Abstract
Sacituzumab govitecan (SG) is an anti-Trop-2 antibody-drug conjugate with an SN-38 payload. In the ASCENT study, patients with metastatic triple-negative breast cancer (mTNBC) relapsed/refractory to ≥2 prior chemotherapy regimens (≥1 in the metastatic setting), received SG or single-agent treatment of physician’s choice (eribulin, vinorelbine, capecitabine, or gemcitabine). This ASCENT safety analysis includes the impact of age and UGT1A1 polymorphisms, which hinder SN-38 detoxification. SG demonstrated a manageable safety profile in patients with mTNBC, including those ≥65 years; neutropenia/diarrhea are key adverse events (AE). Patients with UGT1A1 *28/*28 genotype versus those with 1/*28 and *1/*1 genotypes had higher rates of grade ≥3 SG-related neutropenia (59% vs 47% and 53%), febrile neutropenia (18% vs 5% and 3%), anemia (15% vs 6% and 4%), and diarrhea (15% vs 9% and 10%), respectively. Individuals with UGT1A1 *28/*28 genotype should be monitored closely; active monitoring and routine AE management allow optimal therapeutic exposure of SG.
Subject terms: Breast cancer, Drug safety
Introduction
Triple-negative breast cancer (TNBC) comprises approximately 15% of all breast cancers and is associated with a poor prognosis1–3. Due to absent hormone and HER2 receptors, targeted strategies used in other types of breast cancer are not effective for metastatic TNBC (mTNBC), and current treatment guidelines recommend single-agent chemotherapy for patients without known biomarkers associated with targeted therapy2,4. Patients with TNBC have poorer outcomes compared with those with other breast cancer subtypes, including higher and earlier risk of relapse and shorter survival5,6. Among patients with previously treated mTNBC receiving single-agent chemotherapy, median progression-free survival (PFS) is only approximately 2–3 months, with median overall survival (OS) of approximately 8–15 months7–10. Common adverse events (AEs) with single-agent chemotherapy include hematologic AEs such as neutropenia, as well as diarrhea, nausea, and alopecia8,11. Although TNBC is more common in younger compared with older breast cancer patients, a significant number of TNBCs arise in older patients due to an aging population12,13. Approximately 20% of patients diagnosed with TNBC are ≥65 years old14,15. Furthermore, older patients are generally less fit for chemotherapy due to a greater rate of comorbidities, increased use of concomitant medications, and the potential for greater impact on health-related quality of life16,17. Due to the limited survival outcomes with single-agent chemotherapy regimens and the potential for increased AEs in older patients, there is an unmet medical need for novel targeted agents for all patients with pretreated mTNBC.
Trophoblast cell surface antigen-2 (Trop-2) is a calcium signal transducer that has been linked to poor outcomes in multiple cancer types18. Overexpression of membrane-localized Trop-2 has been associated with poor prognosis and increased tumor growth in breast cancer19,20. Trop-2 is the target of sacituzumab govitecan (SG), an antibody-drug conjugate (ADC) in which the payload of SN-38, a topoisomerase-1 inhibitor and the active metabolite of irinotecan, is conjugated with an anti-Trop-2 humanized monoclonal antibody via a proprietary hydrolyzable linker21. This allows for cytotoxic SN-38 liberation in the acidic tumor microenvironment without prerequisite internalization and subsequent enzymatic cleavage22, enabling “bystander effect” tumor cell killing23. Upon binding, its drug-antibody ratio of 7.6:1 allows the release of a high localized SN-38 concentration from SG without adversely impacting binding or pharmacokinetics21,24.
The safety and efficacy of SG was assessed in a single-arm, phase 1/2 trial in 108 patients with previously treated mTNBC, and reported an objective response rate (ORR) of 33.3% (95% confidence interval [CI], 24.6–43.1) and a median progression-free survival (PFS) of 5.5 months (95% CI, 4.1–6.3), with low rates of discontinuation23. This trial also showed that the 10 mg/kg dose had a manageable safety profile with better efficacy than lower doses24. The pivotal, international, multicenter phase 3 ASCENT (NCT02574455) trial randomized 529 patients with relapsed or refractory mTNBC to receive SG or single-agent treatment of physician’s choice (TPC; eribulin, vinorelbine, gemcitabine, or capecitabine); the clinical benefit seen was consistent with the phase 1/2 study results25. There was a significant ORR (35% vs 5%), PFS (median 5.6 vs 1.7 months; hazard ratio [HR], 0.41; p < 0.001) and overall survival (OS) (median 12.1 vs 6.7 months; HR 0.48; p < 0.001) improvement for SG relative to single-agent chemotherapy. Key SG treatment-related adverse events (TRAEs) of grade ≥3 included neutropenia (51% vs 33%), diarrhea (10% vs <1%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%). SG was subsequently granted full approval by the US Food and Drug Administration (FDA) for patients with unresectable, locally advanced or metastatic TNBC who have received ≥2 prior systemic therapies, at least one of them for metastatic disease26.
Neutropenia and diarrhea are toxicities associated with irinotecan, attributable to its active metabolite, SN-38, which is the cytotoxic payload of SG21,27. The enzyme uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) plays a key role in detoxifying SN-38 by glucuronidation24, producing a metabolite that is then eliminated from the body primarily by biliary excretion28. UGT1A1 activity is reduced in the UGT1A1 *28/*28 phenotype, which is found in approximately 10% of North American patients; this polymorphism leads to a higher risk of neutropenia and diarrhea with irinotecan therapy27–29. For this reason, FDA guidelines call for a reduction in the irinotecan starting dose in patients with colorectal cancer with known UGT1A1 *28/*28 status27,28,30, and European Society for Medical Oncology guidelines recommend UGT1A1 genotyping in patients with metastatic colorectal cancer for whom an irinotecan dose >180 mg/m2 is planned and there is a suspicion of UGT1A1 deficiency, as reflected by low conjugated bilirubin31.
As part of the continuing safety evaluation of SG, we conducted a detailed and updated safety analysis of AEs of interest, an analysis of time to onset and duration of key TRAEs, and examined TRAEs in older patients and by UGT1A1 variant status.
Results
Patient disposition and baseline characteristics
Of 529 patients randomized in ASCENT, 9 (3%) and 38 (15%) in the SG and TPC arms, respectively, did not receive treatment (Supplementary Fig. 1). The safety population consisted of 482 patients (of whom 53 had brain metastases) who received at least one dose of study treatment, including 258 in the SG arm and 224 in the TPC arm. Among patients in the safety population who were treated with TPC, 123 (55%), 28 (13%), 32 (14%), and 41 (18%) received eribulin, capecitabine, gemcitabine, and vinorelbine, respectively. At data cutoff (March 11, 2020), 17 and 0 patients remained on treatment in the SG and TPC arms, respectively. In both arms, the primary reason for discontinuation (SG vs TPC) was disease progression (86% vs 82%).
Patient baseline characteristics were well-balanced between arms (Table 1). The median age was 54 (range 27–82), the median number of prior anticancer regimens (any setting) was 4 (range 2–17), and all patients had previously received a taxane. There were 49 (19%) and 48 (21%) patients aged ≥65 years in the SG and TPC arms, respectively; of these, 8 and 13 patients were aged ≥75 years.
Table 1.
Patient demographics and disease characteristics at baseline (Safety Population).
| Characteristic | SG (n = 258) | TPC (n = 224) |
|---|---|---|
| Female, n (%) | 256 (99) | 224 (100) |
| Age | ||
| Median, years (range) | 54 (27–82) | 54 (30–81) |
| <50 years, n (%) | 92 (36) | 71 (32) |
| 50–64 years, n (%) | 117 (45) | 105 (47) |
| ≥65 years, n (%) | 49 (19) | 48 (21) |
| ECOG PS, n (%) | ||
| 0 | 117 (45) | 93 (42) |
| 1 | 141 (55) | 131 (58) |
| Race or ethnic group, n (%) | ||
| White | 211 (82) | 172 (77) |
| Black | 25 (10) | 31 (14) |
| Asian | 11 (4) | 9 (4) |
| Other | 11 (4) | 12 (5) |
| Brain metastasis at randomization, n (%) | ||
| Yes | 30 (12) | 23 (10) |
| No | 228 (88) | 201 (90) |
| Median no. prior anticancer regimens1, (range) | 4 (2–17) | 4 (2–14) |
| Prior chemotherapy regimens from randomization stratification, n (%) | ||
| 2–3 | 178 (69) | 158 (71) |
| >3 | 80 (31) | 66 (29) |
| BRCA1/2 mutation status2, n (%) | ||
| Negative | 145 (56) | 123 (55) |
| Positive | 19 (7) | 20 (9) |
| Unknown | 94 (36) | 81 (36) |
| UGT1A1 variant status3, n (%) | ||
| *1/*1 (wild type) | 113 (44) | NA |
| *1/*28 (heterozygous) | 96 (37) | NA |
| *28/*28 (homozygous) | 34 (13) | NA |
| Unknown/other | 15 (6) | NA |
| Original diagnosis of TNBC4, n (%) | ||
| Yes | 184 (71) | 156 (70) |
| No | 74 (29) | 68 (30) |
| Median time from metastatic diagnosis, mo. (range) | 17.1 (0.1–202.9) | 15.5 (−0.4–95.8) |
Assessed in the safety population.
BRCA breast cancer gene, ECOG PS Eastern Cooperative Oncology Group performance status, NA not applicable, SG sacituzumab govitecan, TNBC triple-negative breast cancer, TPC treatment of physician’s choice, UGT uridine diphosphate glucuronosyltransferase.
1Anticancer regimens refer to any prior metastatic/neoadjuvant/adjuvant/locally advanced regimens used to treat an eligible breast cancer patient, including hormonal treatment.
2Approximately 64% of patients in each arm consented and had known BRCA1/2 mutation status.
3Population of patients with known UGT1A1 variant status was 250.
4Patients on study either had TNBC at initial diagnosis or had hormone receptor-positive disease that converted to hormone-negative at time of study entry.
Safety by treatment arm
In the SG arm, median relative dose intensity was 99.7%. As reported previously, the most common TRAEs (all grades) in the SG arm were neutropenia (63%), diarrhea (59%), and nausea (57%); the most common grade ≥3 TRAEs were neutropenia (51%), leukopenia (10%), and diarrhea (10%; Supplementary Table 1)25. In the TPC arm overall, the most common TRAEs (all grades) were neutropenia (43%), fatigue (30%), and nausea (26%); most common grade ≥3 TRAEs were neutropenia (33%), leukopenia (5%), anemia (5%), and fatigue (5%). No patient vs 2 patients (1%) in the SG vs TPC arms had treatment-related grade ≥3 neuropathy. No patients experienced treatment-related interstitial lung disease of any grade with TPC. In the SG arm, there was 1 event (0.4%) of grade 3 pneumonitis complicated by prior radiation and progressive disease. This patient was a 52-year-old woman who experienced the event 14 days after her last dose of SG; it resolved 7 weeks after onset without sequalae25. SG-related grade ≥3 rash (<1%), all-grade ocular disorders (<5%), and hyperglycemia (<2%) were also infrequent. Rates of discontinuation due to TRAEs were low in both the SG (2%) and TPC (3%) arms. No treatment-related deaths were reported in the SG arm, and 1 death (neutropenic sepsis related to eribulin) was reported in the TPC arm. There were no discontinuations due to SG-related neutropenia, febrile neutropenia, or diarrhea.
An assessment of TRAEs by TPC agent (either eribulin alone or vinorelbine, capecitabine, and gemcitabine combined) showed similar key all-grade and grade ≥3 TRAEs as the TPC arm overall (Supplementary Table 1). Minor differences between the eribulin subgroup and the vinorelbine, capecitabine, and gemcitabine combined subgroup included slightly lower rates of all-grade neutropenia (39% vs 48%) and diarrhea (8% vs 17%) and grade ≥3 anemia (2% vs 8%), but higher rates of all-grade alopecia (25% vs 4%), respectively.
Neutropenia
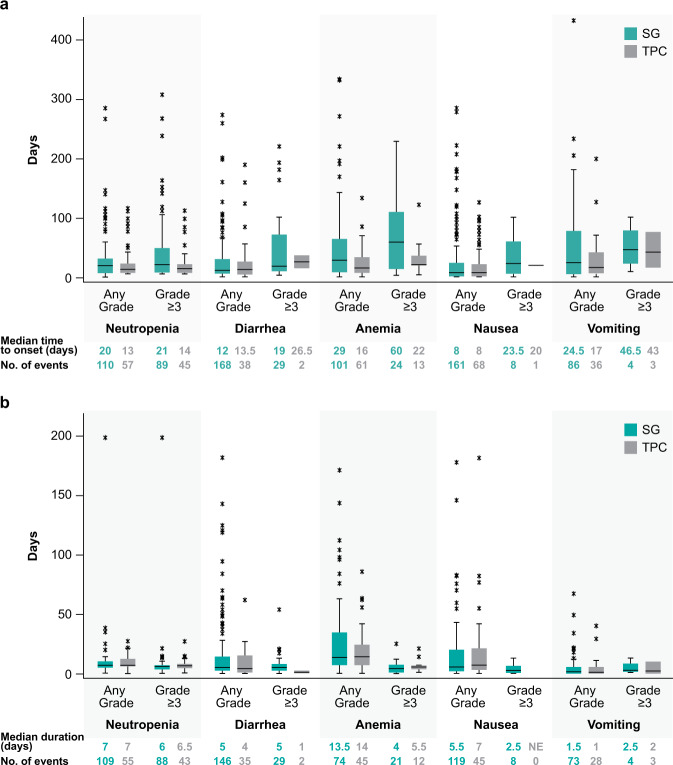
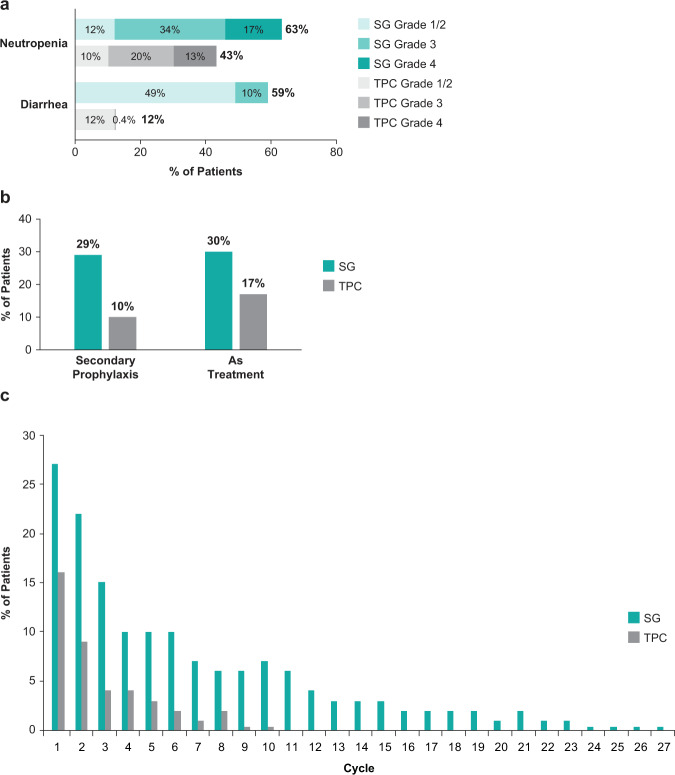
Median time to onset of the first event of treatment-related grade ≥3 neutropenia was 21 days and 14 days for the SG and TPC arms, and median duration of an individual episode of grade ≥3 neutropenia was 6.0 and 6.5 days, respectively (Fig. 1). Treatment-related neutropenia of all grades was more frequent in the SG arm compared with the TPC arm (any grade: 63% vs 43%; grade ≥3: 51% vs 33%) (Fig. 2A). In the first treatment cycle with SG vs TPC, 43% vs 33% and 28% vs 23% of patients had any grade and grade ≥3 neutropenia, respectively; 4% vs 0.4% of patients in the SG vs TPC arms had febrile neutropenia, all grade ≥3. Myeloid growth factor use was more common in the SG vs TPC arm (total use, 49% vs 23%; for secondary prophylaxis, 29% vs 10%; as treatment, 30% vs 17%; Fig. 2B), was most common in the first 3 cycles of treatment, and treatment was less common in later cycles (<10% of SG-treated patients; Fig. 2C). Dose interruptions due to treatment-related neutropenia or febrile neutropenia occurred in 46% and 21% of patients in the SG and TPC arms, respectively, whereas dose reductions for this reason occurred in 11% and 19% of patients, respectively. Treatment-related neutropenia and febrile neutropenia led to discontinuations in 0% vs 1% and 0% vs <1% of patients in the SG vs TPC arms, respectively.
Fig. 1. Time course of treatment-related adverse events of special interest.
a Time to onset of first event of treatment-related AESI and (b) duration of an individual episode of treatment-related AESI of any grade and grade ≥3 in the Safety Population. Box and whisker plots, with upper and lower boundaries of each box plot representing the 25th and 75th percentiles and the horizontal lines within the box representing median values. Whiskers extend to the last observation if it was not an outlier (defined as greater than Q3 + 1.5 × IQR or less than Q1–1.5 × IQR) or to the minimum/maximum values if an outlier was not identified. Outliers are indicated by an asterisk. AESI adverse event of special interest, IQR interquartile range, Q1 first quartile, Q3 third quartile, SG sacituzumab govitecan, TPC treatment of physician’s choice.
Fig. 2. Incidence of neutropenia and diarrhea, and G-CSF use.
a Incidence of neutropenia and diarrhea by grade, b use of G-CSF in the safety population, and c use of G-CSF by cycle in the safety population. Percent of patients using G-CSF by treatment cycle is based on the total number of patients treated in cycle 1 (SG, n = 258; TPC, n = 224). G-CSF granulocyte colony-stimulating factor, SG sacituzumab govitecan, TPC treatment of physician’s choice.
Diarrhea
Median times to onset of the first event of treatment-related grade ≥3 diarrhea were 19 days and 26.5 days for the SG and TPC arms, and median durations of an individual episode of grade ≥3 diarrhea were 5 days and 1 day, respectively (Fig. 1). Treatment-related diarrhea of all grades was more frequent in the SG arm (any grade: 59% vs 12%; grade ≥3: 10% vs 0.4%) (Fig. 2A). Grades 1 and 2 diarrheas occurred in 30% vs 7% and 19% vs 5% of SG- vs TPC-treated patients. There were no events of grade 4 or 5 diarrhea. Concomitant medicine was used for diarrhea management in 55% vs 10% of SG- vs TPC-treated patients. In the SG vs TPC arms, 55% vs 8% received loperamide and 10% vs 2% received atropine, respectively. Dose interruptions due to treatment-related diarrhea occurred in 5% of patients in the SG arm and no patients in the TPC arm, whereas dose reductions for diarrhea occurred in 5% and <1% of patients, respectively. No treatment discontinuations due to treatment-related diarrhea occurred in either arm.
Nausea and vomiting
In the SG vs TPC arms, median time to onset of the first event of any grade nausea and vomiting was 8 vs 8 days and 24.5 vs 17 days after treatment initiation, respectively (Fig. 1); however, the frequencies of grade ≥3 nausea and vomiting were low (<3%). Premedication or concomitant medication for nausea and vomiting, such as 5-HT3 antagonists (e.g., ondansetron) and receptor antagonists (e.g., prochlorperazine), was used in 86% and 63% of patients in the SG and TPC arms, respectively.
Other adverse events
Among other TRAEs, alopecia (46% vs 16%) was more common in the SG arm. Any grade (45% vs 30%) but not grade ≥3 fatigue (3% vs 5%) was also more common in the SG arm.
Safety outcomes in older patients
Patients aged ≥65 years represent approximately 20% of the ASCENT study population. Patients aged ≥65 or ≥75 years treated with SG generally had safety profiles similar to those aged <65 years, with a similar rate of treatment-emergent AEs (TEAEs; all-grade and grade ≥3) and TEAEs leading to dose reduction or treatment discontinuation, as well as a low rate of discontinuations due to AEs (Supplementary Table 2). Although patients aged ≥65 years who received SG had a slightly higher rate of all-grade and grade ≥3 TRAEs compared with those who received TPC (98% vs 83% and 63% vs 54%, respectively), TRAEs leading to dose reduction (35% vs 33%) were generally similar across treatment arms and there were no treatment-related deaths. Patients aged ≥65 years in the SG arm experienced higher rates of TRAEs leading to dose reduction compared with those aged <65 years (35% vs 19%), but patients aged ≥65 years in the TPC arm also had higher rates of TRAEs leading to dose reduction vs those aged <65 years (33% vs 23%). Key TRAEs leading to dose reduction in SG- vs TPC-treated older patients were neutropenia (10% vs 25%), fatigue/asthenia (10% vs 4%), diarrhea (6% vs 0%), febrile neutropenia (6% vs 0%), and nausea (4% vs 0%), respectively; patients aged <65 years had similar key TRAES leading to dose reductions, including neutropenia (9% vs 18%), diarrhea (4% vs 1%), fatigue/asthenia (2% vs 3%), and febrile neutropenia (2% vs 0%), respectively.
In patients aged ≥65 years, key grade ≥3 TRAEs (SG vs TPC) were neutropenia (45% vs 40%), anemia (14% vs 6%), leukopenia (10% vs 4%), diarrhea (10% vs 0%), and febrile neutropenia (8% vs 0%; Table 2), similar to the safety profile in the overall study population. SG-related all-grade ocular disorders and hyperglycemia occurred in 8% and 4% of older patients, respectively. No interstitial lung disease, grade ≥3 cardiovascular toxicity, grade ≥2 peripheral neuropathy, or grade ≥3 rash was reported with SG in older patients.
Table 2.
Treatment-related adverse events of all grades reported in >20% and of grade 3 or 4 reported in >5% of patients by age.
| TRAE1, n (%) | SG | TPC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 years (n = 209) | ≥65 years (n = 49) | <65 years (n = 176) | ≥65 years (n = 48) | |||||||||
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Hematologic | ||||||||||||
| Neutropenia2 | 134 (64) | 76 (36) | 34 (16) | 29 (59) | 12 (24) | 10 (20) | 75 (43) | 30 (17) | 25 (14) | 21 (44) | 15 (31) | 4 (8) |
| Anemia3 | 63 (30) | 13 (6) | 0 | 26 (53) | 7 (14) | 0 | 40 (23) | 8 (5) | 0 | 14 (29) | 3 (6) | 0 |
| Leukopenia4 | 33 (16) | 19 (9) | 2 (1) | 8 (16) | 4 (8) | 1 (2) | 18 (10) | 8 (5) | 2 (1) | 7 (15) | 2 (4) | 0 |
| Febrile neutropenia | 11 (5) | 10 (5) | 1 (<1) | 4 (8) | 2 (4) | 2 (4) | 5 (3) | 4 (2) | 1 (1) | 0 | 0 | 0 |
| Gastrointestinal | ||||||||||||
| Diarrhea | 121 (58) | 22 (11) | 0 | 32 (65) | 5 (10) | 0 | 20 (11) | 1 (1) | 0 | 7 (15) | 0 | 0 |
| Nausea | 123 (59) | 6 (3) | 1 (<1) | 24 (49) | 0 | 0 | 48 (27) | 0 | 0 | 11 (23) | 1 (2) | 0 |
| Constipation | 32 (15) | 0 | 0 | 12 (24) | 0 | 0 | 27 (15) | 0 | 0 | 5 (10) | 0 | 0 |
| Vomiting | 63 (30) | 2 (1) | 1 (<1) | 12 (24) | 0 | 0 | 20 (11) | 0 | 0 | 3 (6) | 1 (2) | 0 |
| Other | ||||||||||||
| Fatigue | 91 (44) | 6 (3) | 0 | 24 (49) | 2 (4) | 0 | 49 (28) | 10 (6) | 0 | 19 (40) | 2 (4) | 0 |
| Alopecia | 101 (48) | 0 | 0 | 18 (37) | 0 | 0 | 27 (15) | 0 | 0 | 8 (17) | 0 | 0 |
| Decreased appetite | 40 (19) | 2 (1) | 0 | 11 (22) | 2 (4) | 0 | 25 (14) | 1 (1) | 0 | 7 (15) | 0 | 0 |
AE adverse event, MedDRA Medical Dictionary for Regulatory Activities, NCI CTCAE National Cancer Institute Common Terminology Criteria for AE, SG sacituzumab govitecan, TPC treatment of physician’s choice, TRAE treatment-related AE.
1Patients may report more than 1 event per preferred term. AEs were coded using MedDRA v22.1, and AE severity was graded per NCI CTCAE v4.03.
2Combined preferred terms of “neutropenia” and “neutrophil count decreased.”
3Combined preferred terms of “anemia”, “hemoglobin decreased”, and “red blood cell count decreased.”
4Combined preferred terms of “leukopenia” and “white blood cell count decreased.”
Efficacy by dose reductions and interruptions
Efficacy endpoints were assessed in the primary study analysis population of brain metastases-negative patients who received at least one dose of SG (10 mg/kg) or TPC. Dose interruptions occurred in 61% and 33% of patients in the SG and TPC arms, respectively, and dose reductions occurred in 22% and 26%, respectively. In the SG arm, efficacy outcomes for patients with dose reductions or interruptions were similar to those of patients without dose reductions or interruptions. SG treatment was associated with improved ORR, clinical benefit rate (CBR), and PFS compared with TPC in patients with and without dose interruptions, and with and without dose reductions (Table 3). Median PFS was 8.3 vs 2.9 months and 4.6 vs 1.5 months in SG- vs TPC-treated patients with and without dose reductions, respectively; it was 5.7 vs 2.7 months and 4.2 vs 1.6 months in SG- vs TPC-treated patients with and without dose interruptions, respectively.
Table 3.
Efficacy outcomes for patients with dose reductions or interruptions (brain metastasis-negative population).
| Outcome | Overall BMNeg population | Dose reductions | No dose reductions | Dose interruptions | No dose interruptions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG (n = 235) | TPC (n = 233) | SG (n = 62) | TPC (n = 52) | SG (n = 173) | TPC (n = 181) | SG (n = 144) | TPC (n = 78) | SG (n = 91) | TPC (n = 155) | |
| ORR (BICR), n (%) | 82 (35) | 11 (5) | 29 (47) | 7 (13) | 53 (31) | 4 (2) | 56 (39) | 5 (6) | 26 (29) | 6 (4) |
| CBR (BICR), n (%) | 105 (45) | 20 (9) | 37 (60) | 11 (21) | 68 (39) | 9 (5) | 71 (49) | 12 (15) | 34 (37) | 8 (5) |
| Best overall response, n (%) | ||||||||||
| CR | 10 (4) | 2 (1) | 5 (8) | 1 (2) | 5 (3) | 1 (1) | 7 (5) | 0 | 3 (3) | 2 (1) |
| PR | 72 (31) | 9 (4) | 24 (39) | 6 (12) | 48 (28) | 3 (2) | 49 (34) | 5 (6) | 23 (25) | 4 (3) |
| Median PFS (BICR), mo (95% CI) | 5.6 (4.3–6.3) | 1.7 (1.5–2.6) | 8.3 (5.4–10.3) | 2.9 (2.7–4.3) | 4.6 (3.5–5.7) | 1.5 (1.4–1.7) | 5.7 (4.3–7.0) | 2.7 (1.7–3.0) | 4.2 (2.9–6.8) | 1.6 (1.5–2.2) |
Assessed in brain metastases-negative population (SG, n = 235; TPC, n = 233).
BICR blind independent central review, BMNeg brain metastases-negative, CBR clinical benefit rate, CR complete response, ORR objective response rate, PFS progression-free survival, PR partial response, SG sacituzumab govitecan, TPC treatment of physician’s choice.
Safety by UGT1A1 variant status
In the SG arm, UGT1A1 variant status was known for 250 patients (97%), of whom 113 (44%), 96 (37%), and 34 (13%) had *1/*1 (wild type; normal activity), *1/*28 (heterozygous; reduced enzymatic activity), and *28/*28 (*28 homozygous; diminished enzymatic activity) genotypes, respectively (Table 1). The median SG relative dose intensity was 99.8%, 99.5%, and 99.8%, and mean time to first dose reduction was 2.7, 2.4, and 1.8 months in the wild type, heterozygous, and homozygous groups, respectively. TEAEs leading to dose reduction occurred in 18%, 19%, and 35% of patients in the wild type, heterozygous, and homozygous groups, respectively. Patients with the *28 homozygous genotype had a slightly higher rate of grade ≥3 treatment-related neutropenia (59%) than those with heterozygous (47%) and wild-type (53%) genotypes, but a considerably higher rate of treatment-related grade ≥3 febrile neutropenia (18% vs 5% and 3%, respectively) (Table 4). Grade ≥3 treatment-related anemia (15% vs 6% and 4%, respectively) and grade ≥3 treatment-related diarrhea (15% vs 9% and 10%, respectively) were also more common in patients with the *28 homozygous genotype. Other TRAEs, including nausea, vomiting, constipation, fatigue, alopecia, and decreased appetite, were not impacted by UGT1A1 variant status. Treatment discontinuation due to TRAEs was more common in patients with the *28 homozygous genotype versus those with heterozygous and wild-type genotypes (6%, 1%, and 2%, respectively). The low frequency of the *28 homozygous polymorphism limited the ability to discern additional differences or draw any firm conclusions about differences in AEs.
Table 4.
Key treatment-related adverse events of all grades in >20% and of grade ≥3 in >5% of patients treated with sacituzumab govitecan significantly impacted by UGT1A1 genotype.
| TRAE2, n (%) | SG (n = 243)1 | |||||
|---|---|---|---|---|---|---|
| *1/*1 wild type (n = 113) | *1/*28 heterozygous (n = 96) | *28/*28 homozygous (n = 34) | ||||
| All grades | Grade ≥ 3 | All grades | Grade ≥ 3 | All grades | Grade ≥ 3 | |
| Hematologic | ||||||
| Neutropenia3 | 76 (67) | 60 (53) | 55 (57) | 45 (47) | 24 (71) | 20 (59) |
| Anemia4 | 37 (33) | 5 (4) | 29 (30) | 6 (6) | 16 (47) | 5 (15) |
| Leukopenia5 | 18 (16) | 10 (9) | 13 (14) | 9 (9) | 8 (24) | 5 (15) |
| Lymphopenia6 | 10 (9) | 1 (1) | 5 (5) | 1 (1) | 4 (12) | 2 (6) |
| Febrile Neutropenia | 3 (3) | 3 (3) | 5 (5) | 5 (5) | 6 (18) | 6 (18) |
| Thrombocytopenia7 | 3 (3) | 0 | 6 (6) | 0 | 4 (12) | 4 (12) |
| Gastrointestinal | ||||||
| Diarrhea | 65 (58) | 11 (10) | 57 (59) | 9 (9) | 21 (62) | 5 (15) |
Assessed in the safety population of patients with UGT1A1 genotype. Shown are key TRAEs substantially impacted by the UGT1A1 *28/*28 genotype. Other TRAEs including nausea, vomiting, constipation, fatigue, alopecia, and decreased appetite were not substantially impacted.
AE adverse event, NCI CTCAE National Cancer Institute Common Terminology Criteria for AE, SG sacituzumab govitecan, TRAE treatment-related AE, UGT uridine diphosphate glucuronosyltransferase.
1Seven patients had UGT1A1 genotypes not listed in the table.
2Patients may report more than 1 event per preferred term. AEs were coded using MedDRA v22.1, and AE severity was graded per NCI CTCAE v4.03.
3Combined preferred terms of “neutropenia” and “neutrophil count decreased.”
4Combined preferred terms of “anemia” and “hemoglobin decreased.”
5Combined preferred terms of “leukopenia” and “white blood cell count decreased.”
6Combined preferred terms of “lymphopenia” and “lymphocyte count decreased.”
7Combined preferred terms of “thrombocytopenia” and “decreased platelet count.”
Discussion
With results from the confirmatory phase 3 ASCENT study25, SG became the first ADC to demonstrate a significant survival improvement relative to standard single-agent chemotherapy in patients with pretreated mTNBC. Key AEs associated with SG included neutropenia and gastrointestinal toxicity, AEs expected with an SN-38 payload. The present analysis further elucidates the SG safety profile and AE management in patients with mTNBC, with special relevance to older individuals and those with UGT1A1 *28 homozygous genotype, due to the potential role of UGT1A1 genotype as a predictor of toxicity.
The safety profile of SG is consistent with previous reports23,32,33. In the overall study population (median age, 54 years; median of 4 prior anticancer regimens), TRAEs were primarily hematologic (neutropenia; grade ≥3, 51%) and gastrointestinal (diarrhea; grade ≥3, 10%). Use of supportive care medications was effective in managing these AEs, including myeloid growth factors and antipropulsive agents, and discontinuation due to toxicity was rare. Interestingly, in a comparison of this study population with the small subgroup of patients in the ASCENT trial who received SG in the second-line (2 L) metastatic setting (i.e., those who received only 1 line of therapy in the metastatic setting following recurrence ≤12 months after [neo]adjuvant chemotherapy; median of 3 prior anticancer regimens in any treatment setting, including the [neo]adjuvant setting), rates of key TRAEs were generally similar34. However, rates of SG-related all-grade alopecia were lower in the overall study population compared with that of the 2 L subgroup (Table 2; 46% vs 70%34). Because older age is a risk factor for chemotherapy toxicity, impacting treatment decisions and increasing the risk for AEs35,36, it is essential to understand the potential safety risks with SG in older patients. Irrespective of age, toxicities associated with SG were manageable, and the safety profile of SG in patients aged ≥65 and ≥75 years was generally similar to that of patients aged <65 years and the overall study population.
Of note, the observed delayed appearance of nausea and vomiting (median of 8 and 24.5 days after initiation of SG treatment, respectively) should be considered when managing treatment-associated symptoms. Patients should be provided with take-home medications for preventing and treating delayed nausea and vomiting, with clear instructions26. Antiemetics should be employed as clinically indicated, and atropine can be used to manage excessive cholinergic response26. Other AEs in the SG arm included alopecia (46%), which may affect quality of life37. Although therapeutic scalp cooling is effective for many chemotherapeutic agents, its efficacy with ADCs is unknown38,39. SG response and PFS advantage relative to TPC was maintained in patients who started at 10 mg/kg and then required SG dose reductions/interruptions to manage AEs; efficacy outcomes for patients with dose reduction or interruptions in the SG arm were similar to those for the overall study population25. In the ASCENT protocol, dose reduction/interruption recommendations for the TPC arm were based on local prescribing information or institutional standard practice, with no specific recommendations for myeloid growth factor use. A higher frequency of patients in the SG vs TPC arms received myeloid growth factors (49% vs 23%) and dose interruptions (61% vs 33%), which may have been due to heterogeneity in local guidelines for the different agents in the TPC arm; the frequency of patients with dose reductions (22% vs 26%, respectively) was similar. However, differences in the frequency of patients with dose reductions/interruptions should not affect the efficacy analysis for these groups, as there was a clinical benefit for SG in both patients with and without dose reductions/interruptions. These results support the recommended initial dosing of SG at 10 mg/kg followed by as-needed dose reductions and interruptions, over initiating treatment at a lower dose, which has the potential to compromise efficacy benefit24. Interestingly, median PFS was numerically higher in patients who received SG or TPC and had dose reductions/interruptions vs those who did not have dose reductions/interruptions. However, lack of pharmacokinetics, the small numbers of patients in these subgroup analyses, and the potential for confounding effects associated with dose reductions/interruptions limit further interpretation of these data.
Although hematologic toxicities are concerns for all the treatments administered in ASCENT, the safety profile of SG is distinct from those of eribulin, vinorelbine, capecitabine, and gemcitabine. Importantly, there were no instances of SG-related grade ≥3 neuropathy and low frequencies of SG-related interstitial lung disease (1 case of grade 3 pneumonitis, <1%), grade ≥3 rash (<1%), all-grade ocular disorders (<5%), and hyperglycemia (<2%); no SG-related deaths occurred. In comparison, peripheral neuropathy is a safety concern for eribulin, with 8% of patients who received single-agent eribulin in a metastatic breast cancer trial experiencing grade ≥3 events; peripheral neuropathy was also the most common adverse reaction resulting in discontinuation of eribulin (5%) in this trial40. Peripheral neuropathy is also a concern for single-agent vinorelbine, with clinical trial data showing 1% of patients experiencing grade ≥3 events41. Pulmonary toxicity, including fatal pneumonitis, has also been reported with vinorelbine41. Pulmonary toxicity and associated respiratory failure are also a major safety concern for gemcitabine42. In patients who receive single-agent capecitabine for metastatic breast cancer, a major safety concern is skin toxicity, with 57% and 11% experiencing capecitabine-related all-grade and grade 3 hand-and-foot syndrome, respectively, during clinical trials43.
Although not yet approved for TNBC, other ADCs being evaluated for breast cancer have distinct safety profiles. As with SG, common AEs with other ADCs include hematologic and gastrointestinal toxicities44,45. AEs associated with ADCs under investigation but not with SG include interstitial lung disease, neuropathy, ocular disorders, rash, and hyperglycemia44,45. These safety profile differences may be due in part to the payload of each ADC; with an SN-38 payload, SG has demonstrated a lower rate of diarrhea than that of SN-38’s prodrug, irinotecan22.
Our examination of potential UGT1A1 polymorphism effects on SG toxicity was prompted by the enzyme’s known role in mediating the toxicity of SN-38, the cytotoxic species delivered to cells by SG. Clearance of SN-38, a lipophilic molecule, largely depends on its glucuronidation by UGT1A1, by which it is made water soluble for subsequent excretion27. In irinotecan-treated patients with colorectal cancer who have a UGT1A1 polymorphism that limits SN-38 glucuronidation, increased SN-38 exposure results in higher rates of neutropenia and late-onset diarrhea29,46, the dose-limiting toxicities associated with irinotecan47. One such report found that patients receiving the combination of capecitabine and irinotecan with the *28 homozygous genotype were 14.2 times more likely to experience febrile neutropenia than those with the wild-type genotype48.
In line with other reports, the *28 homozygous genotype was found in a relatively small percentage of ASCENT patients (13%). As in previous irinotecan studies, rates of hematologic toxicities and diarrhea in ASCENT were modestly higher for patients with the UGT1A1 *28 homozygous genotype than for those with the wild-type genotype. Specifically, rates of grade ≥3 neutropenia, febrile neutropenia, anemia, and diarrhea were higher in the *28 homozygous group compared with the heterozygous and wild-type groups. Despite this, the discontinuation rate for SG due to TRAEs for patients with the *28 homozygous genotype was low (6%), and no patients of any UGT1A1 genotype discontinued SG due to SG-related neutropenia or diarrhea, suggesting favorable tolerability when using the current AE management strategies of active monitoring and early intervention (e.g., dose reductions and supportive medication use). These results imply that the currently recommended SG starting dosage (10 mg/kg) is appropriate regardless of patient UGT1A1 genotype. Although this detailed safety analysis of ASCENT provides evidence of a manageable safety profile in patients with UGT1A1 polymorphisms, the relatively low frequency of the UGT1A1 *28 homozygous mutation limits the ability to discern differences or draw firm conclusions. Although prescreening for UGT1A1 genotype is not required, individuals with known UGT1A1 homozygous *28/*28 genotype should be monitored closely for neutropenia and diarrhea, regardless of age.
In summary, the results of this expanded ASCENT safety analysis show that SG has a manageable safety profile, irrespective of age, with an AE profile consistent with prior reports. These data confirm prior observations24, suggesting that UGT1A1 variant status should not significantly alter recommendations for SG treatment or AE management in this patient population. Initial dosing at 10 mg/kg is recommended, with dose reductions as needed for toxicity, and these data suggest that dose reductions to manage toxicity do not appear to impact efficacy. Active monitoring, prophylaxis, and early intervention with routine AE management strategies like dose reductions allow for optimal therapeutic exposure of SG for patients with pretreated mTNBC.
Methods
Study design and participants
Detailed methods for ASCENT (NCT02574455; registered October 12, 2015), a phase 3 randomized, open-label multicenter study, have been reported25. ASCENT enrolled patients with mTNBC (per American Society of Clinical Oncology/College of American Pathologists criteria49 who relapsed after or were refractory to ≥2 previous standard chemotherapy regimens (no upper limit) for unresectable, locally advanced or metastatic disease. Patients whose disease recurred ≤12 months after completing (neo)adjuvant therapy were considered as having had one prior line of therapy. Screening for brain metastasis was not mandatory, and patients with brain metastases stable for ≥4 weeks before treatment were eligible. Patients were randomized 1:1 to SG 10 mg/kg intravenously on Days 1 and 8 of each 21-day cycle, or TPC (eribulin; vinorelbine; capecitabine; or gemcitabine) and received treatment until disease progression or unacceptable toxicity. Severe neutropenia and non-neutropenic toxicities were managed with dose delays or reductions and medications, as shown (Supplementary Fig. 2). Prophylactic growth factors at study start were not allowed, and antidiarrhea prophylaxis was not recommended.
Crossover to the SG arm was not allowed upon disease progression in the TPC arm. Patients were stratified by number of prior therapies (2–3 vs >3), geographic region (North America vs Europe), and the presence or absence of known brain metastasis (Yes or No). The primary endpoint was PFS in patients without known brain metastases by independent, centralized, and blinded review per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Secondary endpoints included safety. Exploratory endpoints included safety by UGT1A1 variant status.
The study was approved by each institutional review board or ethics committee prior to initiation and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines per the International Conference on Harmonization. A full list of institutions which granted ethical approval can be found in the Supplement. All patients provided written informed consent.
Assessments
Response was evaluated as reported previously50. AEs were assessed at each study visit. Complete blood counts (CBC) and serum chemistries were performed at baseline and at last study visit for all treatments, and prior to infusion of SG or TPC agents administered intravenously (eribulin, gemcitabine, and vinorelbine), and at the start of each treatment cycle and as required per local standard of care for TPC agents administered orally (capecitabine). In the event of grade ≥3 hematologic toxicity, CBCs were obtained more frequently at physician’s discretion until toxicity recovered to baseline or grade 1. Descriptive statistics were used to characterize AEs. AEs were assessed per the National Cancer Institute Common Terminology Criteria for AEs, version 4.03, and coded per the Medical Dictionary for Regulatory Activities, version 22.1. At baseline, a single whole blood sample was collected from all patients receiving SG for determination of UGT1A1 genotype for retrospective assessment of safety. UGT1A1 genotype was determined by Sanger sequencing, performed centrally. The relationship between the incidence of AEs of special interest to SG, including hematologic events and diarrhea, and UGT1A1 genotype were of particular focus.
Statistical analysis
The safety population consisted of all patients who received ≥1 dose of study drug. Relative dose intensity was calculated by dividing the cumulative dosage received (in mg/kg) by the product of the assigned dose (in mg/kg) based on the number of doses the patient was scheduled to receive during the patient’s treatment period. Time to onset of first AEs was defined as time from first dosing date to the start date of the AE. Duration of an individual episode of AE was calculated as the AE end-date minus the AE start-date. Median of the individual episodes of AE was used for calculation at the population level for patients with multiple episodes. PFS was defined as time from randomization until objective tumor progression or death or censored at last radiographic assessment for patients without progression or death. PFS was analyzed by the Kaplan-Meier method; 95% CIs were determined according to the method of Brookmeyer and Crowley.
Supplementary information
Acknowledgements
This study was sponsored by Immunomedics, Inc., a subsidiary of Gilead Sciences, Inc. We thank the ASCENT patients, their caregivers, study investigators, and team members. We thank William A. Wegener, MD, PhD, Robert M. Sharkey, PhD, and Pius Maliakal, PhD, former employees of Immunomedics, for their contributions to the development of the ASCENT protocol and the clinical development of sacituzumab govitecan. Medical writing and editorial assistance were provided by Robert Rydzewski, MS, CMPP, and Shala Thomas, PhD, CMPP, of Team 9 Science, and funded by Immunomedics, Inc., a subsidiary of Gilead Sciences, Inc.
Author contributions
Study design/conduct of the ASCENT trial and conceptualization of this manuscript: H.S.R., A.B., S.A.H., J.O., J.C., L.A.C., L.G., M.J.P., D.M.G., Q.H., L.M.I. and K.K. Acquisition of data: H.S.R., S.M.T., D.L., K.P., A.B., S.A.H., J.O., J.C., V.D., L.A.C., L.G., M.J.P., S.L. and K.K. Formal data analysis, project administration, previous employment by Immunomedics, Inc: DMG., Q.H., M.O. and L.M.I. Obtained funding, company founder while the ASCENT trial was performed; Chairman of the Board and Chief Scientific Officer of Immunomedics, Inc. while the ASCENT trial was performed; former stockholder in Immunomedics, Inc.; and inventor of patents pertaining to sacituzumab govitecan: D.M.G. All authors contributed to the interpretation of data and writing, editing, and review of the final manuscript.
Data availability
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
Competing interests
All authors declare no competing non-financial interests but the following competing financial interests. H.S.R. reports consultancy/advisory roles with Samsung, Mylan, and Puma; institutional research funding from Pfizer, Novartis, Eli Lilly, Genentech/Roche, Macrogenics, OBI, Merck, Immunomedics/Gilead, Daiichi Sankyo, Seattle Genetics, Eisai, Sermonix, AstraZeneca, and Odonate; travel/accommodations/expenses from Daiichi Sankyo, Mylan, Pfizer, Merck, Novartis, AstraZeneca, and Macrogenics. S.M.T. reports institutional research funding from Bristol Myers Squibb, Eisai, Immunomedics/Gilead, Genentech/Roche, Pfizer, Novartis, Nektar, Merck, AstraZeneca, Eli Lilly, Nanostring, Cyclacel, Sanofi, Odonate, Seattle Genetics, and Exelixis; consultancy/advisory roles with Bristol Myers Squibb, Eisai, Immunomedics/Gilead, Genentech/Roche, Pfizer, Novartis, Nektar, Merck, AstraZeneca, Eli Lilly, Nanostring, Sanofi, Odonate, Seattle Genetics, Puma, Daiichi Sankyo, Athenex, Oncopep, Kyowa Kirin, Samsung, CytomX, Certara, Mersana, Ellipses Pharma, 4D Pharma, OncoSec Medical, Chugai, BeyondSpring, OncXerna, Zymeworks, and Zentalis Pharmaceuticals. D.L. reports consultancy/advisory roles with Novartis, MSD, and Roche. K.P. reports consultancy/advisory roles with AstraZeneca, Eli Lilly, Novartis, Teva, MSD, Pierre Fabre, Hoffmann-La Roche and Vifor Pharma; speaker’s bureau with Eli Lilly, Mundi Pharma, Novartis, Pfizer, Seattle Genetics, and Hoffmann-La Roche; institutional research funding from Sanofi and MSD; travel/accommodations/expenses from AstraZeneca, Novartis, Pfizer, PharmaMar, and Hoffmann-La Roche. A.B. reports consultancy/advisory roles with Biotheranostics Inc., Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics/Gilead, Taiho, Sanofi, Daiichi Sankyo/AstraZeneca, Puma, Philips, Eli Lilly, and Foundation Medicine; research funding from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics/Gilead; travel/accommodations/expenses from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics/Gilead, Taiho, and Sanofi. S.A.H. reports research funding from Ambrx, Amgen, Arvinas, Bayer, Daiichi Sankyo, Genentech/Roche, GSK, Immunomedics/Gilead, Eli Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Pieris, PUMA, Radius, Sanofi, Seattle Genetics, and Dignitana; ownership interest with NK Max. J.O. reports consultancy/advisory roles with AbbVie, Agendia, AstraZeneca, Bristol Myers Squibb, Celgene, Eisai, Genentech/Roche, Immunomedics/Gilead, Jounce Therapeutics, Eli Lilly, Merck, Novartis, Pfizer, Puma Biotechnology, and Seattle Genetics. J.C. reports consultancy/advisory role with Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Eli Lilly, Servier, Merck, GSK, Leuko, Bioasis, and Clovis Oncology; speaker’s bureau for Roche, Novartis, Celgene, Eisai, Pfizer, Samsung, Eli Lilly, Merck, and Daiichi Sankyo; institutional research funding from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London; ownership interest in MedSIR. V.D. reports consultancy/advisory roles with Genentech/Roche, Novartis, Eli Lilly, Pfizer, AstraZeneca, Eisai, AbbVie, MSD, Daiichi Sankyo, and Seattle Genetics; speaker’s bureau for Roche, Novartis, Pfizer, Eli Lilly, Astra Zeneca, and Daiichi Sankyo; travel/accommodations/expenses from Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca, and Daiichi Sankyo. L.A.C. reports royalties (spouse) from Falcon Therapeutics; research funding from Innocrin Pharma, Syndax, Immunomedics/Gilead, Novartis, and NanoString Technologies; institutional research funding from Sanofi-Aventis, Novartis, G1 Therapeutics, Genentech/Roche, and GSK. L.G. reports consultancy/advisory roles with ADC Therapeutics, Amgen, AstraZeneca, Biomedical Insights, Celgene, Eli Lilly, F. Hoffmann-La Roche, Forty Seven (CD47), G1Therapeutics, Genenta, Genentech, Genomic Health, Hexal AG Sandoz, Menarini Ricerche Spa, Merck, Metis Precision Medicine, Novartis, Odonate Therapeutics, Oncolytics, Onkaido-Moderna Therapeutics, Pfizer, Revolution Medicine, Sanofi, Seattle Genetics, Synaffix, Synthon, Tahio, and Zymeworks; intellectual property rights/patent holder in conjunction with F. Hoffmann-LaRoche. M.J.P. has no disclosures to report. S.L. reports consultancy/advisory roles with AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Eli Lilly, Medscape, Merck, Novartis, Pfizer, Pierre Fabre, Prime Surgical, Puma, Roche, and Seattle Genetics; speaker’s bureau for AstraZeneca, Daiichi Sankyo, Novartis, Pfizer, Pierre Fabre, Medscape, Roche, and Samsung; institutional research funding from Abbvie, AstraZeneca, Celgene, Novartis, Pfizer, Roche, Daiichi Sankyo, Immunomedics/Gilead. D.M.G. reports intellectual property rights/patent holder at Immunomedics; former ownership interest with Immunomedics; other interests with the Center for Molecular Medicine and Immunology. Q.H., M.O., and L.M.I. report employment with Immunomedics. K.K. reports employment (spouse) with Array Biopharma, Pfizer, and GRAIL; consultancy/advisory role with bioTheranostics, Immunomedics/Gilead, Pfizer, Eisai, Eli Lilly, Novartis, Genentech/Roche, Ipsen, Merck, Seattle Genetics, Cyclacel, OncoSec, 4D Pharma, Puma and Daiichi Sankyo/AstraZeneca; speaker’s bureau for Eli Lilly; institutional research funding from Incyte, Immunomedics/Gilead, Novartis, Incyte, Genentech/Roche, Eli Lilly, Pfizer, Calithera Biosciences, Acetylon, Seattle Genetics, Amgen, Zeno Pharmaceuticals, and CytomX Therapeutics; travel/accommodations/expenses from Eli Lilly, AstraZeneca, and Pfizer; stock and other ownership interests (immediate family member) in Array BioPharma, Pfizer, and GRAIL.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/6/2024
A Correction to this paper has been published: 10.1038/s41523-024-00650-6
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-022-00467-1.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018;62:110–122. doi: 10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gennari A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018;25:S142–S150. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21:1050–1062. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park IH, et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01) Cancer Res Treat. 2019;51:43–52. doi: 10.4143/crt.2017.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robson ME, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugo HS, et al. Efficacy and safety of ixabepilone and capecitabine in patients with advanced triple-negative breast cancer: a pooled analysis from two large phase III, randomized clinical trials. Clin. Breast Cancer. 2018;18:489–497. doi: 10.1016/j.clbc.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Twelves C, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148:553–561. doi: 10.1007/s10549-014-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman PA, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins EO, et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist. 2014;19:1076–1083. doi: 10.1634/theoncologist.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan HG, Malmgren JA, Atwood MK. Triple-negative breast cancer in the elderly: Prognosis and treatment. Breast J. 2017;23:630–637. doi: 10.1111/tbj.12813. [DOI] [PubMed] [Google Scholar]
- 15.Howlader, N. et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst.106, 10.1093/jnci/dju055 (2014). [DOI] [PMC free article] [PubMed]
- 16.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J. Support Oncol. 2003;1:18–24. [PubMed] [Google Scholar]
- 17.Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J. Natl. Compr. Canc Netw. 2013;11:1494–1502. doi: 10.6004/jnccn.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trerotola M, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–233. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogi F, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9:e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9:28989–29006. doi: 10.18632/oncotarget.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015;6:22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin. Biol. Ther. 2020;20:871–885. doi: 10.1080/14712598.2020.1757067. [DOI] [PubMed] [Google Scholar]
- 23.Bardia A, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 24.Ocean AJ, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer. 2017;123:3843–3854. doi: 10.1002/cncr.30789. [DOI] [PubMed] [Google Scholar]
- 25.Bardia A, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 26.TRODELVY® (sacituzumab govitecan-hziy) [package insert]. (Gilead Sciences, Inc., 2022).
- 27.Dean, L. Irinotecan Therapy and UGT1A1 Genotype. In Medical Genetics Summaries (eds. Pratt, V. M. et al.) (National Center for Biotechnology Information, Bethesda, 2012). [PubMed]
- 28.Takano M, Sugiyama T. UGT1A1 polymorphisms in cancer: impact on irinotecan treatment. Pharmgenomics Pers. Med. 2017;10:61–68. doi: 10.2147/PGPM.S108656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Cheng D, Kuang Q, Liu G, Xu W. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in Caucasians. Pharmacogenomics J. 2014;14:120–129. doi: 10.1038/tpj.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CAMPTOSAR® (irinotecan hydrochloride) [package insert]. (Pfizer, Inc., 2022).
- 31.Van Cutsem E, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 32.Bardia A, et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2017;35:2141–2148. doi: 10.1200/JCO.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starodub AN, et al. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin. Cancer Res. 2015;21:3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey LA, et al. Assessment of sacituzumab govitecan (SG) in patients with prior neoadjuvant/adjuvant chemotherapy in the phase 3 ASCENT study in metastatic triple-negative breast cancer (mTNBC): 2nd-line subgroup analysis. J. Clin. Oncol. 2021;39(Suppl):1080. doi: 10.1200/JCO.2021.39.15_suppl.1080. [DOI] [Google Scholar]
- 35.Yancik R, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 36.Muss HB, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J. Clin. Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 37.Haque E, et al. Management of chemotherapy-induced alopecia (CIA): A comprehensive review and future directions. Crit. Rev. Oncol. Hematol. 2020;156:103093. doi: 10.1016/j.critrevonc.2020.103093. [DOI] [PubMed] [Google Scholar]
- 38.Nangia J, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: The SCALP randomized clinical trial. JAMA. 2017;317:596–605. doi: 10.1001/jama.2016.20939. [DOI] [PubMed] [Google Scholar]
- 39.Rugo HS, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. 2017;317:606–614. doi: 10.1001/jama.2016.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.HALAVEN® (eribulin mesylate) [package insert]. (Eisai, Inc., 2021).
- 41.NAVELBINE® (vinorelbine) [package insert]. (Pierre Fabre Pharmaceuticals, Inc., 2020).
- 42.GEMZAR® (gemcitabine) [package insert]. (Eli Lilly, 2019).
- 43.XELODA® (capecitabine) [package insert]. (Genentech, Inc., 2021).
- 44.ENHERTU® (fam-trastuzumab deruxtecan-nxki) [package insert]. (Daiichi Sankyo, Inc., 2022).
- 45.Powles T, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toffoli G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 47.Kweekel D, Guchelaar HJ, Gelderblom H. Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat. Rev. 2008;34:656–669. doi: 10.1016/j.ctrv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Kweekel DM, et al. UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: A Dutch Colorectal Cancer Group study. Br. J. Cancer. 2008;99:275–282. doi: 10.1038/sj.bjc.6604461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.