Abstract
Cortico-cortical paired associative stimulation (ccPAS) is an effective transcranial magnetic stimulation (TMS) method for inducing associative plasticity between interconnected brain areas in humans. Prior ccPAS studies have focused on protocol’s aftereffects. Here, we investigated physiological changes induced “online” during ccPAS administration. We tested 109 participants receiving ccPAS over left ventral premotor cortex (PMv) and primary motor cortex (M1) using a standard procedure (90 paired-pulses with 8-ms interstimulus interval, repeated at 0.1 Hz frequency). On each paired-pulse, we recorded a motor-evoked potential (MEP) to continuously trace the emergence of corticomotor changes. Participant receiving forward-ccPAS (on each pair, a first TMS pulse was administered over PMv, second over M1, i.e., PMv-to-M1) showed a gradual and linear increase in MEP size that did not reach a plateau at the end of the protocol and was greater in participants with low motor threshold. Participants receiving reverse-ccPAS (i.e., M1-to-PMv) showed a trend toward inhibition. Our study highlights the facilitatory and inhibitory modulations that occur during ccPAS administration and suggest that online MEP monitoring could provide insights into the malleability of the motor system and protocol’s effectiveness. Our findings open interesting prospects about ccPAS potential optimization in experimental and clinical settings.
Subject terms: Motor control, Neural circuits, Sensorimotor processing, Synaptic plasticity
Introduction
Cortico-cortical paired associative stimulation (ccPAS) is an effective transcranial magnetic stimulation (TMS) method for inducing associative plasticity between interconnected brain areas in humans1–4, based on the Hebbian principle of spike-timing-dependent plasticity (STDP)5–7. The ccPAS protocol consists in the repeated application of pairs of TMS pulses over two interconnected brain sites1–4,7–15, using an optimal interstimulus interval (ISI) between the pulses so that, for each pair, the first pulse administered over the first site (containing “pre-synaptic neurons”, according to the Hebbian principle5) would induce an activation spread reaching the second site (containing “post-synaptic neurons”) immediately before/simultaneously with the administration of a pulse over that site. This pre- and post-synaptic coupling mimics patterns of neural stimulation instrumental for achieving STDP6,7, thus enhancing (or weakening) the strength of the neural pathway connecting the stimulated brain areas1–4,7–15. Indeed, studies have reported that ccPAS induces changes in functional13,14 and effective2,3,12,13 connectivity of the targeted networks, as well as behavioral effects both in the motor1,11,15, visual9,10,16,17 and executive functions8 domains, suggesting that ccPAS could be a useful tool for investigating and changing behavior following plastic ‘re-wiring’ of the human connectome.
Notably, prior studies have mostly focused on physiological and behavioral aftereffects of ccPAS1–3,8–14, without clarifying whether and how plastic changes build up already during protocol administration. Addressing this issue is the main goal of the present study. Importantly, clarifying the dynamics of physiological changes “online” during ccPAS administration may provide insights into the optimal duration of the protocol. Moreover, while prior studies have suggested that interindividual differences in motor excitability predict sensitivity to exogenous manipulations of STDP11,18, whether individual’s resting motor threshold (rMT) predicts plastic changes during the administration of ccPAS is a relevant and yet largely unexplored issue.
To fill these gaps, we administered ccPAS over a premotor-motor circuit encompassing the left ventral premotor cortex (PMv) and the left primary motor cortex (M1), while continuously monitoring changes in corticomotor excitability via motor-evoked potentials (MEPs) recording. Indeed, because M1 was targeted using suprathreshold intensity, on each pulse a MEP was recorded in the contralateral (right) first dorsal interosseous of participants’ hand (see Methods for details).
The PMv-M1 is a hierarchically organized neural network primarily involved in fine motor control of sensory-guided actions such as grasping and manipulating objects19,20, but has also been implicated in several other functions including action imitation21,22, processing of observed actions23–25 and action-related language26–28. Of particular relevance to the present research, prior studies have established the temporal properties of the PMv-to-M1 pathway, by showing that a conditioning TMS pulse over PMv results in a modulation of MEPs induced by a second pulse over M1 when an ISI of 8 ms is used29,30. Accordingly, previous ccPAS studies targeting the PMv-to-M1 pathway have selected an 8-ms ISI to repeatedly and coherently couple pre- and post-synaptic activity—optimal for inducing STDP2,11,14.
Building on this prior work, we designed a ccPAS protocol consisting in 90 pairs of TMS pulses delivered at 0.1 Hz, adopting an 8-ms ISI to induce STDP in the PMv-to-M1 pathway. Participants were randomly divided into two groups (Fig. 1a): premotor-motor ‘forward-ccPAS’, i.e., a protocol aimed at enhancing the hierarchical organization of the circuit, where PMv conveys signals to M1 for motor command implementation, by repeatedly stimulating PMv before M1, or ‘reverse-ccPAS’, in which the order of the pulses on each pair was reversed-i.e., M1 stimulation was followed by PMv stimulation. Based on the Hebbian rule5–7, forward-ccPAS should induce long-term potentiation-like enhancement of the PMv-to-M1 pathway, resulting in increased corticomotor excitability, whereas reverse-ccPAS would weaken that pathway, resulting in decreased corticomotor excitability.
Figure 1.
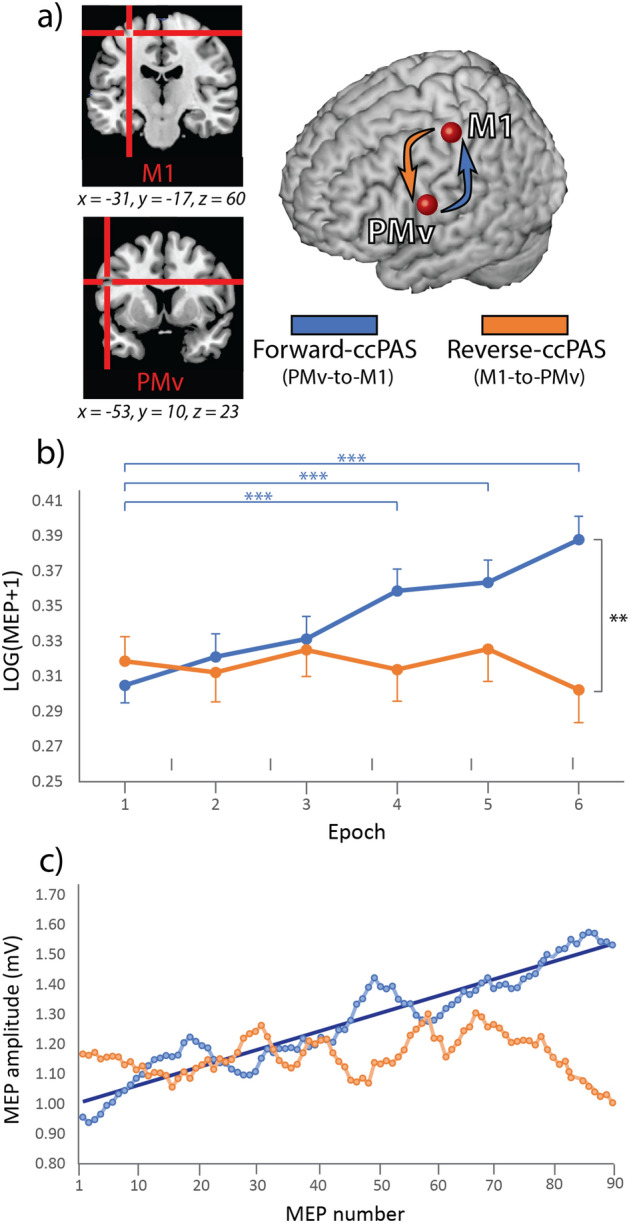
Targeted brain sites and MEP changes during ccPAS. (a) Mean Talairach coordinates of the targeted cortical sites reconstructed using MRIcron. (b) Changes in mean MEPs across Epochs. Error bars denote s.e.m. Asterisks indicate significant post-hoc comparisons: ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001. (c) Gradual changes in MEP size at the single-trial level.
Results
Figure 1b shows changes in corticomotor excitability during the ccPAS protocol in the two groups (see also Table S1). The Protocol (forward-ccPAS, reverse-ccPAS) x Epoch (1–6) ANOVA on MEP amplitudes revealed a significant main effect of Epoch (F5,535 = 8.25; P < 0.001; ηp2 = 0.08), which was qualified by a significant Protocol x Epoch interaction (F5,535 = 13.06; P < 0.001; ηp2 = 0.11). Tukey’s post-hoc tests showed that forward-ccPAS induced a clear increase in MEP amplitudes over time, significant from the fourth epoch onwards (all P < 0.001), while MEPs during reverse-ccPAS did not show consistent changes across epochs (all P ≥ 0.45).
Notably, the excitatory effect of forward-ccPAS was quite consistent across participants, although variable in magnitude. To assess inter-individual variability, we computed a MEP modulation index as the percentage increase of MEP amplitude in the last epoch compared to the first epoch [(last epoch − first epoch)/first epoch * 100]. Figure 2a shows that the vast majority of participants (87.5%) presented larger MEPs at the end of the protocol, 75% showed an increase of at least + 10% and 46% showed a consistent increase of at least + 30% in the last epoch. In contrast, only 3.6% of participants showed a reduction of approximately 10% in the last epoch.
Figure 2.
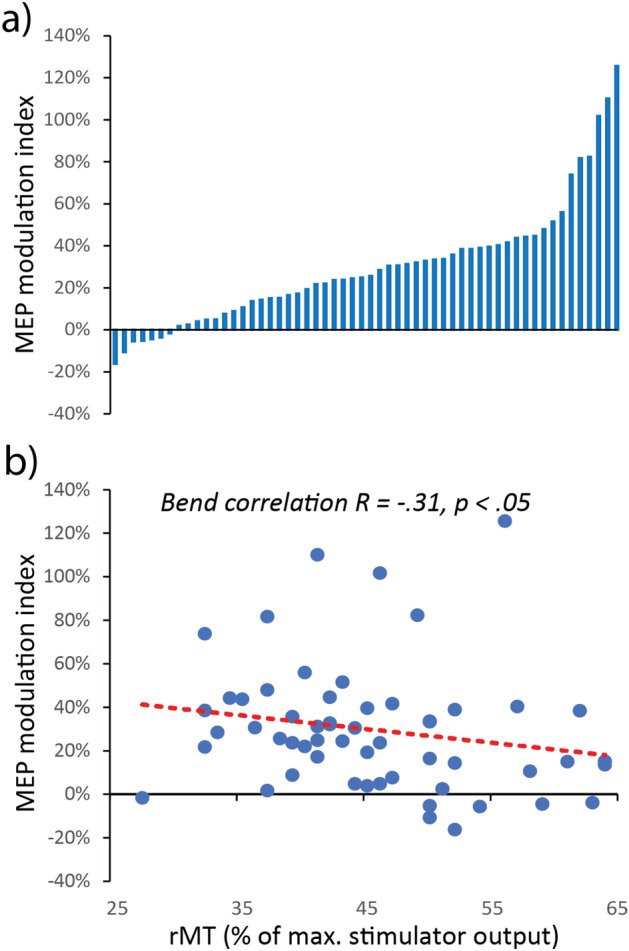
(a) Individual variability in the response to forward-ccPAS as shown by the distribution of individual MEP modulation indices computed as the percentage increase in the last epoch compared to the first epoch. (b) Relation between changes in MEPs and resting motor threshold (rMT) during forward-ccPAS.
Building on previous studies investigating predictors of TMS aftereffects11,18, we also tested whether individual’s rMT-a reliable global measure of motor excitability31-predicted differences in the magnitude of MEP increase during forward-ccPAS. We found that rMT significantly predicted the MEP modulation index (Bend Correlation R = − 0.31; P = 0.01; Fig. 2b), with participants with lower rMT showing the greater increase in corticomotor excitability.
In a control analysis, we checked whether there was an influence of the type of TMS machine, gender and motor activity carried out just before ccPAS and found no evidence supporting a role of these factors in modulating the strength of the ccPAS effect (Fig. S1).
Lastly, to provide more insights into the temporal features of the modulatory effects, we plotted the distribution of the single-trial MEPs (Fig. 1c). Importantly, during the administration of the 90 pulses in the forward ccPAS condition, we observed an increase in MEP size, accurately fitting a linear distribution (f(x) = 0.006*x + 1.002; R2 = 0.89).
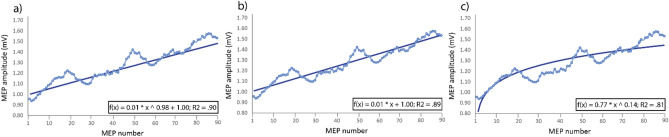
We compared different fittings to establish which one better described changes in MEPs during forward-ccPAS (Fig. 3) and found several adequate equations fittings. The best fitting equation corresponded to a two-term power distribution (f(x) = 0.007 * x0.977 + 0.998; R2 = 0.90; Fig. 3a). However, such an equation is virtually corresponding to a simple linear distribution, which indeed proved to have an almost identical graph and R2 (Figs. 1c, 3b). Lastly, a single term power could also be used to adequately describe our results, although achieving a lower R2 (f(x) = 0.766 * x0.141; R2 = 0.81; Fig. 3c). The linear fitting, therefore, appeared to be the best accurate model and notably, the observed increase suggests that no clear plateau was reached by the end of stimulation (15 min). Moreover, although we observed no significant change across epochs during reverse-ccPAS, the last portion of the graph indicates a clear trend towards reduced corticomotor excitability.
Figure 3.
Fitting equations for single-trial MEPs distribution during forward-ccPAS: (a) two-term Power distribution; (b) linear distribution; (c) single-term power distribution.
Discussion
We highlight the dynamics of changes in corticomotor excitability during ccPAS over PMv and M1. Our study shows a gradual increase in MEP amplitudes during forward-ccPAS, targeting the connection from PMv to M1, with continuous amplitude increase along the stimulation train. In contrast, reverse-ccPAS showed a trend toward inhibition at the end of the train. Thus, pattern of corticomotor excitability was not merely due to repeated stimulation of PMv or M1, and critically depended on the order between each pair of pulses over these two areas.
The gradual changes in MEP amplitudes that we observed are in line with prior work highlighting dose-dependent effects of TMS, and showing larger effects associated with increasing the number of pulses within a single session32,33 or along the number of sessions34–36. Interestingly, MEP increase during forward-ccPAS fitted a linear model and did not reach a plateau at the end of the train, raising the interesting issue of protocol duration: in most of the prior studies, the number of pulses in the ccPAS protocol have been arbitrarily set between 90 and 1001–3,8–14. Our findings of linear, dose-dependent MEPs increase during forward-ccPAS, suggest that increasing the number of paired-stimulations may induce more prominent plastic effects. This possibility is potentially relevant for clinical applications of ccPAS, when stronger alterations could be desirable. Yet, this should be directly tested as the relationship between additional doses and changes in excitability could evolve non-linearly37,38. Moreover, while our study suggests that the number of paired pulses could be a relevant variable to consider to increase ccPAS effectiveness, future studies could also test the role of frequency and intensity of stimulation, as these parameters are known to influence the effects of repetitive TMS31,39; moreover, implementing closed-loop state-dependent paradigms may offer additional specificity and efficiency benefits.
A growing literature shows that the effect of brain stimulation is highly variable across individuals40–43. In keeping, while most participants receiving forward-ccPAS showed a consistent increase in corticomotor excitability, the magnitude of the increase was variable across them. Notably, we found that the magnitude of MEP enhancement during forward-ccPAS was predicted by interindividual differences in rMT, with larger MEP enhancement associated with lower rMT. Because the rMT provides a measure of motor excitability33, these findings lend direct support to the notion that greater motor excitability is associated with higher sensitivity to associative plasticity11,18.
In our control analyses we further explored individual predictors of sensitivity to ccPAS manipulation by testing the influence of gender. These analyses suggest no influence of this factor (Fig. S1), in keeping with a prior TMS study testing STDP effects44. However, we did not assess the phase of the menstrual cycle of female subjects, and prior reports suggest a possible influence of ovarian hormones on motor system sensitivity to repetitive TMS45. Moreover, we tested young participants only, thus limiting the possibility to investigate the effect of age on STDP. Future studies should further explore inter-individual differences in responsiveness to ccPAS, and factors that account for such variability, such as age, gender or genetic polymorphisms, and, crucially, individual patterns of structural brain connectivity44; finally, because the present work has used a between subject design, it has not been possible to characterize whether each individual’s malleability to forward-ccPAS protocols and reverse-ccPAS protocols correlate. Despite these limitations, our study identifies a common and well-established neurophysiological parameter, namely the individual’s rMT, as a predictor of ccPAS sensitivity, expanding prior studies focusing on forward-ccPAS aftereffects11 and thus providing insights into the issue of individualized approaches to brain stimulation.
While we observed protocol-specific effects, with a clear increase in corticomotor excitability during forward-ccPAS and a tendency toward decrease in the last phases of reverse-ccPAS, our measure (MEPs) does not clarify the precise level at which plastic effects occur (e.g., cortico-cortical connections, M1 corticospinal neurons, or both). We did not include a control condition (i.e., a single-pulse stimulation of M1 to record unconditioned MEPs) interleaved with the protocol’s paired-stimulation, as such control stimulation could potentially interfere with ccPAS efficacy, by reducing the coherence of the repeated paired-stimulation-which is essential for STDP to occur1–7. Moreover, the bulk of available works, including ours, have limited their investigation to the left (dominant) hemisphere of right-handed participants and the malleability of the right hemisphere PMv-to-M1 pathway to ccPAS manipulation remains to be established2,11,12,14. However, prior ccPAS studies targeting interhemispheric and right-hemisphere motor and/or visual circuits have commonly reported results coherent with the notion of STDP1,15,16,46, similarly to studies testing the left hemisphere2,11,12,14; moreover, studies directly testing STDP-effects over the left and right M1 have commonly reported comparable long-term potentiation effects in the two hemispheres47.
Additionally, our study has only assessed one ISI between the two interested nodes, namely 8 ms; this specific timing was chosen based on previous results indicating it as the most effective to probe direct cortico-cortical connections between PMv and M129,30,48. Indeed, prior studies have established that the most effective ISI for driving STDP with ccPAS corresponds to the most effective ISI to probe cortico-cortical connections1–4,7–17. Although we did not investigate further ISIs, our design allows to rule out that the increase of corticomotor excitability that we observed following forward-ccPAS was due to the mere stimulation of PMv and M1, as we observed no increase in excitability in the (control) reverse-ccPAS condition. However, in a previous ccPAS study targeting the PMv-M1 circuit12, we found that a ccPAS using a longer ISI between the pulses, based on long-latency cortico-cortical connectivity49,50, is also able to induce STDP-like effects. Thus, future studies should explore the comparative efficacy of ccPAS protocols informed by different timings and assess whether personalizing the ISI to match individual connectivity patterns could maximize ccPAS efficiency51–53.
Despite these limitations, we can conclude that ccPAS over the PMv-M1 circuit induces a consistent modulation of corticomotor excitability that gradually and linearly builds up already during protocol administration, and depends on stimulation parameters (i.e., order of the paired-pulse) and interindividual differences in motor excitability. All in all, our study suggests that MEP monitoring during STDP manipulation could provide insights into the malleability of the motor system and protocol’s effectiveness, and paves the way to the possibility to adopt real-time physiological monitoring during ccPAS for optimizing individual stimulation parameters in experimental and clinical settings.
Material and methods
Participants
A total sample of 109 right-handed healthy volunteers took part in this study after providing written informed consent. All were right-handed, based on the Edinburgh Handedness Inventory, had normal or corrected-to-normal vision and were naïve to the purpose of the study. All participants gave written informed consent prior to the study and were screened to avoid adverse reactions to TMS31 and exclude individuals with neurological disorders or subject to pharmacological treatment acting on the central nervous system. The study was carried out at the Centro studi e ricerche in Neuroscienze Cognitive, University of Bologna. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and approved by the Bioethics Committee of the University of Bologna (2.6/07.12.16). None of the participants reported adverse reactions or discomfort related to TMS.
The sample was randomly divided into two groups (Fig. 1a). The first group (N = 56, 36 females, mean age ± SD: 22.6 y ± 2.6) underwent premotor-motor ‘forward-ccPAS’: on each TMS pair, a conditioning pulse over PMv was administered immediately before M1 stimulation (ISI = 8 ms), so that the first TMS pulse (PMv) would elicit a cortico-cortical volley reaching M1 slightly before the second TMS pulse (M1), resulting in convergent M1 activation-optimal for inducing STDP1–14. A second group (N = 53, 30 females, mean age ± SD: 22.8 y ± 2.7) underwent ‘reverse-ccPAS’, having each M1 stimulation followed by PMv stimulation. This control condition allows us to rule out that any observed effects of forward-ccPAS may be ascribed to the mere repeated stimulation of PMv or M1, and critically depended on the order between each pair of pulses over these two areas.
Participants in this study were tested in further sessions before and after ccPAS. Specifically, 21 participants were tested in visuomotor dexterity and choice reaction tasks (results from this study have been already published11); 40 and 48 participants were tested in two further experiments testing the effect of ccPAS on imitation and M1 intracortical excitability, respectively. All three studies reported significant and coherent after-effects; these data will be presented in separate publications addressing different and independent research questions and focusing on ccPAS aftereffect. Because the ccPAS procedure was the same across the different experiments (see below), here, we pooled data from the three experiments together to increase sample size and drawn more robust conclusions regarding MEP changes during ccPAS administration.
General experimental design
We administered ccPAS over PMv and M1; the protocol consisted of 90 pairs of TMS pulses over the two areas delivered at 0.1 Hz frequency2,11,12,14. Importantly, M1 stimulation was performed using suprathreshold TMS intensity. Thus, on each paired-stimulation we induced a motor-evoked potential (MEP) in the relaxed right first dorsal interosseous (FDI), allowing to track the emergence of changes in corticomotor excitability during protocol administration.
ccPAS
TMS was administered using two 50-mm butterfly-shaped iron-branding coils. In both forward and reverse-ccPAS protocols, we administered 90 pairs of TMS pulses at a rate of 0.1 Hz for 15 min1–3. Each participant’s rMT was assessed using the established procedure as the minimum stimulator output intensity able to induce MEPs > 50 μV in 5 out of 10 consecutive trials31. In all participants, rMT was assessed immediately before the ccPAS protocol. In the forward-ccPAS protocol a first pulse was administered over the left PMv and the second pulse was administered over the left M1 with an ISI of 8 ms, so to activate short-latency PMv-to-M1 connections20,29,30. In the reverse-ccPAS protocol, instead, the order of stimulation was reversed, with the M1 pulse always preceding the one over PMv. In both groups, the PMv pulse intensity was set to 90% of the individual’s rMT while the M1 stimulation was adjusted to evoke ~ 1 mV MEPs1–3. TMS was performed using either two independent Magstim 200 (monophasic) stimulators (in 88 participants) or a Magstim 200 stimulator for PMv stimulation and a Magstim Rapid2 (biphasic) stimulator for M1 stimulation (see Supplementary Results).
Brain localization
Coil positions were identified using established methods11,12,49,50 as detailed below. The left M1 was identified functionally as the FDI motor hotspot. To target M1, the coil was held at 45° to the sagittal midline inducing a posterior-to-anterior current direction in the brain54. The left PMv was identified using the SoftTaxic neuronavigation system (EMS, Italy). Skull landmarks (nasion, inion, and two pre-auricular points) and about 90 points providing a uniform representation of the scalp were digitized by means of the Polaris Vicra digitizer (Northern Digital INC, Ontario, CA). An individual estimated magnetic resonance image (MRI) was obtained for each subject through a 3D warping procedure fitting a high-resolution MRI template with the participant’s scalp model and craniometric points. The targeted an anterior sector of the PMv at the border with the posterior part of the inferior frontal gyrus using the following Talairach coordinates: x = − 52, y = 10, z = 24. These coordinates were obtained by averaging the coordinates reported in previous studies55–59; these studies showed that stimulating this ventral frontal site affected planning, execution and perception of hand actions, confirming the functional relevance of the PMv site. The selected PMv coordinates are consistent with those used in previous ccPAS2,13,14 and dual-site TMS studies targeting the PMv-to-M1 circuit16–48. The coil over PMv was placed at ~ 45° to the midline to induce a ventro-lateral to medio-posterior current49,50,55.
The scalp locations that corresponded best to left M1 and left PMv coordinates were identified and marked with a pen. Then, the SofTaxic Navigator system was used to estimate the projection of all targeted scalp positions on the brain surface, confirming correct coil placement for all the sites. Across the forward-ccPAS and reverse-ccPAS groups, the estimated Talairach coordinates for the left M1 were (mean ± S.D.): x = –30.6 ± 5.5, y = –17.1 ± 6.8, z = 59.6 ± 3.9; for the left PMv were: x = –53.4 ± 1.8, y = 10.1 ± 1.7, z = 23.4 ± 1.9.
Data analysis
Peak-to-peak MEP amplitudes induced by M1 stimulation in the FDI muscle were automatically extracted from EMG signals using a custom MatLab code (MathWorks, USA) and measured in mV. Trials showing EMG activity 100 ms prior to TMS were discarded from further analysis (4.7%). The 90 MEPs recorded during the ccPAS were divided into 6 epochs of 15 MEPs each, and the mean MEP amplitude in each epoch was transformed using the formula Log10(value + 1) to address lack of normality. These data were analyzed using a Protocol x Epoch ANOVA, whose results are reported in the main text.
To explore predictors of MEP changes, we first calculated a modulation index for each subject as the MEP amplitude in the last epoch divided by the MEP amplitude in the first epoch. Then, we computed robust correlations between such MEP modulation index and individual’s rMT using MatLab Toolbox60.
Supplementary Information
Author contributions
S.T.: Methodology, Software, Investigation, Data Curation, Formal analysis, Visualization, Writing - Original Draft. F.F.: Methodology, Software, Investigation, Data Curation, Writing - Review & Editing. E.C.: Methodology, Software, Investigation, Data Curation, Writing - Review & Editing. E.S.: Supervision, Writing - Review & Editing. V.R.: Supervision, Writing - Review & Editing. A.A.: Conceptualization, Formal analysis, Supervision, Project administration, Funding acquisition, Writing - Original Draft.
Funding
This work was supported by grants from the Bial Foundation [347/18], Fondazione del Monte di Bologna e Ravenna [1402bis/2021] and the Ministero dell’Istruzione, dell’Università e della Ricerca [2017N7WCLP] awarded to Alessio Avenanti.
Data availability
The data that support the findings of this study are available from the corresponding author (AA), upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-18774-9.
References
- 1.Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a hebbian mechanism. Cereb. Cortex. 2009;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- 2.Buch ER, Johnen VM, Nelissen N, O’Shea J, Rushworth MFS. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J. Neurosci. 2011;31:17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veniero D, Ponzo V, Koch G. Paired associative stimulation enforces the communication between interconnected areas. J. Neurosci. 2013;33:13773–13783. doi: 10.1523/JNEUROSCI.1777-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romei V, Thut G, Silvanto J. Information-based approaches of noninvasive transcranial brain stimulation. Trends Neurosci. 2016;39:782–795. doi: 10.1016/j.tins.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Hebb D. The Organisation of Behaviour. Wiley; 1949. [Google Scholar]
- 6.Caporale N, Dan Y. Spike timing-dependent plasticity: A Hebbian learning rule. Annu. Rev. Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 7.Koch G. Cortico-cortical connectivity: The road from basic neurophysiological interactions to therapeutic applications. Exp. Brain Res. 2020;238:1677–1684. doi: 10.1007/s00221-020-05844-5. [DOI] [PubMed] [Google Scholar]
- 8.Momi D, Neri F, Coiro G, Smeralda C, Veniero D, Sprugnoli G, Santarnecchi E. Cognitive enhancement via network-targeted cortico-cortical associative brain stimulation. Cereb. Cortex. 2020;30:1516–1527. doi: 10.1093/cercor/bhz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiappini E, Silvanto J, Hibbard PB, Avenanti A, Romei V. Strengthening functionally specific neural pathways with transcranial brain stimulation. Curr. Biol. 2018;28:R735–R736. doi: 10.1016/j.cub.2018.05.083. [DOI] [PubMed] [Google Scholar]
- 10.Romei V, Chiappini E, Hibbard PB, Avenanti A. Empowering reentrant projections from V5 to V1 boosts sensitivity to motion. Curr. Biol. 2016;26:2155–2160. doi: 10.1016/j.cub.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Fiori F, Chiappini E, Avenanti A. Enhanced action performance following TMS manipulation of associative plasticity in ventral premotor-motor pathway. Neuroimage. 2018;183:847–858. doi: 10.1016/j.neuroimage.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Chiappini E, Borgomaneri S, Marangon M, Turrini S, Romei V, Avenanti A. Driving associative plasticity in premotor-motor connections through a novel paired associative stimulation based on long-latency cortico-cortical interactions. Brain Stimul. 2020;13:1461–1463. doi: 10.1016/j.brs.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Santarnecchi E, Momi D, Sprugnoli G, Neri F, Pascual-Leone A, Rossi A, Rossi S. Modulation of network-to-network connectivity via spike-timing-dependent noninvasive brain stimulation. Hum. Brain Map. 2018;39:4870–4883. doi: 10.1002/hbm.24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnen VM, Neubert FX, Buch ER, Verhagen L, O’Reilly JX, Mars RB, Rushworth MF. Causal manipulation of functional connectivity in a specific neural pathway during behaviour and at rest. Elife. 2015;4:e04585. doi: 10.7554/eLife.04585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koganemaru S, Mima T, Nakatsuka M, Ueki Y, Fukuyama H, Domen K. Human motor associative plasticity induced by paired bihemispheric stimulation. J. Physiol. 2009;587:4629–4644. doi: 10.1113/jphysiol.2009.174342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiappini, E., Sel, A., Hibbard, P., Avenanti, A., & Romei. Increasing interhemispheric connectivity between human visual motion areas uncovers asymmetric sensitivity to horizontal motion. Curr. Biol., In press. [DOI] [PubMed]
- 17.Di Luzio P, Tarasi L, Silvanto J, Avenanti A, Romei V. Human perceptual and metacognitive decision-making rely on distinct brain networks. PLoS Biol. 2022;20:e3001750. doi: 10.1371/journal.pbio.3001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller-Dahlhaus JFM, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp. Brain Res. 2008;187:467–475. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- 19.Castiello U. The neuroscience of grasping. Nat. Rev. Neurosci. 2005;6:726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- 20.Davare M, Kraskov A, Rothwell JC, Lemon RN. Interactions between areas of the cortical grasping network. Curr. Opin. Neurobiol. 2011;21:565–570. doi: 10.1016/j.conb.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brass M, Ruby P, Spengler S. Inhibition of imitative behaviour and social cognition. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2359–2367. doi: 10.1098/rstb.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr. Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 25.Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others’ action. Curr. Opin. Neurobiol. 2005;15:213–218. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/S0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- 27.Vitale F, Padrón I, Avenanti A, De Vega M. Enhancing motor brain activity improves memory for action language: A tDCS study. Cereb. Cortex. 2021;31:1569–1581. doi: 10.1093/cercor/bhaa309. [DOI] [PubMed] [Google Scholar]
- 28.Vitale F, Monti I, Padrón I, Avenanti A, de Vega M. The neural inhibition network is causally involved in the disembodiment effect of linguistic negation. Cortex. 2022;147:72–82. doi: 10.1016/j.cortex.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–1057. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davare M, Rothwell JC, Lemon RN. Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr. Biol. 2010;20:176–181. doi: 10.1016/j.cub.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Peinemann A, Reimer B, Löer C, Quartarone A, Münchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004;115:1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: A randomized trial. Neurology. 2012;78:256–264. doi: 10.1212/WNL.0b013e3182436558. [DOI] [PubMed] [Google Scholar]
- 35.Schulze L, Feffer K, Lozano C, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul. 2018;11:327–336. doi: 10.1016/j.brs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Valero-Cabré A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur. J. Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- 37.Müller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur. J. Neurosci. 2007;25:3461–3468. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- 38.Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: Reversal of theta burst after-effect with prolonged stimulation. Exp. Brain Res. 2010;204:181–187. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suppa A, Quartarone A, Siebner H, Chen R, Di Lazzaro V, Del Giudice P, Classen J. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clin. Neurophysiol. 2017;128:2140–2164. doi: 10.1016/j.clinph.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valchev N, Tidoni E, Hamilton AFDC, Gazzola V, Avenanti A. Primary somatosensory cortex necessary for the perception of weight from other people’s action: A continuous theta-burst TMS experiment. Neuroimage. 2017;152:195–206. doi: 10.1016/j.neuroimage.2017.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paracampo R, Pirruccio M, Costa M, Borgomaneri S, Avenanti A. Visual, sensorimotor and cognitive routes to understanding others’ enjoyment: An individual differences rTMS approach to empathic accuracy. Neuropsychologia. 2018;116:86–98. doi: 10.1016/j.neuropsychologia.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Jones CB, Lulic T, Bailey AZ, Mackenzie TN, Mi YQ, Tommerdahl M, Nelson AJ. Metaplasticity in human primary somatosensory cortex: Effects on physiology and tactile perception. J. Neurophysiol. 2016;115:2681–2691. doi: 10.1152/jn.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minkova L, Peter J, Abdulkadir A, Schumacher LV, Kaller CP, Nissen C, Lahr J. Determinants of inter-individual variability in corticomotor excitability induced by paired associative stimulation. Front. Neurosci. 2019;13:841. doi: 10.3389/fnins.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability An rTMS study in humans. Clin. Neurophysiol. 2014;115:1063–1068. doi: 10.1016/j.clinph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Nord CL, Popa T, Smith E, Hannah R, Doñamayor N, Weidacker K, Voon V. The effect of frontoparietal paired associative stimulation on decision-making and working memory. Cortex. 2019;117:266–276. doi: 10.1016/j.cortex.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridding MC, Flavel SC. Induction of plasticity in the dominant and non-dominant motor cortices of humans. Exp. Brain Res. 2006;171:551–557. doi: 10.1007/s00221-005-0309-2. [DOI] [PubMed] [Google Scholar]
- 48.Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J. Physiol. 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiori F, Chiappini E, Soriano M, Paracampo R, Romei V, Borgomaneri S, Avenanti A. Long-latency modulation of motor cortex excitability by ipsilateral posterior inferior frontal gyrus and pre-supplementary motor area. Sci. Rep. 2016;6:38396. doi: 10.1038/srep38396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiori F, Chiappini E, Candidi M, Romei V, Borgomaneri S, Avenanti A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: a dual site TMS study. Sci. Rep. 2017;7:14936. doi: 10.1038/s41598-017-13708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carson RG, Kennedy NC. Modulation of human corticospinal excitability by paired associative stimulation. Front. Hum. Neurosci. 2013;7:823. doi: 10.3389/fnhum.2013.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: A pharmaco-TMS study. Neuropsychopharmacology. 2011;36:1894–1902. doi: 10.1038/npp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: A transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin. Neurophysiol. 2001;112:250–258. doi: 10.1016/S1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 55.Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J. Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dafotakis M, Sparing R, Eickhoff SB, Fink GR, Nowak DA. On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res. 2008;1228:73–80. doi: 10.1016/j.brainres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 57.Jacquet PO, Avenanti A. Perturbing the action observation network during perception and categorization of actions’ goals and grips: State-dependency and virtual lesion TMS effects. Cereb. Cortex. 2015;25:598–608. doi: 10.1093/cercor/bht242. [DOI] [PubMed] [Google Scholar]
- 58.Avenanti A, Annela L, Serino A. Suppression of premotor cortex disrupts motor coding of peripersonal space. Neuroimage. 2012;63:281–288. doi: 10.1016/j.neuroimage.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 59.Avenanti A, Paracampo R, Annella L, Tidoni E, Aglioti SM. Boosting and decreasing action prediction abilities through excitatory and inhibitory tDCS of inferior frontal cortex. Cereb. Cortex. 2018;28:1282–1296. doi: 10.1093/cercor/bhx041. [DOI] [PubMed] [Google Scholar]
- 60.Pernet CR, Wilcox R, Rousselet GA. Robust correlation analyses: false positive and power validation using a new open source Matlab toolbox. Front. Psychol. 2013;3:606. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (AA), upon request.