Abstract
Background
Little is known about rates of invasive procedures and associated complications after lung cancer screening (LCS) in nontrial settings.
Research Question
What are the frequency of invasive procedures, complication rates, and factors associated with complications in a national sample of veterans screened for lung cancer?
Study Design and Methods
We conducted a retrospective cohort analysis of veterans who underwent LCS in any Veterans Health Administration (VA) facility between 2013 and 2019 and identified veterans who underwent invasive procedures within 10 months of initial LCS. The primary outcome was presence of a complication within 10 days after an invasive procedure. We conducted hierarchical mixed-effects logistic regression analyses to determine patient- and facility-level factors associated with complications resulting from an invasive procedure.
Results
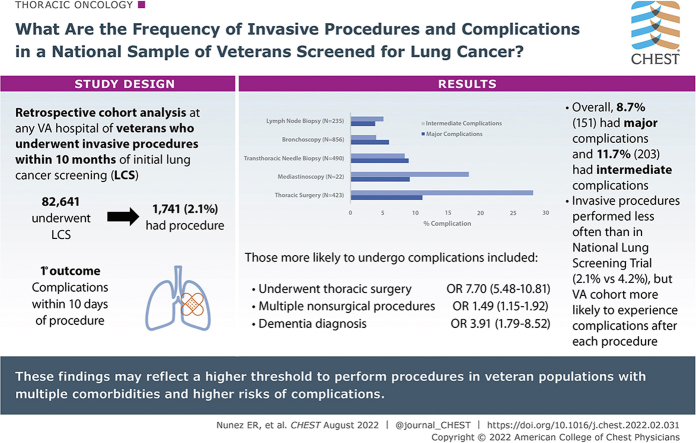
Our cohort of 82,641 veterans who underwent LCS was older, more racially diverse, and had more comorbidities than National Lung Screening Trial (NLST) participants. Overall, 1,741 veterans (2.1%) underwent an invasive procedure after initial screening, including 856 (42.3%) bronchoscopies, 490 (24.2%) transthoracic needle biopsies, and 423 (20.9%) thoracic surgeries. Among veterans who underwent procedures, 151 (8.7%) experienced a major complication (eg, respiratory failure, prolonged hospitalization) and an additional 203 (11.7%) experienced an intermediate complication (eg, pneumothorax, pleural effusion). Veterans who underwent thoracic surgery (OR, 7.70; 95% CI, 5.48-10.81), underwent multiple nonsurgical procedures (OR, 1.49; 95% CI, 1.15-1.92), or carried a dementia diagnosis (OR, 3.91; 95% CI, 1.79-8.52) were more likely to experience complications. Invasive procedures were performed less often than in the NLST (2.1% vs 4.2%), but veterans were more likely to experience complications after each type of procedure.
Interpretation
These findings may reflect a higher threshold to perform procedures in veteran populations with multiple comorbidities and higher risks of complications. Future work should focus on optimizing the identification of patients whose chance of benefit likely outweighs the complication risks.
Key Words: complications, lung cancer screening, procedures, veterans
Abbreviations: CDW, Corporate Data Warehouse; CPT, Current Procedural Terminology; LCS, lung cancer screening; NLST, National Lung Screening Trial; VA, Veterans Health Administration
Graphical Abstract
Take-home Points.
Study Question: What are the frequency of invasive procedures, complication rates, and factors associated with complications in a national sample of veterans screened for lung cancer?
Results: Overall, 82,641 veterans underwent lung cancer screening, of whom 1,741 (2.1%) underwent an invasive procedure after initial screening. Among veterans who underwent a procedure, 20.4% experienced a procedural complication. Invasive procedures were performed half as frequently as in the National Lung Screening Trial (2.1% vs 4.2%), but veterans were more likely to experience complications after each type of procedure.
Interpretation: These findings may reflect a higher threshold to perform procedures in veterans with multiple comorbidities and higher risks of complications. Future work should focus on optimizing the identification of patients whose chance of benefit likely outweighs the complication risks.
FOR EDITORIAL COMMENT, SEE PAGE 274
Lung cancer remains the leading cause of cancer mortality, with 1.8 million deaths annually worldwide.1 The National Lung Screening Trial (NLST) was the largest randomized trial of lung cancer screening (LCS), with > 53,000 participants. The NLST showed a 20% relative reduction in mortality with annual LCS,2 a reasonable risk to benefit ratio on average,3 and more a favorable risk to benefit ratio for those at higher risk of lung cancer.4 The NLST has been used as the benchmark for LCS guidance, providing the data used in decision aids to inform patients and providers of the expected trade-offs from LCS, including the anticipated frequency and complications of invasive procedures to evaluate screen-detected findings.5,6
Yet, the NLST may not be an ideal benchmark from which to gauge the anticipated frequency and complications of LCS for several reasons. First, the NLST primarily comprised high-volume academic medical centers, which typically have lower complication rates (eg, 7.8% of NLST participants experienced a major complication after an invasive procedure).7,8 Second, NLST benefitted from the healthy volunteer effect: trial participants were younger, were less racially and ethnically diverse, were better educated, harbored fewer comorbidities, and were more likely to have quit smoking than the LCS-eligible US population.9,10 Third, both rates and complications of procedures often differ in nontrial settings.11,12 Finally, the NLST was conducted more than a decade ago. As data have accumulated on LCS, rates of performing procedures may have changed as providers and patients have become more comfortable with surveillance of nodules and the Lung CT Screening Reporting and Data System has standardized recommendations for evaluation.13, 14, 15
Therefore, we sought to analyze the frequency of complications resulting from invasive procedures in a national cohort of veterans who underwent LCS outside the trial setting and to identify factors associated with development of complications. The nationally integrated Veterans Health Administration (VA) health care system is an ideal setting for studying use and complications of invasive procedures in current practice, with robust clinical and administrative data from > 140 VA medical centers and early adoption, but variable implementation, of LCS and downstream evaluation across sites.16, 17, 18 Our hypothesis was that older veterans and those with pulmonary or cardiovascular comorbidities would be more likely to experience complications resulting from invasive procedures and that substantial variability in rates of procedures and associated complications would be found across sites.
Study Design and Methods
Study Design and Population
We conducted a retrospective cohort analysis of veterans 55 to 80 years of age with > 30 years of smoking who underwent initial LCS in any VA facility from January 1, 2013, through December 31, 2019. Using the VA’s Corporate Data Warehouse (CDW), we identified veterans who underwent an initial LCS examination in the VA system based on either (1) a health factor for “agrees to screening” generated by VA’s LCS clinical reminder embedded in the electronic health record, followed by chest CT imaging within 3 months; or (2) first chest CT scan for LCS based on Current Procedural Terminology (CPT) codes or CDW radiology tables (e-Fig 1) that specifies the chest CT scan as a screening LDCT. We excluded veterans with a prior lung cancer diagnosis or who previously had undergone LCS outside of the VA, based on CPT codes for LCS in Medicare claims. We then identified the subset of veterans who underwent an invasive procedure typically performed for evaluation of LCS-detected findings in the 10 months after an initial LCS examination. We chose 10 months to allow time for evaluation in cases where abnormal screen-detected findings initially may have been managed with repeat imaging before an invasive procedure, without overlapping with the time frame that a repeat annual LCS would be expected. Specifically, we queried the VA CDW and Medicare claims data for International Classification of Diseases or CPT codes for bronchoscopy, transthoracic needle biopsy, mediastinoscopy, or lung resection. We excluded veterans who visited the ED in the 5 days before the procedure or underwent a procedure that occurred more than 2 days after inpatient admission date, because these procedures likely were performed in response to an acute illness unrelated to LCS findings.
Primary Outcome
The primary outcome was the presence of a complication within 10 days of an invasive procedure. We queried VA CDW and Medicare claims data (available through December 31, 2018) for evidence of complications defined by International Classification of Diseases and CPT codes (e-Tables 1, 2). Like in the NLST, we classified our complications into major and intermediate complications (e-Table 2). For procedures that occurred within 10 days of each other, we assigned complications to the more invasive procedure (eg, if a bronchoscopy occurred on the same day or in the 10 days after a lung resection, all complications would be attributed to the lung resection). We accounted for the timing of the complication: for a less invasive procedure that occurred 10 days before a more invasive procedure, complications occurring before the more invasive procedure were attributed to the initial less-invasive procedure, whereas complications that occurred after two or more procedures were attributed to the most invasive procedure. Procedures in order of invasiveness were: thoracic surgery (thoracotomy, thoracoscopy), mediastinoscopy, transthoracic needle biopsy, bronchoscopy including endobronchial ultrasound bronchoscopy, and extrathoracic lymph node biopsy. Of note, expected outcomes of procedures were not considered complications; for example, mechanical ventilation after thoracic surgery was not labeled as acute respiratory failure.
Covariates
We a priori selected clinically relevant covariates based on prior studies of procedural complications.19,20 For each veteran, we extracted data from the VA CDW on demographic characteristics and derived comorbidities and Elixhauser comorbidity index scores based on International Classification of Diseases codes in the VA CDW or Medicare files (e-Table 3).21,22
We assigned veterans to the VA facility at which they underwent their procedure. Facility-level covariates included geographic location (ie, US census region) and procedure volume. We performed log transformation of one variable, number of procedures performed, because of its right-skewed distribution.
Statistical Analyses
We conducted mixed-effects logistic regression analyses, including both patient- and facility-level variables, to determine factors associated with complications. Our models assumed random coefficients to patient-level variables that varied by facility. We report the mean patient effects averaged across facilities, accounting for facility-specific random effects, in our analytic summaries. To address potential issues of multicollinearity, we computed variance inflation factors for each patient-level variable in the model. No variables had a variance inflation factor of > 10, a conventional threshold for collinearity concerns, suggesting that collinearity was not problematic.23 To quantify facility-level variation, we calculated an intraclass correlation coefficient on the model with patient fixed effects and only random intercepts for each facility.24 Missing data for race and BMI were filled in using regression imputation, given the low rate of missing data on these variables. Our multivariate model excluded 84 veterans who were included in the descriptive analyses, but whose procedure was identified through Centers for Medicare and Medicaid Services data, because we could not assign facilities to these veterans. All analyses were performed using SAS version 9.4 software (SAS Institute).
Sensitivity Analyses
To explore the possibility that our study may not capture procedures provided through private insurers, we performed a sensitivity analysis restricting our analysis to Medicare-eligible veterans (age ≥ 65 years) because veterans with VA or Medicare coverage are less likely to obtain screening through the private sector.25,26 Additionally, because Medicare claims data were available only through 2018, we performed a sensitivity analysis restricting the date of procedures from January 1, 2013, through December 31, 2018. We also performed a sensitivity analysis of our multivariate model, stratifying our cohort by whether veterans underwent surgical or nonsurgical procedures.
Results
Overall, 82,641 veterans underwent an initial LCS examination between 2013 and 2019. Compared with NLST participants, the present cohort was older (mean age, 66.9 years vs 61.4 years), comprised more men (95.6% vs 59%), was more racially diverse, and was more likely to harbor comorbid medical disorders (Table 1). Among the 82,641 veterans in this cohort, 1,741 (2.1%) underwent 2,026 invasive procedures within 10 months of the LCS examination. Yearly rates of invasive procedures performed ranged from 1.8% to 3.5%, and although yearly increases occurred in the number of screenings performed, no trend was found in the proportion of veterans receiving an invasive procedure across the years in the study period. Facilities varied in their rates of performing invasive procedures among screened veterans from 0% to 5%.
Table 1.
Characteristics of Veterans Who Underwent Lung Cancer Screening and Subsequent Procedures Compared With Participants in the National Lung Screening Trial
| Characteristic | Veterans Who Underwent LCS (n = 82,641) | Veterans Who Underwent Procedures (n = 1,741) | NLST (n = 26,732) |
|---|---|---|---|
| Age, mean (SD) | 65.2 ± 5.6 | 66.9 ± 5.4 | 61.4 ± 5.0 |
| Female sex | 3,818 (4.6) | 76 (4.4) | 10,952 (41.0) |
| Race | |||
| White | 63,823 (77.2) | 1,416 (81.3) | 24,289 (90.9) |
| Black | 15,142 (18.3) | 265 (15.2) | 1,195 (4.5) |
| Hispanic | 2,162 (2.6) | 32 (1.8) | 479 (1.8) |
| Other | 1,514 (1.8) | 29 (1.6) | 1,075 (4.0) |
| Missing | 1,423 (1.7) | 38 (2.2) | 163 (0.6) |
| Married | 35,380 (42.8) | 779 (44.7) | 17,815 (66.6) |
| Heart disease or heart attack | 13,758 (16,6) | 300 (17.2) | 3,445 (12.9) |
| Stroke | 3,356 (4.1) | 85 (4.9) | 753 (2.8) |
| COPD | 27,474 (33.2) | 698 (40.1) | 4,674 (17.5) |
| Interstitial lung disease | 1,299 (1.6) | 49 (2.8) | 70 (0.3) |
| Dementia | 2,136 (2.6) | 40 (2.3) | Not available |
| Depression | 22,081 (26.7) | 444 (25.5) | |
| Posttraumatic stress disorder | 13,981 (16.9) | 279 (16.0) | |
| Substance use disorder | 22,210 (26.9) | 442 (25.4) | |
| BMI, kg/m2 | 28.88 ± 6.31 | 27.82 ± 7.65 | |
| Elixhauser comorbidity index score | 4.25 ± 3.12 | 4.44 ± 3.26 |
Data are presented as No. (%) or mean ± SD. LCS = lung cancer screening; NLST = National Lung Screening Trial. Variables with missing data include: BMI screening (n = 379 [0.46%]) and race (n = 1,423).
The overall rates of procedures in the VA cohort was lower than that in the NLST (2.1% vs 4.2%) as well as for each individual procedure except extrathoracic lymph node biopsy (0.29% vs 0.30%): bronchoscopy (1.1% vs 2.5%), transthoracic needle biopsy (0.2% vs 0.9%), thoracic surgery (0.5% vs 2.8%), and mediastinoscopy (0.03% vs 2.8%). Of the 2,026 invasive procedures undergone in the study cohort, the most frequent was bronchoscopy (n = 856 [42.3%]) followed by transthoracic needle lung biopsy (n = 490 [24.2%]), thoracic surgery (n = 423 [20.9%]), lymph node biopsy (n = 235 [11.6%]), and mediastinoscopy (n = 22 [1.1%]) (Table 2).
Table 2.
Rates of Invasive Procedures in Veterans Who Underwent LCS Between 2013 and 2019 Compared With Participants in the NLST
| Invasive Procedure | Veterans Who Underwent LCS (n = 82,641) | Percentage of Total No. of Procedures (n = 2,026) | Participants in the NLST Low-Dose CT Arm (n = 26,453) | Percentage of Total No. of NLST Procedures (n = 1,718) |
|---|---|---|---|---|
| Bronchoscopy | 856 (1.04) | 42.3 | 671 (2.5) | 39.1 |
| Transthoracic needle lung biopsy | 490 (0.59) | 24.2 | 254 (1.0) | 14.8 |
| Thoracic surgery for pulmonary nodule (thoracotomy or thoracoscopy) | 423 (0.51) | 20.9 | 596 (2.3) | 34.7 |
| Biopsy or excision of lymph node (not in the mediastinum or hilum) | 235 (0.28) | 11.6 | 80 (0.3) | 4.7 |
| Mediastinoscopy or mediastinotomy | 22 (0.03) | 1.1 | 117 (0.44) | 6.8 |
| No. of invasive procedures | 2,026 | 100 | 1,718 | 100 |
Data are presented as No. (%), No., or percentage. LCS = lung cancer screening; NLST = National Lung Screening Trial. At the patient level, 1,741 veterans (2.1%) underwent a total of 2,026 procedures, and in the NLST, a total of 1,106 participants (4.2%) underwent a total of 1,718 procedures.
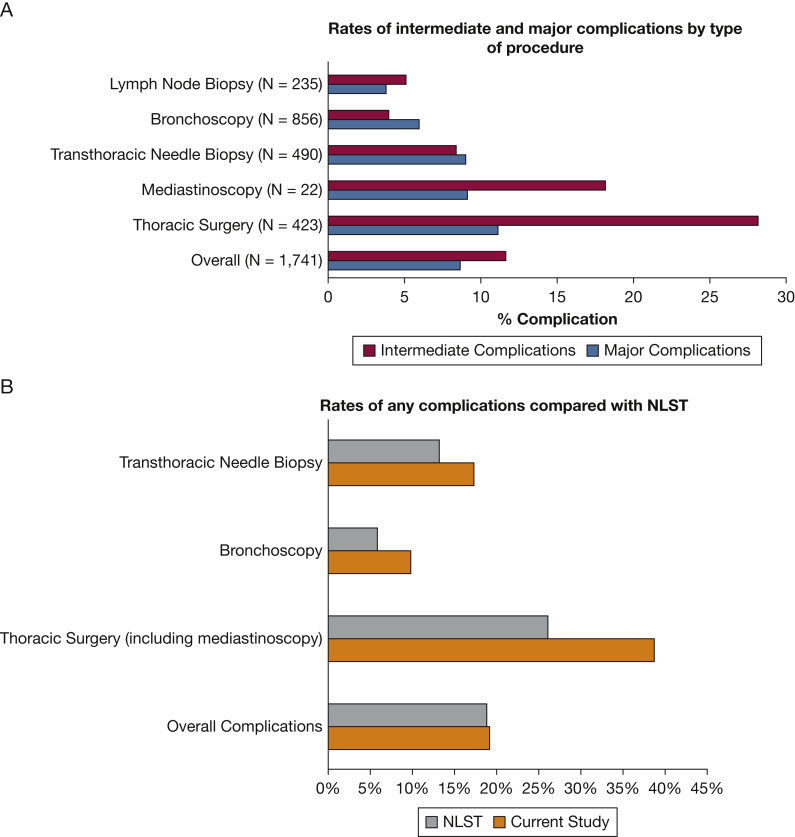
Of the 1,741 veterans who underwent an invasive procedure, 151 (8.7%) experienced a major complication (Fig 1A). The most common major complications were acute respiratory failure (n = 58/1,741 [3.3%]), followed by pneumothorax requiring a chest tube (n = 32/1,741 [1.8%]), prolonged mechanical ventilation (n = 23/1,741 [1.3%]), and sepsis (n = 15/1,741 [0.9%]) (e-Table 4). Additionally, 200 veterans (11.5%) experienced a prolonged hospitalization of > 1 week after the invasive procedure and 18 veterans (1.0%) died within 30 days of an invasive procedure, compared with 1.5% of NLST participants who died within 60 days of the most invasive procedure. An additional 203 of the 1,741 veterans (11.7%) who underwent an invasive procedure experienced an intermediate complication. The most common intermediate complications were cardiac arrhythmias (n = 79/1,741 [4.5%]), pneumothorax not requiring a chest tube (n = 51/1,741 [2.9%]), pleural effusion (n = 36/1,741 [2.1%]), and hemorrhage or hemoptysis (n = 36/1,741 [2.1%]).
Figure 1.
A, B, Bar graphs showing rates of major and intermediate complications developing stratified by type of procedure (A) and compared with the NLST (B). Of note, the NLST reported procedures and complications as a percentage of screenings with positive results, whereas the present study reported complications at the individual patient level. NLST = National Lung Screening Trial.
Although no difference was found in overall complications rates compared with the NLST, when stratified by procedure type, veterans in this cohort more commonly experienced complications for each type of procedure (Fig 1B). Thoracic surgery showed the highest frequencies of major (n = 47 [11.1%]) and intermediate (n = 119 [28.1%]) complications (Fig 1). After thoracic surgery, mediastinoscopy showed the highest presence of complications (major, 9.1%; intermediate, 18.2%), followed by transthoracic needle biopsy (major, 9.0%; intermediate, 8.4%), bronchoscopy (major, 6.0%; intermediate, 4.0%), and lymph node biopsy (major, 3.8%; intermediate, 5.1%). These frequencies of complications by procedure type were higher than those resulting from procedures reported in the NLST: thoracic surgery (major, 11.9%; intermediate, 14.0%), transthoracic needle biopsy (major, 0%; intermediate, 13.1%), and bronchoscopy (major, 1.3%; intermediate, 4.6%) (Fig 1B). Overall, the rate of major plus intermediate complications after invasive procedures in the NLST was similar to that in the present cohort (18.9% for NLST vs 19.1% for the VA group). This can be attributed to the fact that a higher proportion of invasive procedures in the NLST were surgeries (34.7% vs 20.9% in the VA cohort) (Table 2), which are more likely to result in complications than lower-risk procedures.
Multivariate Analysis
Patient-Level Factors
In the multivariate model, veterans were more likely to experience either a major or intermediate complication if they had a history of dementia (OR, 3.91; 95% CI, 1.79-8.52), if the procedure was a thoracic surgery (ie, lung resection or mediastinoscopy; OR, 7.70; 95% CI, 5.48-10.81), or for each additional nonsurgical procedure a veteran underwent (OR, 1.49; 95% CI, 1.15-1.92). Veterans were less likely to experience complications if they harbored comorbid interstitial lung disease (OR, 0.29; 95% CI, 0.10-0.88). No statistically significant differences were found among veterans of different ages or race, nor among cardiovascular comorbidities. Also, no significant differences were found among facility-level covariables (Table 3).
Table 3.
Patient- and Facility-Level Factors Associated With a Complication Developing From a Downstream Procedure of LCSa
| Variable | ORb | 95% CI |
|---|---|---|
| Age | 1.01 | 0.98-1.03 |
| Female sex | 0.61 | 0.29-1.27 |
| Race (White as reference) | ||
| Black | 0.89 | 0.59-1.34 |
| Hispanic | 0.32 | 0.07-1.45 |
| Other | 0.74 | 0.25-2.16 |
| Married | 0.67 | 0.51-0.89 |
| BMI | 1.01 | 0.99-1.02 |
| Thoracic surgery | 7.70 | 5.48-10.81 |
| No. of nonthoracic surgery procedures | 1.49 | 1.15-1.92 |
| Comorbidities | ||
| Elixhauser comorbidity index score | 1.04 | 0.98-1.10 |
| Major adverse cardiac event | 0.64 | 0.38-1.08 |
| Stroke | 1.53 | 0.83-2.80 |
| COPD | 1.17 | 0.87-1.58 |
| Congestive heart failure | 1.05 | 0.58-1.91 |
| Dementia | 3.91 | 1.79-8.52 |
| Depression | 0.95 | 0.67-1.35 |
| Posttraumatic stress disorder | 1.20 | 0.82-1.76 |
| Substance use disorder | 0.93 | 0.66-1.32 |
| Interstitial lung disease | 0.29 | 0.10-0.88 |
| Facility-level characteristics | ||
| Geography (East as reference) | ||
| Midwest | 0.91 | 0.49-1.68 |
| South | 1.01 | 0.58-1.76 |
| West | 1.27 | 0.65-2.47 |
| No. of LCS examinations performedc | 0.96 | 0.78-1.17 |
LCS = lung cancer screening.
No missing covariables are included in the model that are not reported in Table 3.
Boldface values are statistically significant at P < .05.
Reflects log-transformed variables.
Facility-Level Variation
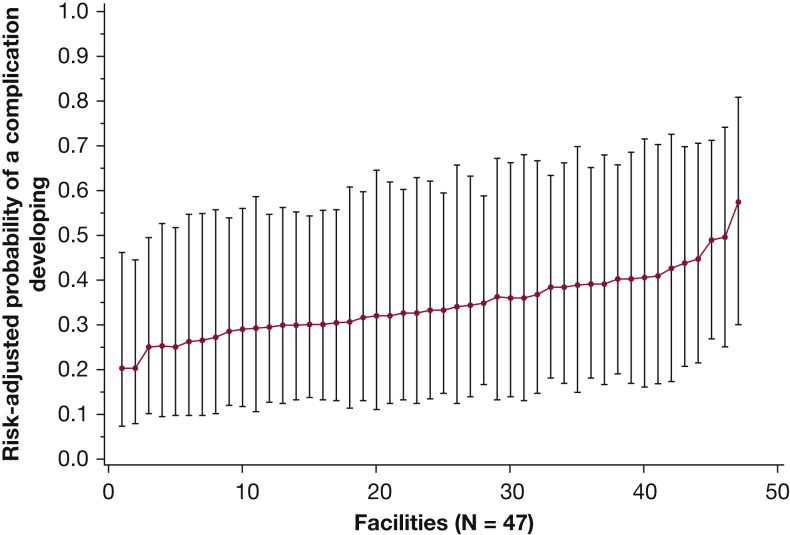
The multivariate model with no facility effects other than intercepts varying by facility showed an intraclass correlation coefficient of 0.07, suggesting that 7% of the variation in the odds of a complication developing is attributed to facilities, rather than to differences in the veterans who receive care at those facilities. To examine variation by facility further, we plotted this variation across facilities in odds of complications developing, with point estimates for the probability of a complication developing varying from 20.3% to 57.4% across facilities (although the CI for even the highest complication-rate facility overlapped with the point estimate from the lowest complication-rate facility) (Fig 2).
Figure 2.
Graph showing facility-level variation in the development of complications after a downstream procedure from lung cancer screening for a veteran with mean characteristics in the study cohort. Facilities are ranked by patient-adjusted probability of a complication developing. Bars represent 95% CIs.
Sensitivity Analyses
No changes were found in the direction of statistically significant mean effects in multivariate models separating thoracic surgery (including mediastinoscopy) and nonsurgical procedures (bronchoscopy, transthoracic needle biopsy, and lymph node biopsy) (e-Table 5). Other sensitivity analyses showed similar proportions of veterans who experienced complications when excluding those ineligible for Medicare (8.5% experienced major complications and 11.9% experienced intermediate complications) or when restricting the study period to fiscal years 2015 through 2018 (9.1% and 12.2% of veterans with major and intermediate complications, respectively) (e-Table 6).
Discussion
To our knowledge, this study is the largest analysis of complications from invasive procedures in a national cohort of patients undergoing LCS in a nontrial setting and demonstrated that complications resulting from LCS-related procedures in practice occur more frequently than observed in clinical trials for each type of invasive procedure. The higher complication rate in this cohort likely reflects the fact that patients undergoing screening in practice are older, more racially diverse, and harbor more cardiopulmonary comorbidities than NLST participants (Table 1). Veterans were less likely to undergo an invasive procedure after initial LCS than NLST participants, particularly surgical procedures, but were more likely to experience a complication if they did (Table 2, Fig 1). These findings may reflect a higher threshold of VA providers to perform invasive procedures, perhaps because of concerns about complications when procedures are performed on veterans with multiple comorbidities.27,28 In nontrial populations with complex, multimorbid disease, it is unclear that the low postprocedural complication rates achieved by the NLST can be replicated.
This study also supported findings from Zhao et al,13 who showed that commercially insured patients undergoing an initial LCS underwent invasive procedures less frequently (2.5%), but demonstrated an overall 77% higher complication rate than those in the NLST. Similar to Zhao et al’s work, our study showed lower procedural rates and higher rates of postprocedure complications in clinical practice compared with the NLST, but our study has a few key differences. The present study cohort was derived from veterans who were eligible for LCS and analyzed factors associated with the development of complications, whereas Zhao et al’s study focused on participants enrolled in private insurance and was unable to verify LCS eligibility. Additionally, the methodology varied, with our study identifying complications occurring within 10 days of an invasive procedure, whereas the work by Zhao et al13 identified complications occurring within 3 months of a procedure with a 1:1 case-control matching methodology. Overall, the presence of similar findings between these two studies with different methodologies strengthens the evidence that complications occurring after invasive procedures are more common in clinical practice than those reported in the NLST.
Not surprisingly, procedures that are considered more invasive, such as thoracic surgery and mediastinoscopy, showed higher rates of complications. This was supported further by multivariate models showing that the highest odds of a complication developing were associated with undergoing thoracic surgery. These findings support the American College of Chest Physicians recommendation to perform noninvasive tests (ie, PET/CT imaging) or nonsurgical biopsies (ie, transthoracic needle biopsy or endobronchial ultrasound bronchoscopy) as first-line diagnostic tests for nodules with an intermediate risk of cancer and to reserve immediate surgical resection for patients in whom the probability of malignancy before testing is high.29 Indeed, in our study, an increasing number of yearly LCS examinations was found without an increase in yearly procedures, suggesting that procedures occur less frequently in clinical practice compared with clinical trials as comfort level with screen-detected findings and diagnostic technologies evolve over time.
In multivariate analyses, veterans with dementia were more likely to experience complications, highlighting the importance of considering comorbidities when deciding if a patient is appropriate for screening.30 Interestingly, our study also found that veterans with interstitial lung disease, were less likely to experience complications resulting from invasive lung procedures, which is contrary to prior studies showing an increased risk of surgical complications and the potential for exacerbation in patients with interstitial lung disease.31,32 Surprisingly, our work did not confirm our hypothesis and prior findings that COPD or age were associated with increased risk of complications developing as a result of surgical and nonsurgical lung procedures.19,20 Although we are unable to quantify the severity of lung disease, it may be that given the knowledge of increased surgical risks, older veterans with more severe lung disease and their providers are choosing not to perform LCS or downstream procedures, leaving only a subset of relatively healthier veterans with lower risk of complications to undergo invasive procedures. Our study also found that both COPD and interstitial lung disease were more frequent in the cohort undergoing invasive procedures compared with all veterans who underwent screening, suggesting that patients with underlying lung disease are more likely to demonstrate abnormal LCS results.33, 34, 35 Our study highlights the complexity of accurately predicting who will experience a complication resulting from screening and that older age and some pulmonary diseases may not be as predictive of complications as some clinicians may assume. However, given the higher rate of procedural harm in clinical practice, our study also underlined the importance of accurately identifying those patients at high risk of lung cancer and with reasonable life expectancy for whom the benefits of screening outweigh the harms.36, 37, 38, 39
The facility at which a veteran underwent a procedure was responsible for 7% of the variation in a complication developing. This relatively low level of variation in complication rates may be explained by the VA’s large integrated health care network, which typically has reduced disparities and improved outcomes in lung cancer.40, 41, 42, 43 For example, referral pathways exist for veterans cared for in smaller facilities to undergo the initial LCS at their home facility, but receive downstream evaluation at a larger VA facility with more experience in performing invasive procedures.44 Studies assessing complications of invasive procedures for pulmonary nodules in the private sector have shown greater variation.19
This study has limitations. First, we relied on administrative data, which can underestimate the presence of downstream complications if complications were undercoded. We also were unable to capture care received outside of VA or Medicare, which may have resulted in an underestimate of procedures and complications if substantial care was received through private insurers. Indeed, procedure rates may be higher outside of the VA system, where providers are incentivized by fee-for-service payment structures to perform more procedures.45,46 However, our sensitivity analysis excluding veterans younger than 65 years, who are more likely to be dual users of VA and private insurance, showed no differences in rates of procedures or complications. Our results may not be generalizable outside of the VA. In particular, veterans are not representative of the entire US population, with veterans more likely to be men, to be White, and more frequently to have comorbidities than the LCS-eligible US population.27 Additionally, the VA’s national integrated health care system is unique and has established processes to facilitate multidisciplinary cancer care and pulmonary nodule evaluation, which could affect the rates of invasive procedures.43,47,48 Finally, the COVID-19 pandemic may have impacted the frequency of downstream procedures of screen-detected findings if patients and providers elected to defer evaluation of screen-detected findings to reduce infection risk.49 However, our sensitivity analysis restricting the study period to the end of 2018 showed no differences in the rates of procedures performed.
Interpretation
Our study represents the largest analysis of complications from downstream procedures in lung cancer screening. We found that in clinical practice, veterans undergo an invasive procedure half as frequently as in the NLST, but that those who do undergo procedures are more likely to experience a complication for every type of procedure. These findings may reflect greater discrimination in decision-making about when to perform invasive procedures for a veteran population at higher risk of complications because of multiple comorbidities. Future work should focus on minimizing procedural complications and optimizing the identification of patients whose chance of benefit likely outweighs the complication risks.38
Acknowledgments
Author contributions: E. R. N., T. J. C., S. Z., M. E. G., D. R. M., and R. S. W. conceived and designed the study. E. R. N., S. X. Q., J. H. B., S. Z., D. R. M., and R. S. W. acquired study data. All authors analyzed and interpreted the data. E. R. N., T. J. C., S. Z., M. E. G., and R. S. W. drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. R. S. W. obtained funding and supervised the study.
Funding/support: This work was supported by Veterans Affairs Health Services Research and Development Service Investigator Initiated Research [Grant 18-075 (R. S. W.]), the National Heart, Lung, and Blood Institute [Grant 5T32HL007035], and by resources from the VA Bedford, VA Boston, and VA Ann Arbor Healthcare Systems. Support for Veterans Affairs and Centers for Medicare and Medicaid Services data provided by the Department of Veterans Affairs, the Veterans Affairs Health Services Research and Development Service, and the Veterans Affairs Information Resource Center [project nos. SDR 02-237 and 98-004].
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclaimer: The views expressed in this article do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
Additional information: The e-Figure and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.World Health Organization, International Agency for Research on Cancer . International Agency for Research on Cancer website; 2020. Global cancer observatory: cancer today.https://gco.iarc.fr/today/home [Google Scholar]
- 2.National Lung Screening Trial Research Trial. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey L.L., Deffebach M., Pappas M., et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 4.Kovalchik S.A., Tammemagi M., Berg C.D., et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau YK, Caverly TJ, Cherng ST, et al. Development and validation of a personalized, web-based decision aid for lung cancer screening using mixed methods: a study protocol. JMIR Res Protoc. 2014;3(4):e78. doi: 10.2196/resprot.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effective Health Care Program, Agency for Healthcare Research and Quality. Overview: Is Lung Cancer Screening Right for Me? Accessed October 1, 2020. https://effectivehealthcare.ahrq.gov/products/lung-cancer-screening/overview
- 7.Burke L.G., Frakt A.B., Khullar D., Orav E.J., Jha A.K. Association between teaching status and mortality in US hospitals. JAMA. 2017;317:2105–2113. doi: 10.1001/jama.2017.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke L., Khullar D., Orav E.J., Zheng J., Frakt A., Jha A.K. Do academic medical centers disproportionately benefit the sickest patients? Health Aff (Millwood) 2018;37:864–872. doi: 10.1377/hlthaff.2017.1250. [DOI] [PubMed] [Google Scholar]
- 9.Aberle D.R., Adams A.M., Berg C.D., et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iaccarino J.M., Steiling K.A., Wiener R.S. Lung cancer screening in a safety-net hospital: implications of screening a real-world population versus the National Lung Screening Trial. Ann Am Thorac Soc. 2018;15:1493–1495. doi: 10.1513/AnnalsATS.201806-389RL. [DOI] [PubMed] [Google Scholar]
- 11.Dhruva S.S., Redberg R.F. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy-Martin T., Curtis S., Faries D., Robinson S., Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H., Xu Y., Huo J., Burks A.C., Ost D.E., Shih Y.-C.T. Updated analysis of complication rates associated with invasive diagnostic procedures after lung cancer screening. JAMA Netw Open. 2020;3:e2029874. doi: 10.1001/jamanetworkopen.2020.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo J., Xu Y., Sheu T., Volk R.J., Shih Y.T. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179:324–332. doi: 10.1001/jamainternmed.2018.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinsky P.F., Gierada D.S., Black W., et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162:485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsinger L.S., Anderson C., Kim J., et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 17.Boudreau J.H., Miller D.R., Qian S., Nunez E.R., Caverly T.J., Wiener R.S. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358–367. doi: 10.1016/j.chest.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Núñez E.R., Caverly T.J., Zhang S., et al. Adherence to follow-up testing recommendations in US veterans screened for lung cancer, 2015-2019. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener R.S., Schwartz L.M., Woloshin S., Welch H.G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain Z.A., Kim A.W., Yu J.B., Decker R.H., Corso C.D. Defining the high-risk population for mortality after resection of early stage NSCLC. Clin Lung Cancer. 2015;16:e183–e187. doi: 10.1016/j.cllc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 21.van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 22.Gould M.K., Munoz-Plaza C.E., Hahn E.E., Lee J.S., Parry C., Shen E. Comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Ann Am Thorac Soc. 2017;14:1571–1580. doi: 10.1513/AnnalsATS.201701-030OC. [DOI] [PubMed] [Google Scholar]
- 23.Craney T.A., Surles J.G. Model-dependent variance inflation factor cutoff values. Quality Engineering. 2002;14:391–403. [Google Scholar]
- 24.Wu S., Crespi C.M., Wong W.K. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33:869–880. doi: 10.1016/j.cct.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holder K.A., Cheeseman Day J. Health insurance coverage of veterans. US Census Bureau website. 2017. https://www.census.gov/newsroom/blogs/random-samplings/2017/09/health_insurancecov0.html#
- 26.Zelaya C.E., Nugent C.N. Trends in health insurance and type among military veterans: United States, 2000-2016. Am J Public Health. 2018;108:361–367. doi: 10.2105/AJPH.2017.304212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agha Z., Lofgren R.P., VanRuiswyk J.V., Layde P.M. Are patients at Veterans Affairs Medical Centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi R.B., Post E.P., Sun H., et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564–2569. doi: 10.2105/AJPH.2015.302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould M.K., Donington J., Lynch W.R., et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyer V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 31.Lettieri C.J., Veerappan G.R., Helman D.L., Mulligan C.R., Shorr A.F. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127:1600–1605. doi: 10.1378/chest.127.5.1600. [DOI] [PubMed] [Google Scholar]
- 32.Kreider M.E., Hansen-Flaschen J., Ahmad N.N., et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Jin G.Y., Lynch D., Chawla A., et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatabu H., Hunninghake G.M., Richeldi L., et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med. 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruparel M., Quaife S.L., Dickson J.L., et al. Prevalence, symptom burden, and underdiagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc. 2020;17:869–878. doi: 10.1513/AnnalsATS.201911-857OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiener R.S., Gould M.K., Arenberg D.A., et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzone P.J., Silvestri G.A., Patel S., et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2018;153:954–985. doi: 10.1016/j.chest.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Mazzone P.J., Silvestri G.A., Souter L.H., et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427–e494. doi: 10.1016/j.chest.2021.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caverly T.J., Cao P., Hayward R.A., Meza R. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;169:1–9. doi: 10.7326/M17-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganti A.K., Subbiah S.P., Kessinger A., Gonsalves W.I., Silberstein P.T., Loberiza F.R., Jr. Association between race and survival of patients with non–small-cell lung cancer in the United States Veterans Affairs population. Clin Lung Cancer. 2014;15:152–158. doi: 10.1016/j.cllc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 41.May F.P., Yano E.M., Provenzale D., Neil Steers W., Washington D.L. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62:1923–1932. doi: 10.1007/s10620-017-4607-x. [DOI] [PubMed] [Google Scholar]
- 42.Landrum M.B., Keating N.L., Lamont E.B., et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30:1072–1079. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keating N.L., Landrum M.B., Lamont E.B., et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Ann Intern Med. 2011;154:727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 44.Greenstone C.L., Peppiatt J., Cunningham K., et al. Standardizing care coordination within the Department of Veterans Affairs. J Gen Intern Med. 2019;34:4–6. doi: 10.1007/s11606-019-04997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueroa J.F., Blumenthal D.M., Feyman Y., et al. Differences in management of coronary artery disease in patients with Medicare Advantage vs traditional fee-for-service Medicare among cardiology practices. JAMA Cardiol. 2019;4:265–271. doi: 10.1001/jamacardio.2019.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matlock D.D., Groeneveld P.W., Sidney S., et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310:155–162. doi: 10.1001/jama.2013.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landrum M.B., Keating N.L., Lamont E.B., et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30:1072–1079. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmons J., Gould M.K., Iaccarino J., Slatore C.G., Wiener R.S. Systems-level resources for pulmonary nodule evaluation in the United States: a national survey. Am J Respir Crit Care Med. 2016;193:1063–1065. doi: 10.1164/rccm.201511-2163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzone P.J., Gould M.K., Arenberg D.A., et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST expert panel report. Chest. 2020;158:406–415. doi: 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.