Abstract
Saccharomyces cerevisiae, along with other eukaryotes, is resistant to tetracyclines. We found that deletion of SOD1 (encoding Cu/Zn superoxide dismutase) rendered S. cerevisiae hypersensitive to oxytetracycline (OTC): a sod1Δ mutant exhibited a >95% reduction in colony-forming ability at an OTC concentration of 20 μg ml−1, whereas concentrations of up to 1,000 μg ml−1 had no effect on the growth of the wild type. OTC resistance was restored in the sod1Δ mutant by complementation with wild-type SOD1. The effect of OTC appeared to be cytotoxic and was not evident in a ctt1Δ (cytosolic catalase) mutant or in the presence of tetracycline. SOD1 transcription was not induced by OTC, suggesting that constitutive SOD1 expression is sufficient for wild-type OTC resistance. OTC uptake levels in wild-type and sod1Δ strains were similar. However, lipid peroxidation and protein oxidation were both enhanced during exposure of the sod1Δ mutant, but not the wild type, to OTC. We propose that Sod1p protects S. cerevisiae against a mode of OTC action that is dependent on oxidative damage.
Reactive oxygen species (ROS) are generated during normal cellular respiratory metabolism, but their damaging effects are generally suppressed by antioxidant defenses. Protective enzymes operating in the budding yeast Saccharomyces cerevisiae are well characterized (30); these include superoxide dismutases (SODs) and catalases, which specifically protect against O2·− and H2O2, respectively. S. cerevisiae mutants lacking the principal cellular SOD (Cu/Zn SOD; encoded by SOD1) display certain aerobic growth defects, e.g., reduced growth rate, and methionine and lysine auxotrophies (4). Moreover, sod1Δ strains are hypersensitive to several types of stress, including oxidative stress (16), metal toxicity (6, 36), prolonged stationary incubation (24), and freeze-thaw stress (28). Such evidence has underscored the central role of ROS in mediating various stresses. However, there is presently no evidence to suggest that antioxidant defenses play a role in the insensitivity of eukaryotes, such as S. cerevisiae, to the action of prokaryote-specific antibiotics.
The tetracyclines (e.g., tetracycline, doxycycline, and oxytetracycline [OTC]) are classic broad-spectrum bacteriostatic antibiotics. They are commonly thought to act by inhibiting protein synthesis, through inhibition of binding by aminoacyl-tRNA to the ribosomal A site (22). Although binding of the tetracyclines to eukaryotic ribosomes occurs in vitro, the in vivo insensitivity of eukaryotes to these antibiotics is usually considered a reflection of the inaccessibility of tetracyclines to the eukaryotic intracellular environment (19); genes conferring OTC resistance to prokaryotes generally encode OTC export proteins (18, 32). The generality of fungal tetracycline resistance is exemplified by the use of OTC in fungiselective growth media (Difco manual, Difco, Detroit, Mich.) and the opportunistic yeast infections that commonly arise following tetracycline administration to humans (22).
Inhibition of protein synthesis as the key mode of tetracycline action has not been confirmed (31). Among other suggested mechanisms, ROS generation, as indicated by a few reports, may be increased in the presence of certain tetracyclines, e.g., OTC (21, 29). However, a role for ROS in the mechanism of OTC action has yet to be clearly established. In this report, we show that the absence of a functional SOD1 gene renders S. cerevisiae hypersensitive to OTC. The results reveal a novel role for eukaryotic SODs and shed new light on the mode of OTC action.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
S. cerevisiae S150-2B (MATa leu2-3,112 ura3-52 trp1-289 his3-Δ1) was the parental background from which the isogenic mutants DJY122 (sod1Δ::TRP1) and DJY145 (ctt1Δ::TRP1) were derived (16). Organisms were routinely maintained on yeast extract-peptone-dextrose (YEPD) agar (2). Appropriate phenotypes (e.g., methionine and lysine auxotrophies in DJY122) were confirmed during experimentation. For experimental purposes, cultures were grown in liquid YEPD, as described previously (3). Where specified (see Fig. 1B), plasmids pVC734 (6) and YEp600 (27), which both harbor functional SOD1 sequences, were transformed into lithium acetate-treated DJY122 (11) and maintained by selection.
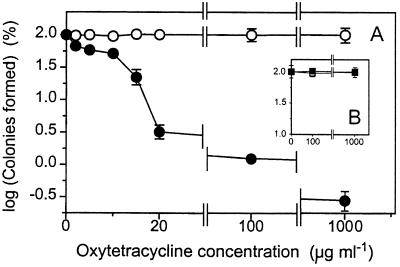
FIG. 1.
Sensitivity of S. cerevisiae DJY122 (sod1Δ) to OTC. (A) Exponential-phase S. cerevisiae S150-2B (wild type) (○) and DJY122 (sod1Δ) (●) cultures were plated on YEPD agar supplemented with OTC. (B) OTC resistance was restored to DJY122 by complementation with plasmids pVC734 (centromeric; □) and YEp600 (multicopy; ■) bearing wild-type SOD1 sequences. CFUs determined after 5 days are expressed as percentages of values obtained in the absence of OTC. Shown are means from two sets of triplicate determinations from independent experiments ± standard errors of the means (n = 6) where these values exceed the dimensions of the symbols.
Growth and viability determination.
The influence of OTC on growth was initially assessed as colony-forming ability on solid YEPD medium supplemented with the desired concentration of OTC hydrochloride (Sigma) supplied from a filter-sterilized stock solution. Colonies were enumerated after 5 days of incubation at 25°C (incubation for >5 days yielded no further CFUs. To examine the effect of OTC on viability during growth in YEPD broth, exponential-phase DJY122 cells were inoculated to a density of approximately 5 × 105 cells ml−1 in liquid YEPD either containing or lacking OTC (100 μg ml−1). CFUs were determined at intervals by plating DJY122 cultures on YEPD agar lacking OTC. To determine whether growth inhibition in OTC-containing solid medium was cytostatic or cytotoxic, cultures were replica plated to fresh YEPD agar, either not supplemented or supplemented with OTC (the latter served as a control to determine transfer efficiency). All solutions and media containing OTC were maintained in the dark to eliminate photochemical ROS generation (21).
SOD1 expression.
Green fluorescent protein (Gfp) was used as a reporter of SOD1 expression. The fluorescence-activated cell sorter- and yeast-optimized Gfp open reading frame (yEGFP3) from pYGFP3 (5) was tagged with AscI and PacI sites by PCR and inserted in place of the wild-type GFP sequence in pFA6a-GFPMT-HIS3MX6 (35), creating pSVA12 (all primer sequences are available on request). Sequence fidelity of PCR products was routinely confirmed with an ABI 377 automated DNA sequencer. A 600-bp SOD1 promoter fragment was amplified from yeast genomic DNA and inserted between the BamHI and PacI sites of pSVA12, creating a transcriptional fusion with yEGFP3. To integrate this construct into the yeast genome (at the nonessential HO locus), the SOD1-yEGFP3-HIS3MX6 module was amplified from pSVA12 with Vent DNA polymerase (New England Biolabs) by short flanking homology PCR (35). Flanking sequences targeted the PCR product to HO. After transformation of the product into S. cerevisiae S150-2B and DJY122 (11), His+ colonies were selected and appropriate integration of the module was confirmed by PCR. Quantitative determination of cellular Gfp production was performed with a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 15-mW, 488-nm argon ion laser. Mean green fluorescence (FL1) values were corrected for the minor contribution of autofluorescence, determined with non-GFP-containing S. cerevisiae.
OTC uptake.
Exponential-phase cultures were supplemented with OTC at 100 μg ml−1. Samples were removed at intervals, and cells were separated by microcentrifugation. Supernatants were retained at 4°C until analysis. OTC in the supernatants was determined as described previously (17). Briefly, 100 μl of sample was mixed with 0.9 ml of a reaction solution, giving final concentrations of the reaction components of 5 mM Na2MoO4 · 2H2O, 100 mM NaNO3, and 10 mM sodium acetate buffer. After 5 min, OTC was determined spectrophotometrically from A404, with reference to a standard curve prepared from OTC solutions of known concentrations. Cellular OTC accumulation was calculated by subtraction from OTC determinations for control incubation mixtures lacking cells.
Measurement of oxidative damage.
For lipid peroxidation determinations, late-exponential-phase cells were harvested by centrifugation (1,200 × g; 8 min), washed twice, and suspended in 10 mM MES (morpholineethanesulfonic acid) buffer (Sigma), pH 5.5, supplemented with 1% (wt/vol) glucose. After 10 min of equilibration with shaking (120 rpm), OTC was added to a final concentration of 100 μg ml−1 where specified (see Fig. 4). Lipid peroxidation was assessed at intervals with the ferric thiocyanate assay for lipid hydroperoxides (25). The assay was performed as described previously (25) but with the following modifications: 1 volume of metaphosphoric acid-saturated methanol was added to samples prior to cell breakage with glass beads (0.5-mm diameter) by using a mini-bead-beater (Biospec Products). After the homogenate had been mixed with 1 volume of chloroform and then centrifuged (12,000 × g; 1 min), the lower chloroform layer (500 μl) was removed and mixed with 450 μl of a solution composed of chloroform-methanol (2:1 [vol/vol]). Chromogen solution (50 μl), a mixture of equal volumes of 3% methanolic KSCN, and 4.5 mM FeSO4 · 7H2O in 0.2 M HCl was added to each sample. Absorbance at 500 nm was determined after 5 min, and lipid hydroperoxide content was calculated by reference to a standard curve prepared with 13-hydroperoxy-octadecadienoic acid (Cayman Chemical). Control samples in which hydroperoxides were reduced by triphenylphosphine addition showed that non-hydroperoxide-generated color in the assay was insignificant.
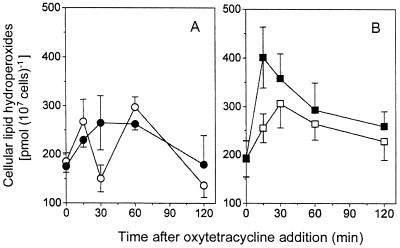
FIG. 4.
OTC-induced lipid peroxidation. Exponential-phase S. cerevisiae S150-2B (A) and DJY122 (B) cultures were incubated in MES buffer (pH 5.5)–1% (vol/vol) glucose in either the absence (open symbols) or presence (solid symbols) of OTC (100 μg ml−1). Shown are mean values for lipid peroxidation from two sets of triplicate determinations from independent experiments ± standard errors of the means (n = 6).
Protein oxidation was determined as protein carbonyl content with an Oxyblot (Oncor) kit. Protein extracts were prepared with standard procedures (2) from cells exposed to OTC in YEPD medium. Carbonyl groups were derivatized with 2,4-dinitrophenyhydrazine (DNPH) according to the manufacturer's (Oncor) protocol. Proteins (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) with a Bio-Rad mini-PROTEAN II system and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Blots were probed with rabbit anti-2,4-dinitrophenyl antibody (Oncor; 1:150 dilution) and anti-rabbit alkaline phosphatase-conjugated immunoglobulin G antibody (Promega; 1:3,000). Oxidized proteins were detected using the BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium system (Promega). Blots were analyzed quantitatively by densitometry with LabWorks (Ultraviolet Products) software. Control samples that were not derivatized with DNPH were confirmed to exhibit no anti-DNP reactivity.
RESULTS AND DISCUSSION
Absence of functional Cu/Zn SOD renders S. cerevisiae hypersensitive to OTC.
The OTC concentration generally used in yeast-selective media (100 μg ml−1) (Difco manual, Difco) was confirmed to have no inhibitory effect on the colony-forming ability of wild-type S. cerevisiae S150-2B (Fig. 1A). Even at 1,000 μg ml−1 (an OTC concentration approaching the antibiotic's solubility limit in YEPD), no decline in CFUs was evident. In contrast, DJY122 (sod1Δ) exhibited a very sharp decline in colony formation as OTC concentrations were increased from 0 to 20 μg ml−1. Thus, approximately 50 and 97% reductions in CFUs were evident at 10 and 20 μg ml−1 OTC, respectively (Fig. 1A). Greater than 98% inhibition of colony formation by DJY122 was evident with further increases in OTC concentration up to 100 and 1,000 μg ml−1. The persistence of a small number of cells at these high concentrations is similar to what was previously observed with bacteria (19). We subcultured six of the resistant isolates and found that their OTC resistance was inheritable, suggesting that the cells either had undergone reversion events or had acquired secondary suppressor mutations (23). To eliminate the possibility that a secondary mutation (nonsuppressor) might be responsible for the OTC-sensitive phenotype of DJY122, we complemented the sod1 mutation. Introduction of functional SOD1 to DJY122, on either a centromeric or a multicopy plasmid, fully restored OTC resistance to the yeast (Fig. 1B). Thus, the OTC sensitivity of the mutant was attributable specifically to the absence of SOD1.
The differential effects against the wild-type and the sod1Δ strains were specific for OTC, as tetracycline (at 100 μg ml−1) exerted no inhibition of colony formation by either strain (data not shown). This result was unexpected, as certain previous evidence indicates that tetracycline also has the potential to promote ROS formation, albeit in the presence of metal salts (29). The single additional OH group of OTC must be critical in eliciting the hypersensitivity of DJY122.
To test whether the dependence of OTC resistance on the possession of a functional SOD1 gene reflected a general dependence on protection against ROS, we also examined a deletion mutant defective in cytosolic catalase (Ctt1) activity. S. cerevisiae DJY145 showed no reduction in colony-forming ability at an OTC concentration of up to 100 μg ml−1, the highest concentration tested (data not shown). This suggested that OTC has the potential to inhibit the growth of S. cerevisiae by a mechanism that depends specifically on O2·−.
OTC does not induce SOD1 transcription.
Expression of SOD1 in yeast is regulated primarily at the transcriptional level (12). To test whether constitutive SOD1 expression was sufficient for the OTC resistance of S150-2B, we monitored transcription from the SOD1 promoter before and after OTC (100 μg ml−1) addition. We confirmed appropriate function in the constructed SOD1-GFP reporter fusion by determining cellular green fluorescence, which was approximately 55-fold greater (before induction) than autofluorescence and which was inducible with copper (10). In contrast to copper, OTC did not influence expression from the SOD1 promoter. Thus, the average cellular green fluorescence remained approximately constant for up to 3 h following OTC exposure (increased Gfp was evident within 30 min of Cu addition) (data not shown). We obtained similar results when the construct was introduced into DJY122. In the latter case, constitutive Gfp levels were approximately 2.3-fold higher than those in the wild type, which could be a reflection of elevated O2·− in the sod1Δ strain. The results indicate that SOD1 transcription is not induced by OTC and that constitutive SOD1 transcription is sufficient for the OTC resistance of wild-type S. cerevisiae.
OTC uptake is not influenced by deletion of SOD1.
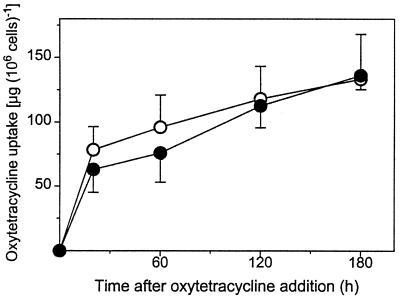
One proposed reason for the OTC resistance of eukaryotes is their ability to exclude the antibiotic (19). To test whether the hypersensitivity of DJY122 could be related to diminished OTC exclusion, e.g., as a result of enhanced constitutive oxidation of plasma membrane lipids, we compared OTC uptake in S150-2B with that in DJY122. Cells exhibited a rapid initial phase of OTC uptake followed by a slower, approximately linear accumulation (Fig. 2). Maximally, only approximately 5% of the OTC supplied (100 μg ml−1) was removed by the cells within 3 h of OTC exposure. Moreover, OTC uptake by DJY122 was no greater than that by S150-2B. Thus, approximately 78 and 63 μg of OTC (106 cells)−1 were accumulated within 20 min by S150-2B and DJY122, respectively, and approximately 135 μg of OTC (106 cells)−1 was accumulated by both cell types after 3 h (Fig. 2). Therefore, the OTC sensitivity of DJY122 does not appear to be attributable to a decreased ability to exclude the antibiotic.
FIG. 2.
OTC uptake by S. cerevisiae. Exponential-phase cultures of S. cerevisiae S150-2B (○) and DJY122 (●) in YEPD medium were supplemented with OTC at 100 μg ml−1. Uptake was calculated from residual OTC concentrations in the medium and by reference to control incubation mixtures lacking cells. Shown are means from sextuplet determinations ± standard errors of the means. Typical results from one of three independent experiments are shown.
Cytotoxic effect of OTC against S. cerevisiae DJY122.
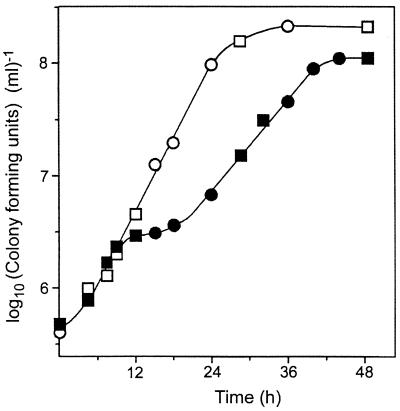
Since the action of OTC as a protein synthesis inhibitor is cytostatic and can be reversed by removal of the antibiotic (19), we tested whether inhibition of the sod1Δ mutant by OTC also occurred by a cytostatic mechanism. First, we determined the strain's ability to form colonies after removal from OTC-containing YEPD broth. In the absence of OTC, an exponential rise in CFUs was evident over the initial 24 h of incubation (Fig. 3). A calculated generation time of approximately 3 h was consistent with the slow aerobic growth rate expected of sod1Δ mutants (12). In the presence of OTC, viable cell numbers increased normally until around 10 h, when there was a slowing of growth. Appreciable increases in viable numbers again were evident by 24 h. However, the subsequent growth rate was slower than that prior to 10 h and than that of cells incubated in the absence of OTC (Fig. 3). Although no reduction in viable cell number was evident at any stage, we hypothesized that outgrowth of OTC-resistant subpopulations (Fig. 1A) could be masking a cytotoxic effect of OTC at around 10 h. Therefore, to provide a more rigorous test, we examined the survival of isolated cells on OTC-supplemented solid medium. Our rationale was that since approximately three or four rounds of cell division occurred before inhibition by OTC became evident (Fig. 3), the minicolonies that would form on OTC-containing agar during this period should be transferable (albeit with limited efficiency) by replica plating. We tested whether such minicolonies could be resuscitated by replica plating to fresh YEPD agar lacking OTC. Additional cultures were also replica plated to YEPD with OTC at each sampling time to determine transfer efficiency. At all of the sampling times tested (14, 24, 36, and 48 h after initial plating on OTC), there was no significant difference in the numbers of CFUs transferred to replica plates containing or lacking OTC (data not shown); control experiments with the wild type confirmed that CFUs were transferable after only 9 h of growth on YEPD agar. The results indicated that cells of DJY122 that are inhibited by OTC cannot be resuscitated after 14 h. This suggests that OTC exerts a cytotoxic action against DJY122, although we have not ruled out the possibility that DJY122 might retain OTC more effectively than S150-2B on dilution and plating, as has been reported for paraquat in Escherichia coli B and K12 (20). A cytotoxic action of OTC would contrast with the antibiotic's cytostatic action as a protein synthesis inhibitor (19). Yeast cells maintain their viability for at least 24 h in the presence of other protein synthesis inhibitors (D. G. Ahearn [Georgia State University], personal communication). Our results are more in keeping with a model in which the killing of DJY122 by OTC is oxidative.
FIG. 3.
Effect of OTC on the growth of S. cerevisiae DJY122 in YEPD broth. Cells were incubated in YEPD in either the absence (○, □) or presence (●, ■) of OTC (100 μg ml−1) (circles and squares distinguish staggered cultures). Colony-forming ability was determined at intervals by plating samples on YEPD agar lacking OTC. Typical results from one of six independent experiments are shown. Points represent means from triplicate determinations. Standard errors of the means were smaller than the dimensions of the symbols.
OTC induces lipid peroxidation in S. cerevisiae DJY122.
As lipid peroxidation is a major consequence and useful marker of ROS-mediated oxidative damage (8, 15), we tested whether lipid peroxidation in OTC-treated S. cerevisiae was enhanced. There was a negligible difference between the background lipid-hydroperoxide content of the wild type and that of sod1Δ strains prior to OTC exposure (Fig. 4), implying that Sod1p is not required for suppression of lipid peroxidation under nonstressed conditions. As lipid peroxidation would be the most likely cause of altered membrane permeability in a ROS-sensitive yeast (15), the results are consistent with the uptake data (Fig. 2), i.e., the OTC sensitivity of DJY122 is not due to a compromised ability to exclude the antibiotic. During the experimental time course, some changes in lipid peroxidation occurred independently of OTC addition. Thus, a general rise in lipid hydroperoxide content from approximately 180 pmol (107 cells)−1 at time zero to between 260 and 300 pmol (107 cells)−1 after 30 min or 1 h occurred during incubation of S150-2B in the absence or presence of OTC (Fig. 4A), and during incubation of DJY122 in the absence of OTC (Fig. 4B). Such a rise in “background” lipid peroxidation (i.e., in the absence of a putative stressor) may be a consequence of transfer from oxygen-depleted growth medium to air-saturated buffer. The maximum lipid hydroperoxide content observed in S. cerevisiae S150-2B was no greater in the presence of OTC than in its absence (although the latter values were more variable [Fig. 4A]), indicating that OTC did not induce lipid peroxidation in this strain. In contrast, lipid peroxidation was rapidly induced following exposure of the sod1Δ mutant to OTC (Fig. 4B). Thus, the cellular lipid hydroperoxide content of DJY122 increased by approximately 210 pmol (107 cells)−1 after 15 min of incubation in the presence of OTC, but only by approximately 60 pmol (107 cells)−1 in the absence of OTC. A subsequent decline in lipid peroxidation was particularly evident in OTC-exposed cells, and after 2 h the level of lipid peroxidation in DJY122 incubated in the presence of the antibiotic was only slightly greater than that in DJY122 incubated in its absence (Fig. 4B). It is stressed that a transient elevation of lipid peroxidation is sufficient for eventual yeast killing (15). The general decline in lipid hydroperoxide content evident in all cells at between 1 and 2 h of incubation could reflect induction of antioxidant defense and/or repair systems (15, 30). Alternatively, and considering that growth was not affected until ∼10 h (see above), this decline may simply reflect breakdown of lipid hydroperoxides to more-reactive downstream intermediates in the oxyradical cascade (14).
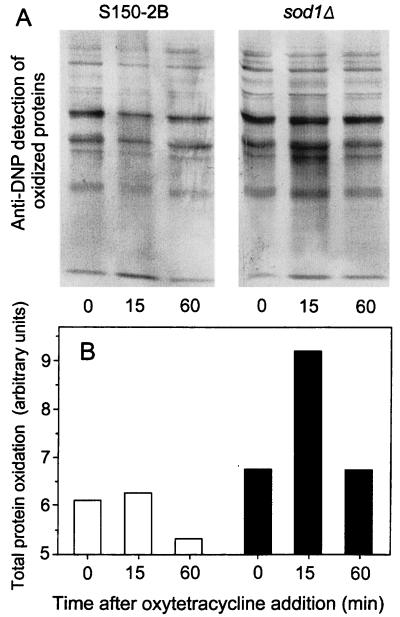
OTC induces protein oxidation in S. cerevisiae DJY122.
To support further our conclusions from the lipid peroxidation data, we measured protein oxidation as an alternative index of oxidative damage. The anti-DNP reactivity (carbonyl content) of total proteins extracted from DJY122 was increased by approximately 1.4-fold within 15 min of OTC exposure (Fig. 5). Similar to the situation observed with lipid peroxidation (see above), the protein carbonyl content had declined and returned to the preexposure level after 60 min. This decline probably did not reflect repair since oxidatively damaged proteins are more readily degraded than repaired (13, 33). Moreover, the only known oxidized-protein repair enzyme (peptide methionine sulfoxide reductase) is highly specific in its action and does not affect carbonyl lesions (26). Thus, degradation may mask any continued oxidative protein damage beyond 1 h of OTC exposure. In contrast to DJY122 cells, wild-type cells in YEPD showed a negligible increase in protein oxidation following OTC addition, and a slight overall decline in protein oxidation was evident during the 60-min exposure. The levels of protein oxidation prior to OTC addition were similar for both cell types (Fig. 5). This observation also was in agreement with the lipid peroxidation data (Fig. 4). The results further strengthen our view that the OTC hypersensitivity of S. cerevisiae DJY122 is attributable to an inability of this mutant to cope with OTC-induced oxidative stress. Consistent with data obtained from other systems (9, 15), the elevation of lipid and protein oxidation in the sod1Δ mutant (relative to that for the wild type) here was not proportional to the elevation in OTC-induced killing (Fig. 1). It is possible that our methods may underestimate the difference between the two strains if, despite our precautions, additional oxidation is introduced during sample preparation. However, the data do support the notion that tolerable levels of oxidative damage are limited by thresholds, beyond which extensive cell killing may ultimately occur.
FIG. 5.
OTC-induced protein oxidation. Exponential-phase cultures of S. cerevisiae S150-2B and DJY122 in YEPD medium were supplemented with OTC at 100 μg ml−1. (A) Total protein carbonyls as reflected by anti-DNP reactivities in cellular protein extracts. (B) Quantitative analysis of results shown in panel A. □, S150-2B; ■, DJY122. Typical results from one of three independent experiments are shown.
Concluding remarks.
Our results further extend the range of known stressors against which cell survival depends on antioxidant defenses and reemphasizes the crucial role of SODs in affording such protection. In addition, this is the first report to suggest that the general insensitivity of eukaryotes to OTC may be so markedly dependent on the function of a single gene. This evidence may be pertinent in the context of the hypersensitivity occasionally evident among patients administered OTC (19, 22) and the occasional sensitivity of other eukaryotic systems to this antibiotic (1, 7, 34). The results could also be of relevance to suppression of the opportunistic yeast infections that commonly follow OTC administration (22). Another note of caution is that yeast-selective media containing OTC clearly select against certain genotypes.
ACKNOWLEDGMENTS
We thank Derek Jamieson (Heriot-Watt University, United Kingdom) for kindly providing yeast strains, Valeria Culotta (Johns Hopkins University) for plasmid pVC734, Edith Gralla (University College of Los Angeles) for YEp600, Brendan Cormack (Stanford University) for pYGFP3, and Peter Philipssen (University of Basel) for pFA6a-GFPMT-HISMX6.
REFERENCES
- 1.Abdullahi S U, Adeyanju J B. Adverse reaction to oxytetracycline in a dog. Vet Human Toxicol. 1985;27:390–395. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1999. [Google Scholar]
- 3.Avery S V, Howlett N G, Radice S. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma membrane fatty acid composition. Appl Environ Microbiol. 1996;62:3960–3966. doi: 10.1128/aem.62.11.3960-3966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang E C, Kosman D J. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- 5.Cormack B P, Bertram G, Egerton M, Gow N A R, Falkow S, Brown A J P. Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology. 1997;143:303–311. doi: 10.1099/00221287-143-2-303. [DOI] [PubMed] [Google Scholar]
- 6.Culotta V C, Joh H D, Lin S-J, Slekar K H, Strain J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J Biol Chem. 1995;270:29991–29997. doi: 10.1074/jbc.270.50.29991. [DOI] [PubMed] [Google Scholar]
- 7.Dietz D D, Abdo K M, Haseman J K, Eustis S L, Huff J E. Comparative toxicity and carcinogenicity studies of tetracycline and oxytetracycline in rats and mice. Fundam Appl Toxicol. 1991;17:335–346. doi: 10.1016/0272-0590(91)90223-q. [DOI] [PubMed] [Google Scholar]
- 8.Dix T A, Aikens J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem Res Toxicol. 1993;6:2–18. doi: 10.1021/tx00031a001. [DOI] [PubMed] [Google Scholar]
- 9.Do T Q, Schultz J R, Clarke C F. Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci USA. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galiazzo F, Cirilio M R, Carri M T, Civitareale P, Marcocci L, Marmocchi F, Rotilio G. Activation and induction by copper of Cu/Zn superoxide dismutase in Saccharomyces cerevisiae: presence of an inactive proenzyme in anaerobic yeast. Eur J Biochem. 1991;196:545–549. doi: 10.1111/j.1432-1033.1991.tb15848.x. [DOI] [PubMed] [Google Scholar]
- 11.Gietz R D, Woods R A. Transformation of yeast by the lithium acetate/single-stranded carrier DNA/PEG method. In: Brown A J P, Tuite M F, editors. Methods in microbiology. Vol. 26. London, United Kingdom: Academic Press; 1998. pp. 53–66. [Google Scholar]
- 12.Gralla E B. Superoxide dismutase: studies in the yeast Saccharomyces cerevisiae. In: Scandalios J G, editor. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 495–525. [Google Scholar]
- 13.Grune T, Reinheckel T, Davies K J. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge J M C. Free radicals in biology and medicine. 2nd ed. New York, N.Y: Oxford University Press; 1989. [Google Scholar]
- 15.Howlett N G, Avery S V. Induction of lipid peroxidation during heavy-metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol. 1997;63:2971–2976. doi: 10.1128/aem.63.8.2971-2976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson D J, Rivers S L, Stephen D W. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology. 1994;140:3277–3283. doi: 10.1099/13500872-140-12-3277. [DOI] [PubMed] [Google Scholar]
- 17.Jelikic-Stankov M, Veselinovic D, Malesev D, Radovic Z. Spectrophotometric determination of oxytetracycline in pharmaceutical preparations using sodium molybdate as analytical reagent. J Pharm Biomed Anal. 1989;7:1565–1570. doi: 10.1016/0731-7085(89)80166-9. [DOI] [PubMed] [Google Scholar]
- 18.Jewell J E, Orwick J, Liu J, Miller K W. Functional importance and local environments of the cysteines in the tetracycline resistance protein encoded by pBR322. J Bacteriol. 1999;181:1680–1683. doi: 10.1128/jb.181.5.1689-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzung B G, editor. Basic and clinical pharmacology. Englewood Cliffs, N.J: Prentice-Hall; 1992. [Google Scholar]
- 20.Kitzler J W, Minakami H, Fridovich I. Effects of paraquat on Escherichia coli: differences between B and K-12 strains. J Bacteriol. 1990;172:686–690. doi: 10.1128/jb.172.2.686-690.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruk I, Lichszteld K, Michalska T. The influence of some biological antioxidants on the light emission from the oxytetracycline oxidation reaction. Toxicol Environ Chem. 1992;35:167–173. [Google Scholar]
- 22.Lancini G, Parenti F, Gallo G G. Antibiotics. A multidisciplinary approach. New York, N.Y: Plenum Press; 1995. [Google Scholar]
- 23.Liu X F, Elashvilli I, Gralla E B, Valentine J S, Lapinskas P, Culotta V C. Yeast lacking superoxide dismutase: isolation of genetic suppressors. J Biol Chem. 1992;267:18298–18302. [PubMed] [Google Scholar]
- 24.Longo V D, Gralla E B, Valentine J S. Superoxide dismutase activity is essential for stationary-phase survival in Saccharomyces cerevisiae—mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 25.Mihaljevic B, Katusin-Razem B, Razem D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radic Biol Med. 1996;21:53–63. doi: 10.1016/0891-5849(95)02224-4. [DOI] [PubMed] [Google Scholar]
- 26.Moskovitz J, Flescher E, Berlett B S, Azare J, Poston J M, Stadtman E R. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci USA. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida C R, Gralla E B, Valentine J S. Characterization of three yeast copper-zinc superoxide dismutase mutants analogous to those coded for in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 1994;91:9906–9910. doi: 10.1073/pnas.91.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J I, Grant C M, Davies M J, Dawes I W. The cytoplasmic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress—generation of free radicals during freezing and thawing. J Biol Chem. 1998;273:22921–22928. doi: 10.1074/jbc.273.36.22921. [DOI] [PubMed] [Google Scholar]
- 29.Quinlan G J, Gutteridge J M C. Hydroxyl radical generation by the tetracycline antibiotics with free radical damage to DNA, lipids and carbohydrate in the presence of iron and copper salts. Free Radic Biol Med. 1988;5:341–348. doi: 10.1016/0891-5849(88)90106-2. [DOI] [PubMed] [Google Scholar]
- 30.Santoro N, Thiele D J. Oxidative stress responses in the yeast Saccharomyces cerevisiae. In: Hohmann S, Mager W H, editors. Yeast stress responses. R. G. Austin, Tex: Landes; 1997. pp. 171–211. [Google Scholar]
- 31.Schnappinger D, Hillen W. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol. 1996;165:359–369. doi: 10.1007/s002030050339. [DOI] [PubMed] [Google Scholar]
- 32.Tauch A, Krieft S, Puhler A, Kalinowski J. The tetAB genes of the Corynebacterium striatum R-plasmid pTP10 encode an ABC transporter and confer tetracycline, oxytetracycline and oxacillin resistance in Corynebacterium glutamicum. FEMS Microbiol Lett. 1999;173:203–209. doi: 10.1111/j.1574-6968.1999.tb13503.x. [DOI] [PubMed] [Google Scholar]
- 33.Ullrich O, Sitte N, Sommerburg O, Sandig V, Davies K J A, Grune T. Influence of DNA binding on the degradation of oxidized histones by the 20S proteasome. Arch Biochem Biophys. 1999;362:211–216. doi: 10.1006/abbi.1998.1031. [DOI] [PubMed] [Google Scholar]
- 34.van den Bogert C, Lont M, Mojet M, Kroon A M. Impairment of liver regeneration during inhibition of mitochondrial protein synthesis by oxytetracycline. Biochim Biophys Acta. 1983;722:393–400. doi: 10.1016/0005-2728(83)90054-3. [DOI] [PubMed] [Google Scholar]
- 35.Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Wisnicka R, Krzepliko A, Wawryn J, Krawiec Z, Bilinski T. Protective role of superoxide dismutase in iron toxicity in yeast. Biochem Mol Biol Int. 1998;44:635–641. doi: 10.1080/15216549800201672. [DOI] [PubMed] [Google Scholar]