed intensive care management. The cohort death rate was 2.4% (3 patients).
Overall, the clinical course of COVID-19 infection in these IMD patients was relatively mild except for patients with intoxication and energy metabolism disorders who had high risk of developing acute metabolic decompensation with severe complications.
Keywords: COVID-19 infection, Inherited metabolic disorders, SARS-CoV-2
Abbreviations
- ASA
Argininosuccinic aciduria
- BKT
Beta-Ketothiolase deficiency
- CIT-I:
Citrullinemia type I
- COVID-19
Coronavirus disease of 2019
- FBP1
Fructose-1,6-bisphosphatase deficiency
- GA-I:
Glutaric aciduria type I
- GALT
Galactosemia
- GSD-I:
Glycogen storage disease type I
- GSD-III
Glycogen storage disease type III
- HCU
Homocytinurea
- HMG
HMG CoA lyase deficiency
- IMD
Inherited metabolic disorders
- IV
intravenous
- IVA
Isovaloric acidemia
- MMA
Methylmalonic acidemia
- MPS
Mucopolysaccharidosis
- MSUD
Maple syrup urine disease
- PA
Propionic acidemia
- PKU
Phenylketonuria
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- TYR-I:
Tyrosinemia type I
- TYR-II
Tyrosinemia type II
- VLCAD
Very long-chain Acyl-CoA dehydrogenase deficiency
1. Introduction
A block in a metabolic pathway is the biochemical hallmark of inherited metabolic disorders (IMD), and it is generally caused by a defect in either an enzyme or transport protein. The pathophysiology of metabolic diseases is related to excessive accumulation of toxic metabolites and/or deficiency of essential product. Infection is considered a major triggering factor for metabolic decompensation in IMD, especially in patients with intoxication disorders and energy metabolism disorders. This is attributed to the increased metabolic demand during infection leading to catabolic state in these patients overwhelming the block in the metabolic pathway (Saudubray and Cazorla, 2016). SARS-CoV-2 virus and the clinical syndrome of COVID-19 has resulted in a worldwide pandemic since its discovery in late 2019 (Centers for Disease Control and Prevention, 2019; World Health Organization, 2019). Elderly and individuals with comorbidities such as hypertension, heart disease, diabetes, active cancer, and chronic kidney disease have a significant higher risk of severe infection and death due to COVID-19 (World Health Organization, 2019). The risk of serious complications due to COVID-19 infection in IMD patients is still unknown. However, the metabolic community considers this group of patients especially susceptible to COVID-19 disease for two reasons. Firstly, patients with certain types of IMDs are well-known to have a significant risk of developing acute metabolic decompensation during infections, which may lead to life threatening complications during COVID-19 (Saudubray and Cazorla, 2016; Zubarioglu et al., 2021). Secondly, immune system dysregulation has been variably reported among IMD patients, especially patients with organic acidemias, which is theoretically increasing the risk of severe COVID-19 in this group of patients (Altun et al., 2021).
Studies on the impact and the outcome of COVID-19 infection in IMD patients are scarce. There are a few reports on the clinical impact of COVID-19 infection in IMD patients with different clinical responses ranging from mild to severe presentation (Zubarioglu et al., 2021; Sibulo et al., 2021; Saad-Naguib et al., 2021; Kaur et al., 2021; Caciotti et al., 2020; Wongkittichote et al., 2020; Climent et al., 2020; Lampe et al., 2020; Tummolo et al., 2021; Tobór-Świętek et al., 2021). Due to the uniqueness of this group of patients and the lack of extensive literature reports, the management of COVID-19 infection has been challenging for many health care providers (Lampe et al., 2020; Tobór-Świętek et al., 2021). Our pediatric and adult metabolic service at King Faisal Specialist Hospital and Research Centre (KFSHRC) is one of the largest metabolic centers in the world (serving >1000 IMD patients/year). This has provided us an ample opportunity to examine the spectrum of COVID-19 in this rare disease population. In this study, we describe the epidemiology of COVID-19 disease in our IMD cohort to determine the outcome of this infection in this population. We aimed to characterize the clinical presentation of IMD patients in relation to COVID-19 infection, define which group of IMD is more prone to metabolic decompensation and/or COVID-19 related complications, and asses the risk for morbidity and mortality in IMD patients during COVID-19 infection.
2. Methods
2.1. Subjects
A review of IMD patients attending the metabolic center at KFSHRC, Riyadh, Saudi Arabia was conducted for the period from Nov 2019 when COVID-19 pandemic started till Jul 2021. A total of 1058 IMD patients following in our center under the following diagnostic categories were surveyed for their exposure to COVID-19: intoxication disorders 60.3% (n = 638), carbohydrate metabolism disorders 18.6% (n = 197), energy metabolism disorders 17.1% (n = 181), and complex molecules defects 4% (n = 42). In this cohort, there were 19 IMD disorders under the listed diagnostic categories were included in Table 1 . All participants provided their verbal consent before starting the questionnaire.
Table 1.
Positive COVID-19 cases per IMD patients and percentage of asymptomatic, symptomatic, hospitalized, required intensive care, and died patients.
| Total IMD patients (n = 1058) n (%) | IMD diagnosis n | Positive COVID-19 cases n (%) | Asymptomatic n = 17 (13.7%) | Symptomatic n = 107 (86.2%) | Hospitalized n = 22 (17.7%) | Intensive care n = 10 (8%) | Deaths n = 3 (2.4%) |
|---|---|---|---|---|---|---|---|
| Intoxication Disorders 638 (60%) |
MMA (109) | 16 (15%) | 2 (12.5%) | 14 (87.5% | 7 (43.7%) | 3 (18.7%) | 2 (12.5%) |
| PA (33) | 3 (9%) | 0 | 3 (100%) | 3 (100%) | 3 (100%) | 0 | |
| IVA (34) | 4 (12%) | 0 | 4 (100%) | 0 | 0 | 0 | |
| GA-I (58) | 12 (21%) | 3 (25%) | 9 (75%) | 1 (8%) | 0 | 0 | |
| MSUD (78) | 8 (10%) | 1 (12.5%) | 7 (87.5%) | 1 (12.5%) | 0 | 0 | |
| ASA (49) | 7 (14%) | 0 | 7 (100%) | 2 (28.5%) | 2 (28.5%) | 0 | |
| CIT-I (30) | 0 (0%) | 0 | 0 | 0 | 0 | 0 | |
| HCU (85) | 12 (14%) | 0 | 12 (100%) | 2 (16.6%) | 0 | 0 | |
| PKU (99) | 13 (13%) | 5 (38%) | 8 (61.5%) | 0 | 0 | 0 | |
| TYR-I (45) | 7 (16%) | 1 (14.2%) | 6 (85.7%) | 0 | 0 | 0 | |
| TYR-II (18) | 0 (0) | 0 | 0 | 0 | 0 | 0 | |
| Carbohydrate Metabolism Disorders 197 (19%) |
GALT (58) | 7 (12%) | 1 (14.2%) | 6 (85.7%) | 0 | 0 | 0 |
| FBP1 (33) | 5 (15.15%) | 2 (40%) | 3 (60%) | 0 | 0 | 0 | |
| GSD-I (47) | 2 (4.25%) | 0 | 2 (100%) | 0 | 0 | 0 | |
| GSD-III (59) | 8 (13.5%) | 0 | 8 (100%) | 0 | 0 | 0 | |
| Energy Metabolism Disorders 181 (17%) |
BKT (23) | 2 (9%) | 0 | 2 (100%) | 0 | 0 | 0 |
| HMG (60) | 9 (15%) | 2 (22%) | 7 (77%) | 2 (22%) | 0 | 0 | |
| VLCADD (98) | 6 (6%) | 0 | 6 (100%) | 2 (33.3%) | 1 (16.6%) | 0 | |
| Complex Molecules Defects 42 (4%) |
MPS I-IV & VI) (42) |
3 (7%) | 0 | 3 (100%) | 2 (66.6%) | 1 (33.3%) | 1 (33.3%) |
Methylmalonic Acidemia (MMA), Propionic Acidemia (PA), Isovaloric Acidemia (IVA), Glutaric Aciduria type I (GA-I), Maple Syrup Urine Disease (MSUD), Argininosuccinic Aciduria (ASA), Citrullinemia type I (CIT-I), Homocysteinurea (HCU), Phenylketonuria (PKU), Tyrosinemia type I (TYR-I), Tyrosinemia type II (TYR-II); Beta-Ketothiolase deficiency (BKT), HMG-CoA lyase deficiency (HMG), Very long-chain acyl-CoA dehydrogenase deficiency (VLCADD), Galactosemia (GALT), Fructose 1.6 bisphosphates deficiency (FBP1), glycogen storage disease type I (GSD-I), Glycogen Storage Disease type IIII (GSD-III), Mucopolysaccharidosis types (I-IV and VI) (MPS I-IV and VI).
2.2. Inclusion and exclusion criteria
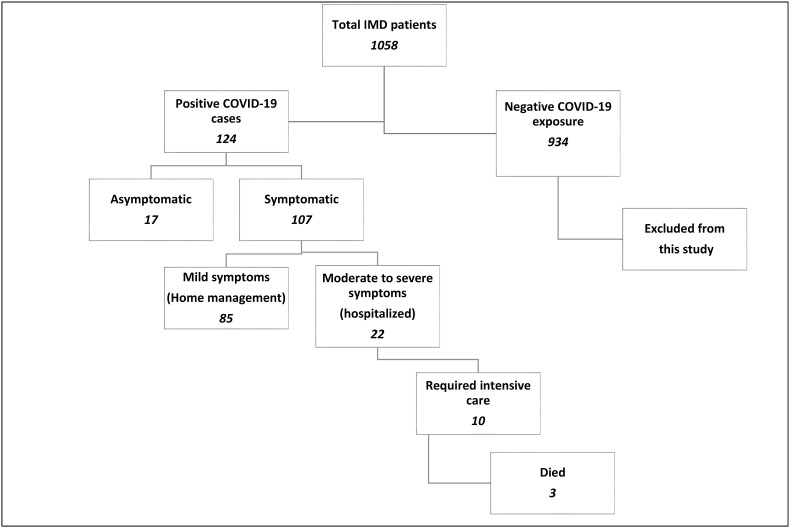
The initial survey was started with contacting all the 1058 patients’ families to inquire about their exposure to COVID-19. Patients with negative exposure/infection were excluded from this study. Only confirmed COVID-19 PCR positive cases were directed to the detailed questionnaire. Fig. 1 shows the schematic graph of the surveyed IMD patients and their outcome.
Fig. 1.
Schematic diagram illustrating the surveyed IMD patients for COVID-19 exposure and their outcome.
2.3. Data collection and design
The data collection was designed as an online questionnaire using Microsoft office forms and consisted of 23 questions. The questionnaire included the demographics data, the IMD diagnosis, the reason for COVID-19 testing, the main COVID-19 infection symptoms, the presence of metabolic decompensation during COVID-19 infection, the IMD management during the infection and the vaccination status for patients aged 12 years and above. It is of note that all the patients developed COVID-19 infection prior to the initiation of the COVID -19 vaccination program except for one Homocystinuria (HCU) patient who had a second COVID-19 infection episode within three months of the first dose of AstraZeneca vaccine (this uncomplicated episode of infection after vaccination was not included in this study). Therefore, this study encompasses an unvaccinated cohort of IMD patients.
The detailed questionnaires for total of 124 patients were completed by certified clinicians through interviewing their families by phone. Further data about the management and hospitalization were obtained from the patients’ medical charts.
2.4. Data selection and extraction
All the extracted data from Microsoft office forms were statistically analyzed using Microsoft Excel and SPSS programs. Statistical data available as a supplementary material.
2.4.1. Ethical consideration
This approved research project (RAC # 2211190) was conducted in accordance with the ethical principles contained in the Declaration of Helsinki (2000), the ICH Harmonized tripartite good clinical practice guidelines, and the policies and guidelines of the research advisory council of KFSHRC.
3. Results
3.1. Rate of IMD patients infected with COVID-19
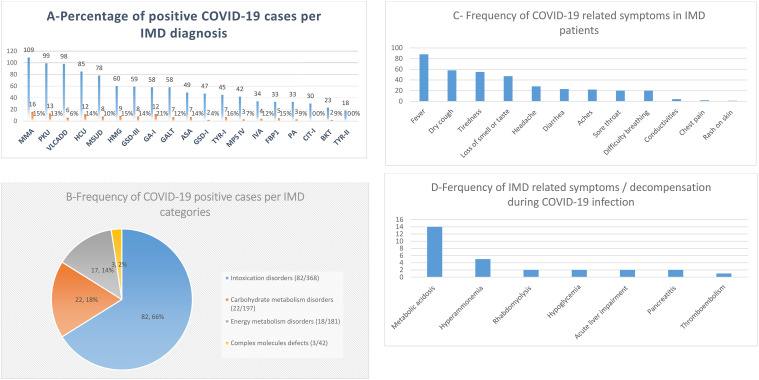
Among total of 1058 IMD patients followed in our center, 11.7% (n = 124) confirmed to have positive COVID-19 infection during the selected study period. The demographic and clinical details of IMD patients with a confirmed COVID-19 infection were summarized in Table 2 . The COVID-19 infection rate among specific IMD disease categories was ranged between 12.6% and 7% (illustrated in Table 1 and Fig. 2 -A and 2-B). Our data showed that patients with Glutaric Acidemia type I (GA-I) had the highest infection rate (21%), while patients with Citrullinemia type I (CIT-I) and Tyrosinemaia Type II (TYR-II) reported no infection (Table 1).
Table 2.
Demographic Characteristics of 124 IMD patients infected with COVID-19.
| Demographic and clinical data | Number (%) | Comment |
|---|---|---|
| Age group | ||
| <16 years | 56 (45%) | Asymptomatic 12 (21.4%); Mild 36 (64%); Mod- severe 8 (14%) |
| >16 years | 68 (55%) | Asymptomatic 5 (7.4%); Mild 49 (72%); Mod- severe 14 (20.5%) |
| Gender | ||
| Male | 71 (57%) | |
| Female | 53 (43%) | |
| IMD Diagnostic category | ||
| Intoxication disorders | 82 (65%) | Hospitalized 16 (20%) |
| Carbohydrate metabolism defects | 22 (18%) | Hospitalized 0 |
| Energy metabolism defects | 18 (14.5%) | Hospitalized 4 (22%) |
| Complex molecules defects | 3 (2.5%) | Hospitalized 2 (67%) |
| Reason for COVID-19 PCR test | ||
| Post-exposure screening | 97 (78) | |
| Diagnostic (symptomatic) | 24 (19) | |
| Pre-hospital visit screening | 3 (2.5) | |
| COVID-19 Symptoms | ||
| Asymptomatic | 17 (13.7%) | Pediatrics 12 (70%), adults 5 (30%) |
| Symptomatic with COVID-19 related infection | 107 (86.3%) | |
| Metabolic Complications frequency | ||
| Metabolic acidosis | 14 (11%) | ((MMA (n = 7); PA (n = 3); HMG (n = 2); ASA (n = 2)) |
| Hyperammonemia | 5 (4%) | (PA (n = 3); ASA (n = 2)) |
| Acute pancreatitis | 2 (1.6%) | ((PA (n = 1); ASA (n = 1)) |
| Rhabdomyolysis | 2 (1.6%) | (VLCADD (n = 2)) |
| Acute liver impairment | 2 (1.6%) | ((ASA n = 1);VLCADD (n = 1)) |
| Hypoglycemia | 2 (1.6%) | (VLCADD (n = 1)) |
| Thrombosis | 1 (0.8%) | (HCU (n = 1)) |
| Management and Outcome | ||
| Home management | 102 (69%) | |
| Hospitalization | 22 (18%) | |
| Intensive care | 10 (8%) | See Table 3 for details |
| Deaths | 3 (2.4%) | |
Fig. 2.
Fig. 2-A Percentage of positive COVID-19 cases per IMD diagnosis; Fig.2-B Frequency of COVID-19 positive case per IMD categories; Fig.2-C Frequency of COVID-19 related symotms in IMD patients; Fig.2-D Frequency of IMD related symotms during COVID-19 infection.
3.2. Asymptomatic and symptomatic rate in IMD patients infected with COVID-19
Only 13.7% (n = 17) of this cohort did not exhibit any symptoms. The majority of symptomatic patients, 68.5% (n = 85) presented with mild symptoms related mainly to COVID-19 infection and required only home management with close follow-up by the specialized Ministry of Health clinics dedicated for COVID-19 infection during the pandemic. Hospitalization was required for 17.8% (n = 22) who presented with moderate to severe symptoms either related to COVID-19 infection or IMD decompensation. All the infected Propionic Acidemia (PA) patients required intensive care, while 33% (1/3) of the Mucopolysarcoidosis type IV (MPS-IV) patients, 28% (2/7) of the Argininosuccinc Acidurea (ASA) patients 18.7% (3/16) of the Methylmalonic acidemia (MMA) patients required intensive care (Table 1, supplementary 2).
3.3. Metabolic decompensation during COVID-19 infection
A proportion of the hospitalized patients had developed metabolic complications (metabolic acidosis, hyperammonemia, acute liver impairment, hypoglycemia, acute pancreatitis, or rhabdomyolysis). Of these, 10 patients (8%) required intensive care management. Details of the patients’ symptomatology related to COVID-19 infection and IMD diagnosis were illustrated in Table 2 and Fig. 2-C and 2-D.
3.4. Management of hospitalized IMD patients with during COVID-19 infection
All admitted patients with intoxication disorders required modification of their metabolic managements including dietary and drug adjustments e.g. IV carnitine, IV scavengers for hyperammonemia, IV fluids and bicarbonate supplements for the metabolic acidosis, in addition to the management of COVID-19 infection as per the World Health Organization (WHO) protocols. Details of the IMD patients who required intensive care hospitalization were summarized in Table 3 .
Table 3.
Clinical summaries of IMD patients who required intensive care hospitalization during COVID-19 infection.
| Patient no. | Age (year) | Gender | IEM diagnosis | COVID-19 related symptoms | IEM related symptoms | Management | Comment |
|---|---|---|---|---|---|---|---|
| 1 | 23 | M | ASA | Fever, shortness of breath & diarrhea | Abdominal pain, nausea, fatigability, hyperammonemia, metabolic acidosis, acute necrotic pancreatitis, acute elevation of liver transaminases | IV hydration, IV ammonia scavengers, IV sodium bicarbonate, IV meropenem, vancomycin, Oxygen support via nasal cannula | |
| 2 | 2 | F | VLCADD | Fever, diarrhea &lethargy | Rhabdomyolysis and acute elevation of liver transaminases | IV hydration Azithromycin, hydroxychloroquine |
|
| 3 | 16 | M | PA | Fever, tiredness, dry cough, & shortness of breath | Metabolic acidosis and acute pancreatitis | IV hydration, sodium bicarbonate | |
| 4 | 17 | M | PA | Fever, tiredness, dry cough, shortness of breath & respiratory failure | Hypperammonemia, metabolic acidosis, and pancytopenia | IV ammonia scavengers, IV sodium bicarbonate, azithromycin, hydroxychloroquine | |
| 5 | 16 | M | PA | Fever, shortness of breath requiring | Hyperammonemia and metabolic acidosis | IV ammonia scavengers, high flow oxygen support via nasal cannula, azithromycin, hydroxychloroquine, ceftriaxone | |
| 6 | 22 | M | ASA | Decrease activity & tiredness | Hyperammoneima, metabolic acidosis, hyponatremia and hypokalemia | IV ammonia scavengers, IV electrolytes | |
| 7 | 28 | M | MMA (k/c of chronic renal failure) | Shortness of breath and pneumonia | Vomiting, decreased activity, refractory metabolic acidosis and hyperkalemia | Hemodialysis, azithromycin, hydroxychloroquine, tocliozumab | |
| 8 | 16 | F | MMA | Fever, shortness of breath, pneumonia & respiratory failure | Hospital course complicated by severe metabolic acidosis. Died from cardiac arrest after a month of stay at local hospital | Invasive ventilator support, IV sodium bicarbonate |
Died |
| 9 | 21 | M | MMA | Decreased activity, uncontrolled seizure episodes and sepsis | Patient died at local hospital | NA | Died |
| 10 | 30 | M | MPS IV (k/c of restrictive lung disease) | Fever and severe shortness of breath | Patient died within 3 h from presentation to local hospital's emergency | NA | Died |
M male; F female; k/c known case; NA data not available; ASA arginioscuccinic aciduria; VLCADD Very long-chain Acyl-CoA dehydrogenase deficiency; PA Propionic Acidemia; MMA Methylmalonic Acidemia; MPS-IV Mucopolysarcoidosis type IV.
3.5. Death rate in IMD patients infected with COVID-19
Three patients (2.4%) died post COVID-19 infection: two Methylmalonic acidemia (MMA) patients died from refractory metabolic acidosis secondary to the sepsis related to COVID-19 infection, and one Mucopolysarcoidosis type IV (MPS IV) patient who was known to have compromised restrictive lunge disease and died from severe respiratory failure that did not respond to the invasive respiratory support.
4. Discussion
The WHO declared the COVID-19 outbreak as pandemic in March 11th, 2020. There is limited information in the literature for health care providers about the clinical course and the outcome of IMD patients who acquired COVID-19 infection. To date, this is the largest study from a single metabolic center in which we evaluated the clinical impact and the outcome of COVID-19 infection in 124 IMD patients. Table 4 highlights the comparison of our data with previous cohorts of IMD patients infected with COVID-19. The incidence of COVID-19 infection in IMD population is estimated to be lower than the general population, this is possibly related to the lower exposure rate (Lampe et al., 2020). In our cohort, 11.7% of IMD patients acquired COVID-19 infection prior to implementing the nationwide vaccination campaign. The negative exposure in the rest of IMD patients following in our center could be related to lower exposure during the national lockdown during the pandemic and the protective measures taken by the families knowing their children's vulnerability to infections in general. The reported asymptomatic rate in general pediatric population infected with COVID-19 from a meta-analysis study was 16% compared to 21.4% in our pediatrics IMD patient (Table 2). In contrast, the asymptomatic rate in general adult population from another meta-analysis study was 40.5% while it was much lower at 7.4% in our adult IMD group (Table 2) (Assaker et al., 2020; Ma et al., 2021). The low asymptomatic rate in our adults IMD group may indicates the contribution of their comorbidities related to their IMDs into their immune response to the infection. Collective asymptomatic rate in previous IMDs' pediatric and adult cohorts was variable between 4.2% and 63% (Table 4). Our cumulative asymptomatic rate was 13.7% (n = 17) patients.
Table 4.
Comparison of our data with the previous cohorts of IMD patients infected with COVID-19 infection.
| Report (year) | Lampe et al. (2020) | Tummolo et al. (2021) | Tobór-Swietek et al. (2021) | Zubarioglu et al. (2021) | This study 2022 |
|---|---|---|---|---|---|
| No. of centers | Multi-centers | 1 Center | Multi-centers | 1 Center | 1 Center |
| Total no. of surveyed IMD patients | 73 | 272 | NA | NA | 1058 |
| Total no. of COVID-19 positive cases | 24 | 19 | 27 | 22 | 124 |
| Pediatrics | 13 | 9 | 10 | 22 | 56 |
| Adult | 11 | 10 | 17 | 0 | 68 |
| Asymptomatic | 1 (4.2%) | 12 (63%) | 0 (0%) | 2 (9%) | 17 (13.7) |
| Hospitalization | 2 (8.3%) | 1 (5.3%) | 4 (14.8%) | 8 (36.4%) | 22 (17.7%) |
| Death | 0 | 0 | 1 (NPC) | 0 | 3 |
NPC Neimann Pick C.
The manifestation of COVID-19 infection reported to be much less severe in children and adolescents than in adults (World Health Organization, 2019; Peng et al., 2020; Alharbi et al., 2021; Shekerdemian et al., 2020). Among the previously reported IMD patients with COVID-19, the overall course of the illness was mild with good recovery [ 4, 12–14]. In line with this finding, the result from this cohort (124 patients) showed that the majority of IMD patients (68.5%; 36 pediatrics, 49 adults) exhibited mild disease related to COVID-19. Interestingly, the rate of COVID-19 infection was variable among various IMD categories, GA-I patients were the most infected group with COVID-19 (21%), while none of the CIT-I and TYR-II patients encountered the infection during the study period. It is not clear if this was stochastic or possibly related to some unknown susceptibility factors.
It is known that infections are major triggers for metabolic decompensation in certain IMD patients especially in patients with intoxication or energy metabolism disorders. Out of this cohort, 17.7% (n = 22) required hospitalization. They presented with either symptom related to COVID-19 infection or with metabolic decompensations triggered by the infection. Most of the hospitalized patients were adults (16/22) with intoxication disorders (MMA, PA, and ASA) and 8 of those patients, in addition to a patient with Very long-chain Acyl-CoA dehydrogenase deficiency (VLCADD), developed severe symptoms and required intensive care management. This echoes the same course of previously reported IMD patients under these two categories (Sibulo et al., 2021; Saad-Naguib et al., 2021; Kaur et al., 2021; Caciotti et al., 2020; Wongkittichote et al., 2020). Metabolic acidosis was mainly reported in patients with organic acidemias, while hyperammonemia was seen in ASA and PA patients. Two adult patients (PA, and ASA) developed acute pancreatitis and it was associated with liver dysfunction in the ASA patient. Acute pancreatitis is a well-known complication seen in organic acidemias and has been reported in urea cycle disorders (Machado et al., 2013). Based on our local experience and current paucity of solid evidence of COVID-19 association with acute pancreatitis, it is reasonable to assume that these two patients developed acute pancreatitis as a complication of the metabolic crisis (Bulthuis et al., 2021). Vascular thrombosis, a complication of COVID-19 infection, which quickly became evident in hospitalized patients since the beginning of the pandemic, was seen in an HCU patient who had developed left femoral vein thrombosis. It is difficult to relate this complication solely to COVID-19 infection as HCU itself predisposes to the development of thromboembolism (Sacharow et al., 2004). This complication was not seen in our cohort with other IMD patients with COVID-19 infection. Other intoxication and energy metabolism disorders in our cohort were hospitalized for COVID-19 related symptoms with no metabolic decompensation.
Patients with certain complex molecules defects like MPS and Pompe disease are at risk of developing cardiac and/or respiratory compromise following infection due to the underling impaired heart and pulmonary function. In our study, only three patients with MPS had COVID-19 infection. Two patients with MPS-IV presented with moderate and severe respiratory symptoms requiring hospitalization. Their chronic restrictive lung disease contributed to their adverse response to the infection. A 16-years old MPS-IV male responded to the non-invasive respiratory support, but a 30-years old MPS-IV patient died within 3 h from presentation to the hospital's emergency, and the death was mainly from respiratory decompensation. The third MPS-IV infected patient presented with mild respiratory symptoms (fever and cough) and he responded to the home management. None of the COVID-19 infected carbohydrate metabolism defects (n = 22) required hospitalization for their mild COVID-19 infection related symptoms.
Death due to complicated cardiomyopathy and heart failure during COVID-19 infection was reported in an infant with MPS-I (Climent et al., 2020). Another reports of death were in a patient with Long-chain fatty-acyl CoA dehydrogenase deficiency due to COVID-19 complications and in another patient with Niemann-Pick C from complicated COVID-19 pneumonia and respiratory failure (Wongkittichote et al., 2020; Tobór-Świętek et al., 2021). None of our patients with energy metabolism disorders died though, two of them developed rhabdomyolysis crisis during their COVID-19 illness. In contrast, two of our hospitalized MMA patients died from severe metabolic decompensation and sepsis and one MPS-IV patient died from severe respiratory decompensation. This may suggest that patients with intoxication disorders and lysosomal storage disorders in general are at a higher risk of severe COVID-19 infection especially if they have comorbidities like chronic kidney disease, cardiomyopathy or restrictive lung disease.
One of the major limitations of this cross sectional study is that the questionnaire was based families recalling of their children's symptoms during COVID-19 infection. The objective data were available only for the hospitalized patients.
5. Conclusion
To date, this is the largest study from a single metabolic center evaluating the impact and the outcome of COVID-19 infection in unvaccinated IMD patients group. Our study indicates that these patients, especially if unvaccinated, should be considered as a high-risk population who requires prompt management during COVID-19 infection. Due to the high risk of metabolic decompensation, IMD patients should be taken care in hospitals equipped with specialized staff and medications needed to treat metabolic decompensations (e.g. IV carnitine, ammonia scavengers and special formulas). In addition, some IMD patients may have an underlying respiratory or cardiac disease which increases their vulnerability to severe COVID-19 disease. Furthermore, clinicians should be aware of the risk of thromboembolism in HCU with COVID-19 infection.
CRediT authorship contribution statement
Ruqaiah Altassan: Methodology, Formal analysis, Visualization, Writing – original draft. Raashda A. Sulaiman: Writing – review & editing. Abdullah Alfalah: Methodology, Investigation. Waad Alwagiat: Methodology, Investigation. Eman Megdad: Investigation. Dana Alqasabi: Investigation. Bedour Handoom: Investigation. Munirah Almesned: Investigation. Hassan Al-Amri: Resources. Zuhair Alhassnan: Resources. Moeen-aldeen Alsayed: Resources. Hamad Alzaidan: Resources. Zuhair Rahbeeni: Resources. Nada Derar: Resources. Mohammed Al-Owain: Conceptualization, Writing – review & editing. Esam Albanyan: Writing – review & editing, Supervision.
Acknowledgment
The authors are thankful to Dr.Gamal Mohamed from Biostatistics Epidemiology & Scientific Computing Department for his great help in the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmg.2022.104602.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alharbi M., Kazzaz Y.M., Hameed T., Alqanatish J., Alkhalaf H., Alsadoon A., Alayed M., Hussien S.A., Shaalan M.A., Al Johani S.M. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J. Infect. Public Health. 2021 Apr;14(4):446–453. doi: 10.1016/j.jiph.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun I., Kiykim A., Zubarioglu T., Burtecene N., Hopurcuoglu D., Topcu B., Cansever M.S., Kiykim E., Cezmi Cokugras H., Aktuglu Zeybek A.C. Altered immune response in organic acidemia. Pediatr. Int. 2021 Dec 3 doi: 10.1111/ped.15082. [DOI] [PubMed] [Google Scholar]
- Assaker R., Colas A.E., Julien-Marsollier F., Bruneau B., Marsac L., Greff B., Tri N., Fait C., Brasher C., Dahmani S. Presenting symptoms of COVID-19 in children: a meta-analysis of published studies. Br. J. Anaesth. 2020 Sep;125(3):e330–e332. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulthuis M.C., Boxhoorn L., Beudel M., Elbers P.W.G., Kop M.P.M., van Wanrooij R.L.J., Besselink M.G. Voermans RP Acute pancreatitis in COVID-19 patients: true risk? Scand. J. Gastroenterol. 2021 May;56(5):585–587. doi: 10.1080/00365521.2021.1896776. [DOI] [PubMed] [Google Scholar]
- Caciotti A., Procopio E., Pochiero F., Falliano S., Indolfi G., Donati M.A., FerriL, Guerrini R., Morrone A. SARS-CoV-2 infection in a patient with propionic acidemia. Orphanet J. Rare Dis. 2020 Oct 28;15(1):306. doi: 10.1186/s13023-020-01563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019 ncov/index.html
- Climent F.J., Calvo C., García-Guereta L., Rodríguez-Álvarez D., Buitrago N.M., Pérez-Martínez A. Fatal outcome of COVID-19 disease in a 5-month infant with comorbidities. Rev. Esp. Cardiol. (Engl. Ed.) 2020 Aug;73(8):667–669. doi: 10.1016/j.recesp.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Campbell S.L., Stockton D.W. Management of COVID-19 infection in organic acidemias. Am. J. Med. Genet. A. 2021 Jun;185(6):1854–1857. doi: 10.1002/ajmg.a.62161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe C., Dionisi-Vici C., Bellettato C.M., Paneghetti L., van Lingen C., Bond S., Brown C., Finglas A., Francisco R., Sestini S., Heard J.M., Scarpa M., MetabERN collaboration group The impact of COVID-19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphanet J. Rare Dis. 2020 Dec 3;15(1):341. doi: 10.1186/s13023-020-01619-x. PMID: 33272301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Liu J., Liu Q., Kang L., Liu R., Jing W., Wu Y., Liu M. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open. 2021 Dec 1;4(12) doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M.C., Fonseca G.M., Jukemura J. Late-onset ornithine carbamoyltransferase deficiency accompanying acute pancreatitis and hyperammonemia. Case Rep. Med. 2013;2013 doi: 10.1155/2013/903546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Gao P., Xu Q., Liu M., Peng J., Wang Y., Xu H. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes. J. Clin. Virol. 2020 Jul;128 doi: 10.1016/j.jcv.2020.104425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad-Naguib M., Barbouth D., Thorson W., Hacker S., Tekin M. COVID-19 in a child with severe propionic acidemia. Pediatr. Int. 2021 May;63(5):606–607. doi: 10.1111/ped.14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharow S.J., Picker J.D., Levy H.L. In: GeneReviews® [Internet] Adam M.P., Mirzaa G.M., Pagon R.A., et al., editors. University of Washington, Seattle; Seattle (WA): 2004 Jan 15. Homocystinuria caused by cystathionine beta-synthase deficiency.https://www.ncbi.nlm.nih.gov/books/NBK1524/ [Updated 2017 May 18] 1993-2022. Available from: [PubMed] [Google Scholar]
- Saudubray J.M., Cazorla A.G. Clinical Approach to Inborn Errors of Metabolism in Pediatrics. Inborn Metabolic Diseases Diagnosis and Treatment. sixth ed. Springer-Verlag; Heidelberg: 2016. pp. 4–69. [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., Heidemann S.M., Kleinman L.C., Sen A.I., Hall M.W., Priestley M.A., McGuire J.K., Boukas K., Sharron M.P., Burns J.P., International COVID-19 PICU Collaborative Characteristics and outcomes of children with Coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020 Sep 1;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibulo L., Kogel W., Landolt L., Seeni S., Markel J., Mlady A. Anesthetic management of a child with propionic acidemia complicated by bacteremia and severe acute respiratory syndrome Coronavirus 2. J. Med. Cases. 2021 Apr;12(4):152–156. doi: 10.14740/jmc3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobór-Świętek E., Sykut-Cegielska J., Bik-Multanowski M., Walczak M., Rokicki D., Ł Kałużny, Wierzba J., Pac M., Jahnz-Różyk K., Więsik-Szewczyk E., Kieć-Wilk B. COVID-19 pandemic and patients with rare inherited metabolic disorders and rare autoinflammatory diseases-organizational challenges from the point of view of healthcare providers. J. Clin. Med. 2021 Oct 22;10(21):4862. doi: 10.3390/jcm10214862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummolo A., Paterno G., Dicintio A., Stefanizzi P., Melpignano L., Aricò M. COVID-19 and inherited metabolic disorders: one-year experience of a referral center. Children (Basel) 2021 Sep 6;8(9):781. doi: 10.3390/children8090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongkittichote P., Watson J.R., Leonard J.M., Toolan E.R., Dickson P.I., Grange D.K. Fatal COVID-19 infection in a patient with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: a case report. JIMD Rep. 2020 Sep 10;56(1):40–45. doi: 10.1002/jmd2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization https://www.who.int/emergencies/diseases/novel-coronavirus-2019/
- Zubarioglu T., Hopurcuoglu D., Ahmadzada S., Uzunyayla-Inci G., Cansever M.S., Kiykim E., Aktuglu-Zeybek C. Inborn errors of metabolism and COVID-19: evaluation of the metabolic outcome. Pediatr. Int. 2021 Jul 30 doi: 10.1111/ped.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.