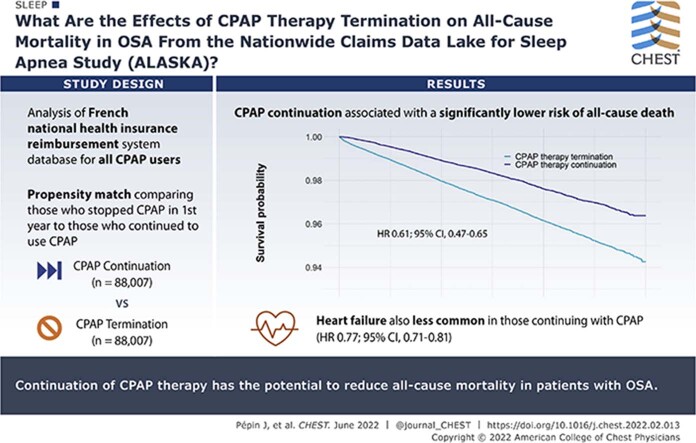
Abstract
Background
Randomized controlled trials have failed to demonstrate an effect of CPAP therapy on mortality. However, these studies have a number of important limitations, including low CPAP adherence, patient selection, and a small number of mortality events.
Research Question
What are the effects of CPAP therapy termination in the first year on all-cause mortality in patients with OSA from the Nationwide Claims Data Lake for Sleep Apnea study?
Study Design and Methods
Data from the Système National des Données de Santé (SNDS) database, the French national health insurance reimbursement system, for all new CPAP users ≥ 18 years of age were analyzed. The SNDS contains comprehensive, individualized, and anonymized data on health spending reimbursements for > 99% of all individuals living in France. OSA diagnosis was based on specific disease codes, whereas CPAP prescription was identified using specific treatment method codes. CPAP therapy termination was defined as the cessation of CPAP reimbursements triggered by the respiratory physician or sleep specialist in charge of follow-up. Patients who terminated therapy in the first year were propensity score matched with those who continued to use CPAP. The primary outcome was all-cause mortality. Three-year survival was visualized using Kaplan-Meier curves. Contributors to mortality also were determined.
Results
Data from two matched groups each including 88,007 patients were included (mean age, 60 years; 64% men). Continuation of CPAP therapy was associated with a significantly lower risk of all-cause death compared with CPAP therapy termination (hazard ratio [HR], 0.61; 95% CI, 0.57-0.65; P < .01, log-rank test). Incident heart failure also was less common in patients who continued vs terminated CPAP therapy (HR, 0.77; 95% CI, 0.71-0.82; P < .01).
Interpretation
These real-world data from a comprehensive, unbiased database highlight the potential for ongoing use of CPAP treatment to reduce all-cause mortality in patients with OSA.
Key Words: adherence, CPAP, mortality, OSA
Abbreviations: HR, hazard ratio; SAVE, Sleep Apnea Cardiovascular Endpoints; SNDS, Système National des Données de Santé
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 1444
Take-home Points.
Study Question: What are the effects of CPAP therapy termination in the first year on all-cause mortality in patients with OSA from the Nationwide Claims Data Lake for Sleep Apnea study?
Results: In matched patient groups, continuation of CPAP therapy was associated with a significantly lower risk of all-cause death compared with CPAP therapy termination. In addition, incidence heart failure was significantly less common in patients who continued vs terminated CPAP therapy in the first year.
Interpretation: These data highlight the potential for ongoing use of CPAP to reduce all-cause mortality in patients with OSA.
OSA is characterized by repeated upper airway collapse during sleep. These episodes are associated with several important consequences, including sympathetic activation, marked negative intrathoracic pressure swings, intermittent oxygen desaturation, hypercapnia, and arousal from sleep. In turn, these effects are thought to contribute to common comorbidities in patients with OSA, including hypertension, cardiovascular and cerebrovascular disease, and metabolic abnormalities.1, 2, 3, 4 These comorbidities could be responsible for the increased all-cause mortality risk that has been reported in patients with OSA.5, 6, 7, 8, 9, 10
Despite the reported association between OSA and mortality, randomized clinical trials evaluating the effects of treating OSA on cardiovascular events and all-cause death have not demonstrated any beneficial effect of CPAP therapy, the gold standard treatment for moderate to severe OSA.11, 12, 13 However, the ability of CPAP to influence hard mortality end points may have been limited by several factors, including low adherence to CPAP and patient selection. In addition, the total number of mortality events was low in all randomized trials, limiting statistical power to detect between-group differences and perhaps not representative of what happens in the real world. Thus, although randomized controlled trials provide a high level of evidence, real-world data may be able to provide a more accurate and generalizable picture of the effects of routine clinical use of CPAP on mortality.
The Nationwide Claims Data Lake for Sleep Apnea study uses data from the Système National des Données de Santé (SNDS) database, the French national health insurance reimbursement system. This analysis investigated all-cause mortality in new users of CPAP who terminated therapy during the first year or continued with long-term CPAP therapy.
Study Design and Methods
Data Source
This analysis included data from the French SNDS database, which contains comprehensive, individualized and anonymized data on health spending reimbursements for > 99% of all individuals living in France. The Nationwide Claims Data Lake for Sleep Apnea project was approved by the Commission Nationale Informatique et Liberté, the French information technology and personal data protection authority. Specific approval was obtained from the Commission Nationale Informatique et Liberté to perform this study (Identifier: DR-2019-78, no. 919194).
Study Population
Eligible patients were adults ≥ 18 years of age who had not previously used CPAP and had initiated CPAP therapy between January 2015 and December 2016. OSA diagnosis was based on specific disease codes, whereas CPAP prescription was identified using specific treatment method codes.14
Patients who terminated CPAP during the first year of therapy were matched with those who continued CPAP therapy for 1 year using propensity score matching to eliminate the influence of biases and confounding factors affecting both therapy termination and mortality rates in the therapy termination and therapy continuation groups. Propensity score matching was based on the following factors: patient demographics (age and sex), insurance coverage, socioeconomic status, and comorbidities (stroke, heart failure, peripheral arterial occlusive disease, hypertension, diabetes mellitus, other cardiovascular diseases, COPD, bariatric surgery, neurotic disorders, use of psychotropic medication, and kidney diseases). To account for possible selection bias, a sensitivity analysis was performed in the untruncated cohort with CPAP initiation being the starting date in a survival Cox model with CPAP continuation as a time-dependent covariate. Variables for adjustments were the same variables used for the propensity score analysis.
Study Parameters and Follow-up
One year after CPAP initiation, the propensity score was applied to select a matched population of CPAP users and nonusers; patients then were followed up for an additional 3 years (e-Fig 1). CPAP therapy termination was defined as the cessation of CPAP reimbursements triggered by the respiratory physician or sleep specialist in charge of follow-up. French national recommendations for reimbursement are CPAP device use of > 4 h/night. Reimbursement rates progressively decrease when very low adherence to CPAP occurs, although delivery and reimbursement of therapy can continue when CPAP use is 2 to 4 h/night, with a requirement for additional patient education and coaching. A mandatory follow-up visit occurs at 4 months after CPAP initiation, then every year thereafter to determine treatment reimbursement renewal.
For this analysis, it was assumed that CPAP termination was linked with nonadherence. Individuals with a valid and documented reason for stopping CPAP therapy (ie, sleep apnea cure after bariatric surgery, otorhinolaryngology surgery, switch to oral appliances, death) were censored in the Kaplan-Meier analysis. In the SNDS database, mortality is defined by the registered date of death, but the cause of death is not available.
Statistical Analysis
Data are expressed as median (interquartile range) for quantitative data and as number (percentage) for qualitative data. Comparisons between groups (CPAP termination vs CPAP continuation) were performed using the Student t test for quantitative data and the χ 2 test for qualitative data. Mortality and the cumulative incidence rate for heart failure were compared using Kaplan-Meier curves, and between-group comparisons were performed by using the log-rank test. These analyses also were performed separately for men and women.
The primary objective was assessed using a propensity score analysis. First, a propensity score model was performed to compute the factors associated significantly with the probability of CPAP termination during the first year. A nonparsimonious multivariate regression model was created including all major factors (list of variables and results in e-Table 1). A 1:1 greedy matching was performed with a caliper of 0.1%. Standardized differences were used to ensure the quality of the propensity score matching. The standardized difference was reduced for all variables after matching (e-Fig 2). Finally, a semiparametric Cox model was used to assess the impact of CPAP termination or continuation on outcomes (mortality, incident heart failure, incident coronary artery disease (CAD), new hospitalization for diabetes, incident arrhythmias, and incident hypertension); cancer was not evaluated because of the comparatively short follow-up time for this analysis. To account for mortality as a competing event for all outcomes, sensitivity analyses were performed considering only patients who were alive. Hazard proportionality assumption was not checked, and hazard ratio (HR) values must be interpreted as an average HR, rather than instantaneous HR.15
Analyses were performed using Python version 3.6.7 software with the libraries Numpy version 1.18.1 and Pandas version 0.24.2 for data management and analysis, Statsmodel version 0.12.1 for logistic regression, and Lifelines version 0.14.1 for Kaplan-Meier curves and Cox models. A P value of .05 was considered statistically significant.
Results
Study Population
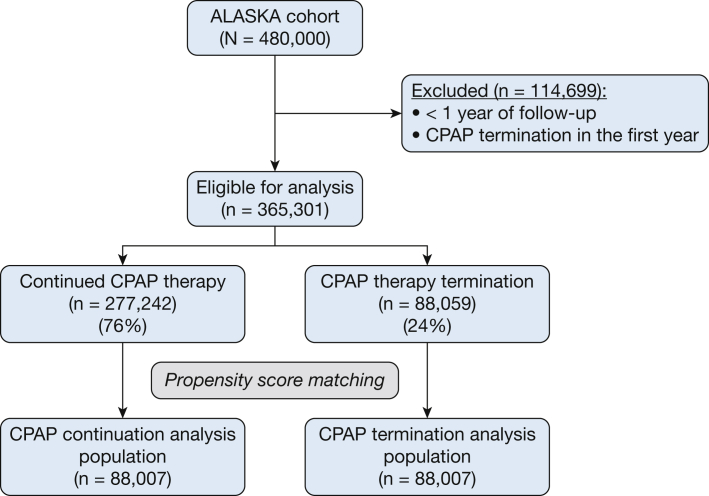
The Nationwide Claims Data Lake for Sleep Apnea cohort includes 480,000 patients, of whom 365,301 had undergone at least 1 year of follow-up and did not have a valid reason for CPAP therapy termination (4,882 patients had a valid reason for CPAP termination during the first year) (e-Table 2). Of these 365,301 patients, 76% (n = 277,242) continued CPAP therapy and 24% (n = 88,059) terminated CPAP therapy. After propensity score matching, the study population for this analysis included 88,007 patients in each group (total of 176,014 patients) (Fig 1). As expected, propensity score matching generated two patient groups that were well matched for baseline characteristics (Table 1).
Figure 1.
Flow chart showing patient inclusion. ALASKA = Nationwide Claims Data Lake for Sleep Apnea.
Table 1.
Baseline Characteristics of the Matched Study Population
| Variable | CPAP Continuation (n = 88,007) | CPAP Termination (n = 88,007) |
|---|---|---|
| Age, y | 60.0 (70.0-50.0) | 59.0 (69.0-49.0) |
| Female sex | 32,227 (36.6) | 31,666 (36.0) |
| Comorbidity | ||
| Chronic psychiatric disorders | 4,621 (5.2) | 4,606 (5.2) |
| Stroke | 2,735 (3.1) | 2,684 (3.1) |
| Heart failure | 2,306 (2.6) | 2,046 (2.3) |
| Coronary heart disease | 8,023 (9.1) | 8,037 (9.1) |
| Hypertension | 42,568 (48.4) | 43,231 (49.1) |
| Diabetes mellitus | 18,610 (21.1) | 18,304 (20.8) |
| COPD | 7,156 (8.1) | 7,387 (8.4) |
Data are presented as No. (%) or median (interquartile range).
All-Cause Mortality
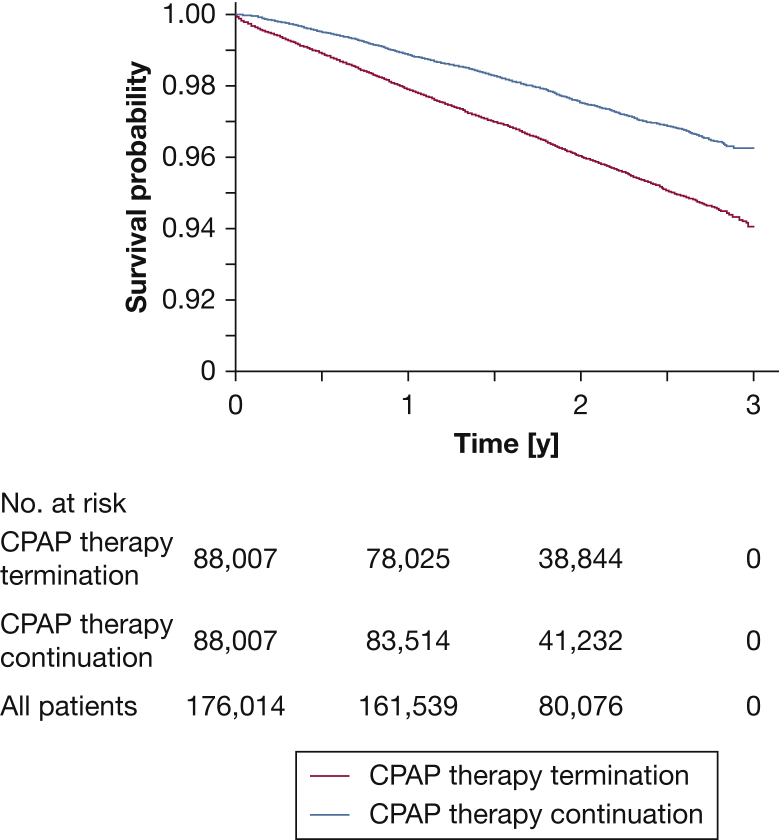
Over a 3-year observation period, death occurred in 3,204 of 88,007 patients (3.6%) in the CPAP therapy termination group compared with 2,053 of 88,007 patients (2.3%) in the therapy continuation group (e-Table 3). Continuation of CPAP therapy was associated with a significantly lower risk of all-cause death compared with CPAP therapy termination (HR, 0.61; 95% CI, 0.57-0.65; P < .01, log-rank test) (Fig 2). The results were similar in men and women (HR, 0.63 [95% CI, 0.57-0.68] and 0.54 [95% CI, 0.47-0.62]; P < .01 for both) (e-Fig 3). The sensitivity analysis also showed a significant reduction in all-cause mortality associated with CPAP continuation, with an HR of 0.73 (95% CI, 0.70-0.76; P < .01 [10,795 events in 336,415 patients, or an event rate of 3.2%]).
Figure 2.
Kaplan-Meier curves showing all-cause mortality.
Factors Contributing to Death
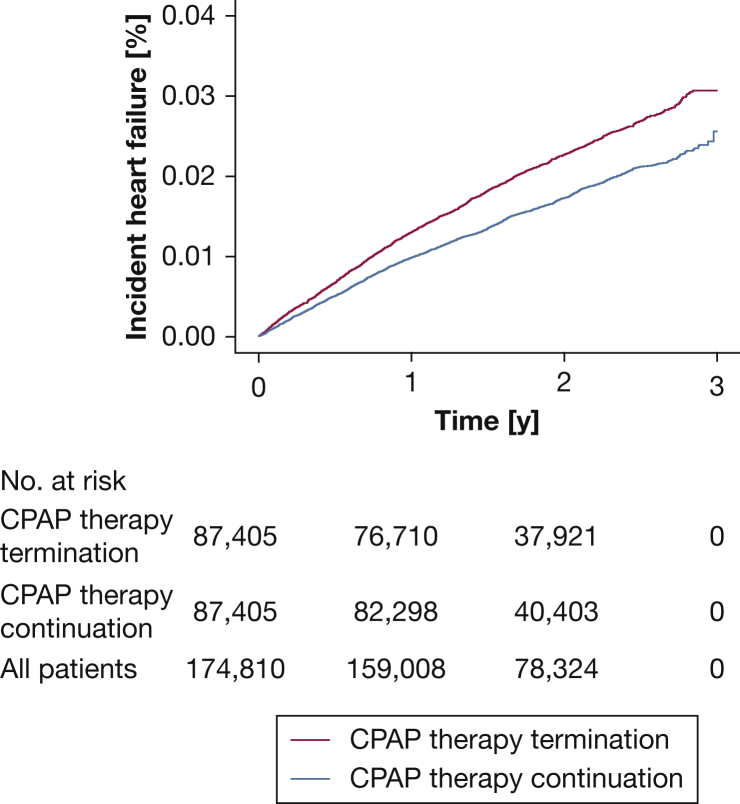
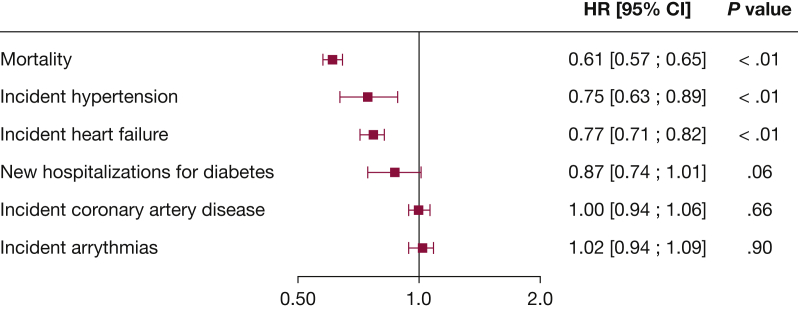
The cumulative incidence of heart failure (based on disease codes entered in the SNDS database) was significantly lower in patients who continued vs terminated CPAP therapy (HR, 0.77; 95% CI, 0.71-0.82; P < .01, log-rank test) (e-Table 3, Fig 3). During follow-up, incident hypertension and heart failure occurred significantly less frequently in patients with OSA who continued vs terminated CPAP therapy (e-Table 3, Fig 4). In addition, a trend toward a lower risk of new hospitalizations for diabetes in the therapy continuation vs therapy termination group was found (P = .06) (e-Table 3, Fig 4). Sensitivity analysis that excluded patients censored for death during the analysis period yielded similar results to the primary analysis (e-Fig 4).
Figure 3.
Line graph showing cumulative incidence of heart failure.
Figure 4.
Forest plot showing risk of all-cause mortality and factors potentially contributing to death. HR of < 1 indicates a lower risk with CPAP continuation. HR = hazard ratio.
Discussion
The results of this analysis of a comprehensive, unbiased national dataset showed a significant association between continuation of CPAP during the first year of therapy and lower all-cause mortality. One potential mechanism underlying this association may be the lower rate of incident heart failure seen in the group who continued CPAP compared with those who terminated CPAP therapy. In our main analysis, only patients who survived long-enough to discontinue the use of CPAP were included. As such, patients who either had died, had < 1 year of follow-up, or who discontinued CPAP during the first year were used for propensity score matching, but were not included in the main all-cause mortality analysis. To avoid potential selection bias resulting from arbitrarily splitting the dataset into two groups that were not created at baseline, we performed a sensitivity analysis using CPAP termination as a time-dependent covariate and evaluated its association with overall survival. The results of this sensitivity analysis confirmed and strengthened the study findings by showing that there was a 27% reduction in all-cause mortality in patients who continued CPAP.
Our findings contrast with those of randomized controlled trials evaluating the effects of CPAP on mortality. The Sleep Apnea Cardiovascular Endpoints (SAVE) study, the Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome—Effect of Intervention With CPAP study, and the Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA study investigated the effects of CPAP on a composite end point that included cardiovascular death and nonfatal cardiovascular events.11, 12, 13 All found no significant difference between the CPAP and usual care groups with respect to the primary end point or for cardiovascular death alone as a secondary end point.11, 12, 13 However, several factors may have limited the ability of these studies to detect any statistically significant effect of CPAP on mortality.
First, adherence to treatment was low (3.3 ± 2.3 h/night in SAVE and 2.78 ± 2.73 h/night in the Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome—Effect of Intervention With CPAP study),11,13 and these levels of adherence do not seem to reflect what is seen with CPAP use in broader clinical settings.16,17 Device use of ≥ 4 h/night may be needed for the benefits of therapy to be realized.12,18 For example, in the Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA study, a preplanned analysis in patient subgroups using CPAP for ≥ 4 h/night vs < 4 h/night showed that those using CPAP for ≥ 4 h/night had a significantly lower rate of composite end point events, including mortality (adjusted HR, 0.29; 95% CI, 0.10-0.86; P = .026).12 Furthermore, in the SAVE study, patients with OSA who were adherent to CPAP therapy showed a lower risk of stroke and the composite end point of cerebral events than those in the usual care group.11 Second, the trials included highly selected patient populations, namely nonsleepy patients with OSA with existing cardiovascular disease. In particular, the noninclusion of patients with excessive daytime sleepiness from randomized controlled trials for ethical reasons might exclude a group likely to adhere and respond well to CPAP therapy.19 Recently, a comparison was made between consecutive sleep clinic patients (n = 3,965) and participants in the prominent recent randomized controlled trials examining the effect of CPAP on adverse cardiovascular outcomes in OSA.20 Less than 20% of real-world patients with OSA presented with all eligibility criteria of randomized controlled trials, and routine clinic patients with OSA were younger, sleepier, and more likely to be women.20 Finally, the total number of mortality events in each study was very small (25 in the CPAP group and 20 in the usual care group in the SAVE study, and 12 in the CPAP group and 14 in the usual care group in the Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome—Effect of Intervention With CPAP study), limiting statistical power for this end point.
In contrast, the current analysis included all patients with OSA in France with an indication for CPAP therapy, making it applicable to general populations, and the large number of deaths provides adequate power for mortality analyses. Furthermore, differentiating between patients who continued using CPAP and those who did not provides a clearer picture of the benefits of CPAP use. Thus, although randomized controlled trials provide the highest level of evidence, real-world data may provide a better indication of overall effectiveness in patient populations likely to be encountered in routine clinical practice. Others also recently suggested that design features and enrolled populations in randomized controlled trials of CPAP therapy in patients with OSA limit the ability of these trials to identify the benefits of treatment.21 Contrary to classical observational studies with exposed and unexposed patients, the discontinuation design provides a more homogeneous initial study population on which the applied propensity score matching further limits unmeasured bias. Specifically, it recently was suggested that observational studies using propensity scores can overcome the ethical limitations around inclusion of patients with sleep apnea who experience excessive daytime sleepiness into randomized controlled trials.22 The US Food and Drug Administration also has indicated that studies using propensity score methods are appropriate to support approval of medical devices such as CPAP.23
Two other recent real-world studies also reported a significant association between CPAP use and lower all-cause mortality.24,25 Similar to our approach, a retrospective analysis of patients from a sleep clinic in Japan used propensity score matching to create two study populations of patients with OSA, in this case, those who used CPAP and those who did not. After a median follow-up of 6 to 7 years, the all-cause mortality rate was significantly lower in those who did vs did not use CPAP (4.2% vs 7.4%; HR, 0.56; 95% CI, 0.41-0.78).25 This approximate doubling of all-cause mortality risk in patients with OSA not using CPAP was similar to that in our study for patients who stopped vs continued CPAP therapy.
The comparison group in a population-based longitudinal study from Spain was patients without OSA, who were found to be at significantly higher risk of all-cause death than patients with OSA prescribed CPAP after adjustment for comorbidities and previous health care resource use (HR, 0.44 [95% CI, 0.36-0.54] in men and 0.44 [95% CI, 0.28-0.68] in women).24
In an earlier study, Woehrle et al26 analyzed a large German health care database and found that patients who received a diagnosis of sleep apnea and were treated with CPAP showed a significantly lower all-cause mortality rate after 3 and 4 years of follow-up compared with control participants who had a diagnosis of sleep apnea, but were not treated with CPAP. Our findings are consistent with those of the German study, which also used propensity score matching to generate the two study groups. However, our sample size is substantially larger and the source of participants more comprehensive than previous similar studies.
A large retrospective cohort study of Medicare beneficiaries with newly diagnosed heart failure found that those who were screened for and had a diagnosis of sleep-disordered breathing (SDB) and were treated with CPAP were significantly less likely to die over 2 years of follow-up than those screened for and with a diagnosis of sleep-disordered breathing who were not treated with CPAP (HR, 0.49; 95% CI, 0.29-0.84).27 These data highlight an important link between sleep-disordered breathing and heart failure that is reflected in our study finding that patients who continued CPAP therapy during the first year were significantly less likely to demonstrate incident heart failure than those who terminated therapy. This result is consistent with a recent study showing a significant association between hypoxic burden and the rate of incident heart failure in patients with OSA.28
A link between CPAP use and lower mortality also was identified in a prospective cohort study from the United Kingdom.29 Patients with OSA syndrome who were treated with CPAP for > 5 years were significantly more likely to be alive at the end of the study (mean follow-up, 14.8 ± 3.7 years), with a relative risk for survival of 5.63 (95% CI, 4.83-6.58; P < .001). Similarly, patients who did not adhere to CPAP therapy in the first year of therapy were at higher risk of death over the subsequent median 2.4-year follow-up period (adjusted HR, 1.74; 95% CI, 1.32-2.28) in a Swedish national registry-based cohort study.30 Health system data from Catalan, Spain, also showed a reduction in population-level mortality with CPAP treatment, but this effect was seen only in men.31 This contrasts with our study, where reductions in mortality were seen consistently in both men and women.
Although we do not currently have precise data on the specific causes of death in our study (the database includes date of death, but not the cause), based on the information available, it seemed that incident hypertension and heart failure potentially were important contributors to death in the group of patients who terminated CPAP therapy. In two other recent real-world studies, the association between CPAP nonuse and mortality seemed to be driven largely by malignancy-related deaths.24,25 However, these studies had a longer duration of follow-up (median, 5.5-6.5 years)24,25 compared with only 3 years for our analysis. Longer follow-up durations also have been used in studies evaluating the link between OSA and cancer.32, 33, 34
The significant negative impact of terminating CPAP therapy in the first year highlights the importance of strategies to improve adherence to, and continuation with, CPAP. A personalized medicine approach using telemedicine-based support programs already has been shown to improve positive airway pressure use and to reduce the number of patients terminating therapy in real-world settings.35,36 Therefore, implementation of these strategies and the associated improvement in CPAP continuation rates have the potential to impact positively on death rates. However, additional prospective studies are needed to determine the effects of different telemonitoring programs and patient engagement tools on hard clinical end points, including mortality.
The database used is an important strength of this study. The French SNDS is currently one of the best anonymized claims databases in the world because of its size (600 terabytes) and its unbiased recruitment (including > 99% of the total French population). It is not specific to any insurer, health care provider, or CPAP device brand. In addition, we performed careful and extended propensity score matching to ensure comparability between the CPAP termination and CPAP continuation groups, and a large number of mortality events were available for analysis (n = 5,257).
Several limitations also need to be considered when interpreting the study findings. As has been highlighted previously,14,36,37 several weaknesses exist in databases that are designed for administrative purposes, rather than clinical research, including a lack of data for some potentially important parameters. In the context of this study, that means that no apnea-hypopnea index data are available, so OSA severity is unknown. However, the fact that all patients fulfilled the criteria for initiation of CPAP provides some indication that OSA was at least moderate in severity. Also some limitations of propensity score matching in this context exist because it was a post hoc process based on available information only, meaning that we do not have data to allow propensity matching on important covariates that might modify the relationship between OSA exposure and outcomes, such as OSA severity, sleepiness, health behaviors, adherence data, and BMI. Several studies have addressed the link between sleepiness phenotypes, OSA severity (ie, hypoxic burden), and incident cardiovascular events.19,38 Contrary to general population cohorts, in routine real-world practice, symptom subtypes were not associated with major adverse cardiovascular events after adjustment for confounders.38 Because our data did not include measurements of sleepiness and hypoxic burden, we did not account for these confounders in our propensity matching. Therefore, potentially relevant covariates such as nutrition, physical activity, and sleep duration (all of which potentially are linked with mortality) were not included. In addition, because of French privacy requirements, the SNDS does not contain data on smoking habits, alcohol intake, or BMI. Furthermore, we do not have any data about the actual hours of CPAP use because the database defines CPAP use as a binary parameter (yes or no). In the future, it may be possible to link SNDS records with CPAP telemonitoring data and to link individual anthropometric and lifestyle profiles, which would allow investigation of the dose-response relationship between CPAP adherence and mortality.
Interpretation
This study showed that continued use of CPAP during the first year after therapy initiation is associated with a significant reduction in mortality in a large national cohort of patients with OSA compared with CPAP therapy termination. This finding adds to a growing body of evidence for the beneficial effects of CPAP use on survival. Additional research is needed to clarify the impact of CPAP on specific causes of death and to determine the relationship between hours of CPAP use and mortality benefit.
Acknowledgments
Author contributions: Study procedures and analyses were undertaken by independent third parties: SEMEIA (P. H. and P. R.), Institut national de la santé et de la recherche médicale Hypoxia-Physiopathology laboratory (S. B.), and artificial intelligence chair trajectories medicine (director and principal investigator, J. L. P.). The first draft of the manuscript was prepared by J. L. P., who had unrestricted access to the data, with the assistance of an independent medical writer funded by ResMed. The manuscript was reviewed and edited by all the authors. All authors made the decision to submit the manuscript for publication. J. L. P. assumes responsibility for the accuracy and completeness of the analyses and for the fidelity of this report to the trial protocol.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. L. P. has received lecture fees or conference traveling grants from ResMed, Perimetre, Philips, Fisher and Paykel, AstraZeneca, Jazz Pharmaceuticals, Agiradom, and Teva and has received unrestricted research funding from ResMed, Philips, GlaxoSmithKline, Bioprojet, Fondation de la Recherche Medicale (Foundation for Medical Research), Direction de la Recherche Clinique du CHU de Grenoble (Research Branch Clinic CHU de Grenoble), and fond de dotation “Agir pour les Maladies Chroniques“ (endowment fund “Acting for Chronic Diseases”). A. M. reports income from Equillium, Corvus, Jazz, and Livanova related to medical education, and ResMed provided a philanthropic donation to the University of California, San Diego. P. A. C. has an appointment to an endowed academic chair at the University of Sydney that was established from ResMed funding; has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies; and is a consultant to ResMed, SomnoMed, Zephyr Sleep Technologies, and Signifier Medical Technologies. R. T. has received consulting fees from ResMed, Inspire, Navigant, and Jazz Pharmaceuticals; lecture fees from Agiradom, Elivie, ResMed, and Philips; conference travel grants from Agiradom; and unrestricted research grants from ResMed, Vitalaire, Philips, APMC foundation Direction de la recherche Clinique du CHU de Grenoble, and interregional research university hospital group. A. V. B., F. L., and A. J. are employees of ResMed. P. R., P. S.-B., and P. H. are employees of SEMEIA. None declared (S. B., D. A.).
Role of sponsors: Representatives of the study sponsor were involved in the design of the study.
Other contributions: Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by ResMed.
Additional information: The e-Figures and e-Tables are available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: J. L. P., S. B., and R. T. are supported by the French National Research Agency in the framework of the Investissements d’Avenir program [Grant ANR-15-IDEX-02] and the e-Health and Integrated Care and Trajectories Medicine and MIAI Artificial Intelligence chairs of excellence from the Grenoble Alpes University Foundation. D. A. is supported by Ligue Pulmonaire Suisse to conduct a program entitled “Integrated Care of Multimorbidity in Patients Surviving Acute Respiratory Failure” [Grant LPS-16/12]. A. M. is funded by the National Institutes of Health. This work has been supported partially by MIAI and the University Grenoble Alpes [Grant ANR-19-P3IA-0003] and an unrestricted grant from ResMed.
Supplementary Data
References
- 1.Bonsignore M.R., Suarez Giron M.C., Marrone O., Castrogiovanni A., Montserrat J.M. Personalised medicine in sleep respiratory disorders: focus on obstructive sleep apnoea diagnosis and treatment. Eur Respir Rev. 2017;26(146):170069. doi: 10.1183/16000617.0069-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knauert M., Naik S., Gillespie M.B., Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lévy P., Kohler M., McNicholas W.T., et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 4.Pepin J.L., Borel A.L., Tamisier R., Baguet J.P., Levy P., Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18(6):509–519. doi: 10.1016/j.smrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Lavie P., Lavie L., Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25(3):514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 7.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Young T., Finn L., Peppard P.E., et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall N.S., Wong K.K., Cullen S.R., Knuiman M.W., Grunstein R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi N.M., Caffo B.S., Goodwin J.L., et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8) doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy R.D., Antic N.A., Heeley E., et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 12.Peker Y., Glantz H., Eulenburg C., Wegscheider K., Herlitz J., Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-de-la-Torre M., Sánchez-de-la-Torre A., Bertran S., et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4):359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 14.Pépin J.L., Bailly S., Rinder P., et al. on behalf of the medXcloud Group CPAP therapy rates by OSA phenotype: a French nationwide database analysis. J Clin Med. 2021;10(5):936. doi: 10.3390/jcm10050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stensrud M.J., Hernán M.A. Why test for proportional hazards? JAMA. 2020;323(14):1401–1402. doi: 10.1001/jama.2020.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cistulli P.A., Armitstead J., Pepin J.L., et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pack A.I., Magalang U.J., Singh B., Kuna S.T., Keenan B.T., Maislin G. To RCT or not to RCT? Depends on the question. A response to McEvoy et al. Sleep. 2021;44(4):zsab042. doi: 10.1093/sleep/zsab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbé F., Durán-Cantolla J., Sánchez-de-la-Torre M., et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 19.Mazzotti D.R., Keenan B.T., Lim D.C., Gottlieb D.J., Kim J., Pack A.I. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynor A., McArdle N., Shenoy B., et al. Continuous positive airway pressure and adverse cardiovascular events in obstructive sleep apnea: are participants of randomized trials representative of sleep clinic patients? Sleep. 2022;45(4):zsab264. doi: 10.1093/sleep/zsab264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labarca G., Dreyse J., Drake L., Jorquera J., Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis. Sleep Med Rev. 2020;52:101312. doi: 10.1016/j.smrv.2020.101312. [DOI] [PubMed] [Google Scholar]
- 22.Pack A.I., Magalang U.J., Singh B., Kuna S.T., Keenan B.T., Maislin G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep. 2021;44(2):zsaa229. doi: 10.1093/sleep/zsaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration Center for Devices and Radiological Health Design considerations for pivotal clinical investigations for medical devices: guidance for industry, clinical investigators, institutional review boards and Food and Drug Administration staff. 2013. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-considerations-pivotal-clinical-investigations-medical-devices Food and Drug Administration website.
- 24.de Batlle J., Bertran S., Turino C., et al. Longitudinal analysis of causes of mortality in continuous positive airway pressure-treated patients at the population level. Ann Am Thor Soc. 2021;18(8):1390–1396. doi: 10.1513/AnnalsATS.202007-888OC. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura K., Nakamura H., Tohyama K., et al. Survival benefit of continuous positive airway pressure in Japanese patients with obstructive sleep apnea: a propensity-score matching analysis. J Clin Sleep Med. 2021;17(2):211–218. doi: 10.5664/jcsm.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woehrle H., Schoebel C., Oldenburg O., et al. Low long-term mortality in patients with sleep apnoea and positive airway pressure therapy: analysis of a large German healthcare database. Somnologie. 2020;24(3):151–158. [Google Scholar]
- 27.Javaheri S., Caref E.B., Chen E., Tong K.B., Abraham W.T. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 28.Azarbarzin A., Sands S.A., Taranto-Montemurro L., et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest. 2020;158(2):739–750. doi: 10.1016/j.chest.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodds S., Williams L.J., Roguski A., et al. Mortality and morbidity in obstructive sleep apnoea-hypopnoea syndrome: results from a 30-year prospective cohort study. ERJ Open Res. 2020;6(3):00057-2020–02020. doi: 10.1183/23120541.00057-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palm A., Midgren B., Theorell-Haglöw J., et al. Factors influencing adherence to continuous positive airway pressure treatment in obstructive sleep apnea and mortality associated with treatment failure—a national registry-based cohort study. Sleep Med. 2018;51:85–91. doi: 10.1016/j.sleep.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 31.de Batlle J., Bertran S., Turino C., et al. Mortality in patients treated with continuous positive airway pressure at the population level. Am J Respir Crit Care Med. 2018;197(11):1486–1488. doi: 10.1164/rccm.201709-1889LE. [DOI] [PubMed] [Google Scholar]
- 32.Lee E.J., Suh J.D., Cho J.H. The incidence of prostate cancer is increased in patients with obstructive sleep apnea: results from the national insurance claim data 2007-2014. Medicine (Baltimore) 2021;100(6) doi: 10.1097/MD.0000000000024659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendzerska T., Povitz M., Leung R.S., et al. Obstructive sleep apnea and incident cancer: a large retrospective multicenter clinical cohort study. Cancer Epidemiol Biomarkers Prev. 2021;30(2):295–304. doi: 10.1158/1055-9965.EPI-20-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Justeau G., Gervès-Pinquié C., Le Vaillant M., et al. Association between nocturnal hypoxemia and cancer incidence in patients investigated for OSA: data from a large multicenter French cohort. Chest. 2020;158(6):2610–2620. doi: 10.1016/j.chest.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 35.Woehrle H., Arzt M., Graml A., et al. Effect of a patient engagement tool on positive airway pressure adherence: analysis of a German healthcare provider database. Sleep Medicine. 2018;41:20–26. doi: 10.1016/j.sleep.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra A., Crocker M.E., Willes L., Kelly C., Lynch S., Benjafield A.V. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. 2018;153(4):843–850. doi: 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woehrle H., Arzt M., Graml A., et al. Predictors of positive airway pressure therapy termination in the first year: analysis of big data from a German homecare provider. BMC Pulm Med. 2018;18(1):186. doi: 10.1186/s12890-018-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trzepizur W., Blanchard M., Ganem T., et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med. 2022;205(1):108–117. doi: 10.1164/rccm.202105-1274OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.