Abstract
The present study evaluated the anti-cancer activity of histone deacetylase (HDAC)-inhibiting CKD-581 in multiple myeloma (MM) and its pharmacological mechanisms. CKD-581 potently inhibited a broad spectrum of HDAC isozymes. It concentration-dependently inhibited proliferation of hematologic cancer cells including MM (MM.1S and RPMI8226) and T cell lymphoma (HH and MJ). It increased the expression of the dishevelled binding antagonist of β-catenin 3 (DACT3) in T cell lymphoma and MM cells, and decreased the expression of c-Myc and β-catenin in MM cells. Additionally, it enhanced phosphorylated p53, p21, cleaved caspase-3 and the subG1 population, and reversely, downregulated cyclin D1, CDK4 and the anti-apoptotic BCL-2 family. Finally, administration of CKD-581 exerted a significant anti-cancer activity in MM.1S-implanted xenografts. Overall, CKD-581 shows anti-cancer activity via inhibition of the Wnt/β-catenin signaling pathway in hematologic malignancies. This finding is evidence of the therapeutic potential and rationale of CKD-581 for treatment of MM.
Keywords: CKD-581, DACT3, HDAC, Wnt/β-catenin pathway, Hematologic cancer
INTRODUCTION
Multiple myeloma (MM) is a cancer originating from plasma cells (Bergsagel and Kuehl, 2005). MM is the second most frequent hematologic malignancy; approximately 10% of the all hematologic malignancy patients are diagnosed with MM (Rajkumar et al., 2014). Although the survival rate of patients has been improved due to the development of effective agents such as proteasome inhibitors, immunomodulatory drugs and monoclonal antibodies, MM patients eventually become resistant to all forms of chemotherapies (de la Puente et al., 2014; Giuliani et al., 2019; Imai et al., 2019). Hence, new therapeutic options for resistant patients are urgently needed.
The diverse roles of epigenetic modifications of DNA and histones in cancer development have been extensively studied (Li and Seto, 2016). Among them, histone acetylation is controlled by two enzymes, histone acetyltransferase (HAT) and histone deacetylases (HDAC) (Sanchez et al., 2011; Singh et al., 2018). HATs are enzymes that acetylate lysine residues of the target proteins, and HDACs remove acetyl groups from lysine residues (Singh et al., 2018). Imbalances between HAT and HDAC activities are associated to the development and progression of many cancers (Sanchez et al., 2011; Dawson and Kouzarides, 2012). Also, loss of acetylation and aberrant expression of various HDACs seem to be related to poor prognosis in cancer patients. For example, loss of acetylation in histone H4 lysine 16 (H4K16) is considered to be a tumor hallmark (Fraga et al., 2005) and a predictive marker of poor prognosis in breast cancer (Elsheikh et al., 2009). Moreover, high expression levels of HDAC1, 2 and 3 are closely related to poor outcomes in ovarian and gastric cancer patients (Weichert et al., 2008a, 2008b). In MM, dysregulation of histone acetylation is also believed to be a critical pathogenic process (Imai et al., 2019). HDACs are frequently overexpressed in plasma cells sampled from MM patients (Yuan et al., 2019), and higher HDAC expression is correlated with poor overall-survival and progression-free survival outcomes in MM patients (Mithraprabhu et al., 2014). Hence, it has been suggested that HDACs are an attractive therapeutic target for MM treatment (Sivaraj et al., 2017).
CKD-581 is a pan-HDAC inhibitor that targets class I–II HDACs (Kim et al., 2020). A phase I clinical study was conducted to evaluate the safety and tolerability of CKD-581 for patients with lymphoma and MM. CKD-581 was well tolerated, and 44.4% patients achieved a stable disease (SD) or better (Cho et al., 2018). CKD-581 combination with standard chemotherapy was evaluated in patients with previously-treated MM. Combination of CKD-581, lenalidomide and dexamethasone was safe and objective response rate (ORR) was 70% (Min et al., 2019).
Wnt/β-catenin signal transduction plays a critical role in the regulation of cell proliferation, differentiation and apoptosis in MM cells (Kim et al., 2011). Dishevelled binding antagonist of β-catenin 3 (DACT3) is known to be a key regulator of Wnt/β-catenin signaling in esophageal squamous cell carcinoma, colorectal, breast and lung cancer types (Jiang et al., 2008; Xi et al., 2010; Beltran et al., 2011; Ren et al., 2017; Zhao et al., 2017). Notwithstanding a recent report demonstrating DACT3 to be a negative regulator of the Wnt/β-catenin pathway (Jiang et al., 2008), HDAC-dependent DACT3 regulation and its function on the Wnt/β-catenin pathway have yet to be studied in hematologic malignancies. In the present study, we revealed that CKD-581 (alteminostat), a novel pan-HDAC inhibitor, downregulated oncoproteins with DACT3 induction in MM cells, and further, we elucidated its pharmacological effectiveness in MM xenograft experiments.
MATERIALS AND METHODS
Antibodies and compounds
Suberoylanilide hydroxamic acid (SAHA, Vorinostat) was obtained from Sigma-Aldrich (St. Louis, MO, USA). CKD-581 (purity 98.80%, M.W. 588.72) was supplied from CKD Pharmaceutical Corporation (Seoul, Korea). CellTiter-Glo luminescent cell viability assay kit was purchased from Promega (Madison, WI, USA). Antibodies recognizing DACT1, DACT3, β-catenin, Cyclin D1, CDK4, p53, BCL-xL and BCL-2 were supplied from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against histone H3, acetylated histone H3, histone H4, acetylated histone H4, acetylated tubulin, p21, phospho-p53 cleaved-caspase 3, AKT, phospho-AKT, p70S6K, phospho-p70S6K (Thr389), mTOR and phospho-mTOR (Ser2448) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies targeting DACT2, c-Myc and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
HDAC enzyme assay
In vitro HDAC enzyme assays were performed by Reaction Biology Corporation (Malvern, PA, USA). Ten points dose response curve for CKD-581 was evaluated for each HDAC isozymes. IC50 values for HDAC isozymes were calculated using the GraphPad Prism 5 program (Irvine, CA, USA). The values are presented as average of at least three different experiments.
Cell culture
Human multiple myeloma cell lines (MM.1S and RPMI8226) and human T cell lymphoma cell lines (HH and MJ) were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). MM.1S, RPMI8226 and HH cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific) that is supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific) at 37°C in 5% CO2. MJ cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM, Thermo Fisher Scientific) containing 20% FBS.
Cell proliferation assay
Cells cultured in 96-well plates were treated with vehicle (0.1% DMSO), CKD-581 or SAHA. Cell viability was determined by CellTiter-Glo assay system (Promega) 72 h after compound treatment. Luminescence was determined using FlexStation 3 (Molecular Devices, San Jose, CA, USA).
Histone protein extraction
Histone proteins were extracted in MM.1S cells treated with CKD-581 or SAHA for 6 h. Histone protein extraction was performed as previously described (Kim et al., 2020). The extracted proteins were subjected to analysis of histone protein acetylation status.
cDNA microarray and gene expression analysis
Total RNA was isolated and purified using Trizol (Thermo Fisher Scientific) and RNeasy Mini Kit (Qiagen, Hilden, NRW, Germany), respectively. The microarray hybridization was performed using the Infinium® HumanHT-12 BeadChip (Illumina, San Diego, CA, USA). Analyses and figure generation were conducted using R (https://www.r-project.org/, version 3.5.2). Raw data were normalized using quantile algorithm and heatmaps were plotted using heatmap.2 from gplots R package (https://cran.r-project.org/web/packages/gplots/index.html, version 3.0.1.1).
Western blot analysis
Cell lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) supplemented with protease and phosphatase inhibitor cocktail (Roche, Basel, Switzerland). BCA protein assay kit (Thermo Fisher Scientific) was used for quantification of protein concentration. Western blotting was performed as previously described (Kim et al., 2020). GAPDH was used for normalization of all immunoblot results. ChemiDoc (Biorad, Hercules, CA, USA) was used to detect chemiluinescence signals.
Flow cytometry analysis
MM.1S and RPMI8226 cells were incubated with vehicle or CKD-581 for 24 h. Cells were harvested and fixed with 70% ethanol in phosphate-buffered saline at –20°C for 24 h. The fixed cells were stained with propidium iodide staining solution at room temperature, and the stained cells were analyzed by flow cytometry (FACSCalibur, BD Bioscience, NJ, USA).
Xenograft assay
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of CKD Research Institute (Approval # S014-037, July 21, 2014). Six-week-old NOD.CB17 SCID mice were subcutaneously implanted with 2×107 MM.1S cells suspended in Matrigel (Trevigen, Gaithersburg, MD, USA). Tumor volume and body weight gain were monitored two times per week.
Statistical analysis
One-way ANOVA was used to assess the differences in all experiments except tumor xenograft experiment. Two-way ANOVA or t-test was used for tumor xenograft experiment. Significance was expressed as *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
HDAC inhibition by CKD-581
To investigate the inhibitory activities of CKD-581 (Fig. 1A) on HDACs, In vitro HDAC inhibition assays using purified HDAC isozymes were conducted. CKD-581 treatment markedly inhibited most of the tested HDAC isozymes, and especially, it potently inhibited HDAC1, 2, 3, 6, 9, 10 and 11 at a concentration as low as 30 nM (Table 1). Further, the results of enzyme assays confirmed that CKD-581 is a potent pan-HDAC inhibitor (Kim et al., 2020).
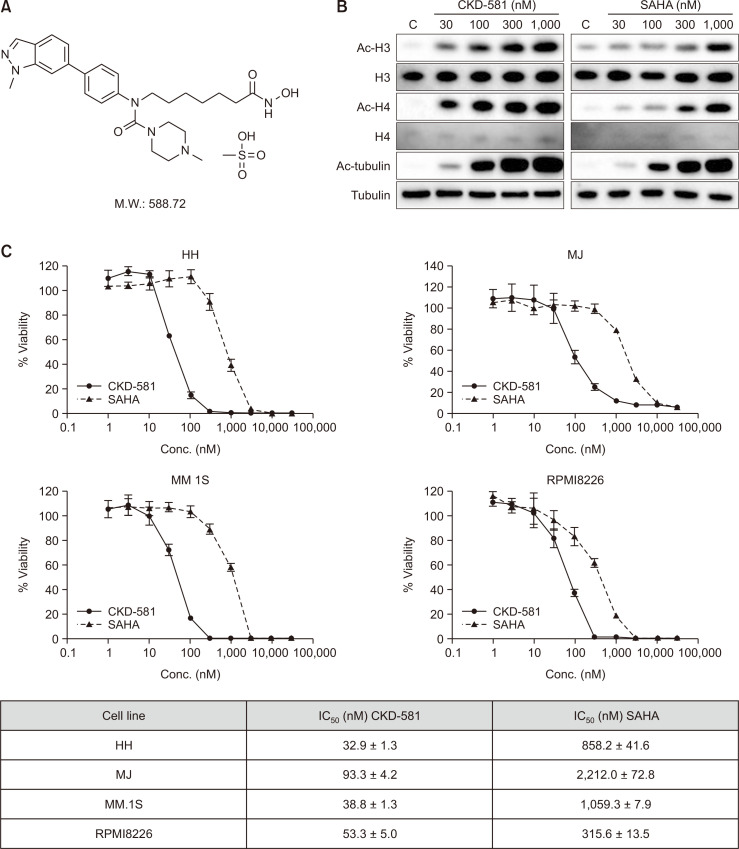
Fig. 1.
CKD-581 increases acetylated proteins and suppresses cell proliferation in hematologic cancer cells. (A) The chemical structure of CKD-581. (B) Acetylation of histone H3, H4 and tubulin. MM.1S cells were treated with 30-1000 nM CKD-581 or SAHA for 6 h. Acetylation of proteins was analyzed by immunoblottings. (C) Inhibitory effects of CKD-581 and SAHA on cell viabilities of hematologic cancer cell lines. All data represent mean ± SEM of at least three independent experiments.
Table 1.
Effects of CKD-581 on HDAC enzymes. HDAC enzyme activity was determined by fluorescence based-enzymatic assays
| HDAC enzyme assay IC50 (nM) | ||
|---|---|---|
| Class I | HDAC1 | 0.50 ± 0.11 |
| HDAC2 | 2.22 ± 0.39 | |
| HDAC3 | 0.61 ± 0.09 | |
| HDAC8 | 49.82 ± 18.24 | |
| Class IIa | HDAC4 | 1502 ± 109 |
| HDAC9 | 0.95 ± 0.69 | |
| HDAC11 | 26.91 ± 19.12 | |
| Class IIb | HDAC6 | 2.62 ± 0.32 |
| HDAC10 | 1.14 ± 0.76 | |
SAHA (Vorinostat®), a HDAC inhibitor, has been approved for the treatment of refractory cutaneous and peripheral T cell lymphoma (Dawson and Kouzarides, 2012; Singh et al., 2018). The acetylation intensity of histone H3 and H4 was increased following exposure to CKD-581 or SAHA in MM.1S cells. Additionally, the acetylation of tubulin also was increased by CKD-581 or SAHA, and the acetylation intensity was stronger by CKD-581 than by SAHA in histone H3 and H4 (Fig. 1B). According to the overall results, CKD-581 potently inhibited both class I and class II HDAC enzyme activities and subsequently induced acetylation of target molecules in MM cells.
Suppression of cell viabilities of T cell lymphoma and MM cells by CKD-581
The inhibitory effects of CKD-581 on the cell viabilities of T cell lymphoma cell lines (HH and MJ) and MM cell lines (MM.1S and RPMI8226) were assessed using the CellTiter-Glo luminescent cell viability assay system. CKD-581 concentration-dependently inhibited the cell viabilities of the T cell lymphoma and MM cell lines. The IC50 values of CKD-581 in HH, MJ, MM.1S and RPMI8226 were 32.9 ± 1.3, 93.3 ± 4.2, 38.8 ± 1.3 and 53.3 ± 5.0 nM, respectively (Fig. 1C). Moreover, The IC50 values of CKD-581 were at least 6-fold lower than those of SAHA. The data indicated that CKD-581 had potently induced cancer cell death in both T cell lymphoma and MM cells.
Selective induction of DACT3 by CKD-581 and subsequent inhibition of Wnt/β-catenin signaling
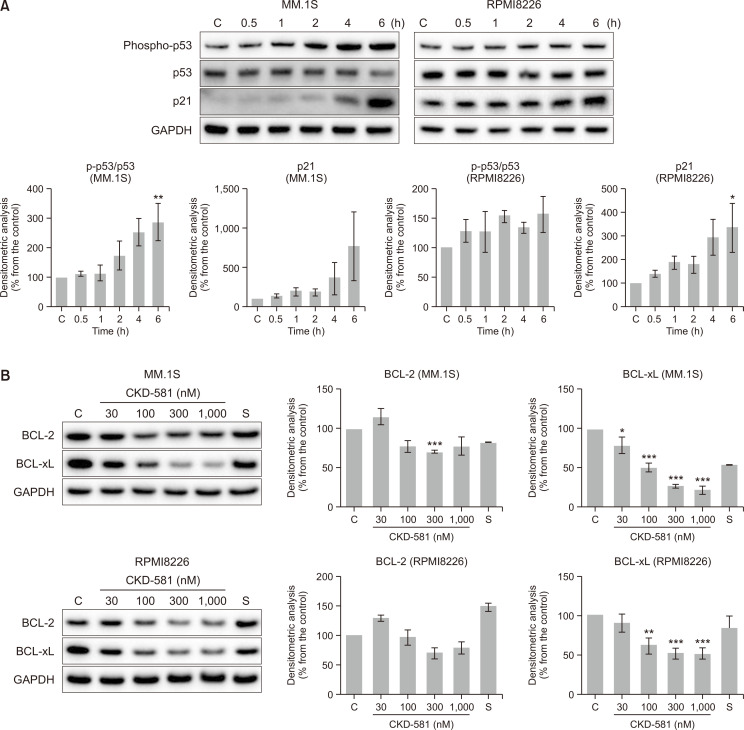
To characterize the pharmacological mechanism of the anti-cancer activities of CKD-581 in hematologic malignancies, a cDNA microarray was performed in the two T cell lymphoma cell lines (HH and MJ cells) exposed to vehicle or CKD-581 for 6, 12 and 24 h. The mRNA expression patterns were analyzed by Infinium® HumanHT-12 BeadChip (48K probes). Heatmap analysis of the gene expression profiles showed that the genes related to the Wnt/β-catenin pathway were significantly affected by CKD-581 treatment (Fig. 2A). Wnt/β-catenin signaling is one of the key signaling pathways for control of the development and stemness of cancer in hematologic malignancies (Wang et al., 2009). Interestingly, DACT3 expression was most distinctly increased in the two cell lines at all of the tested time points (Fig. 2A).
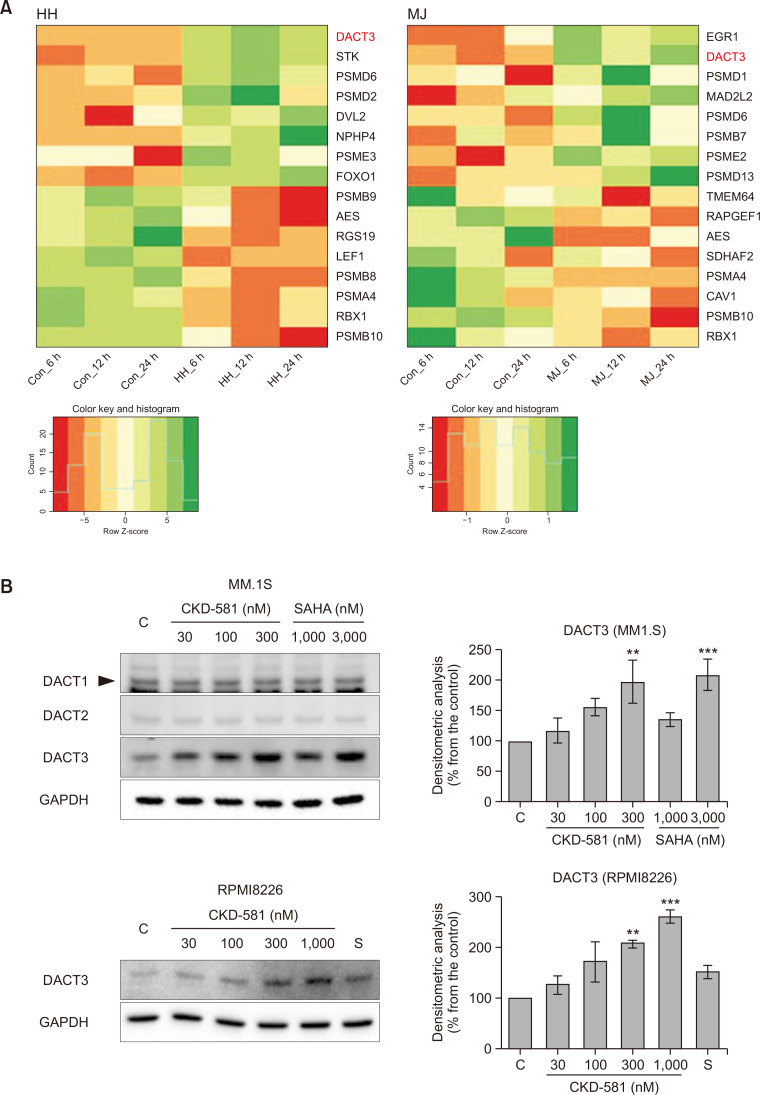
Fig. 2.
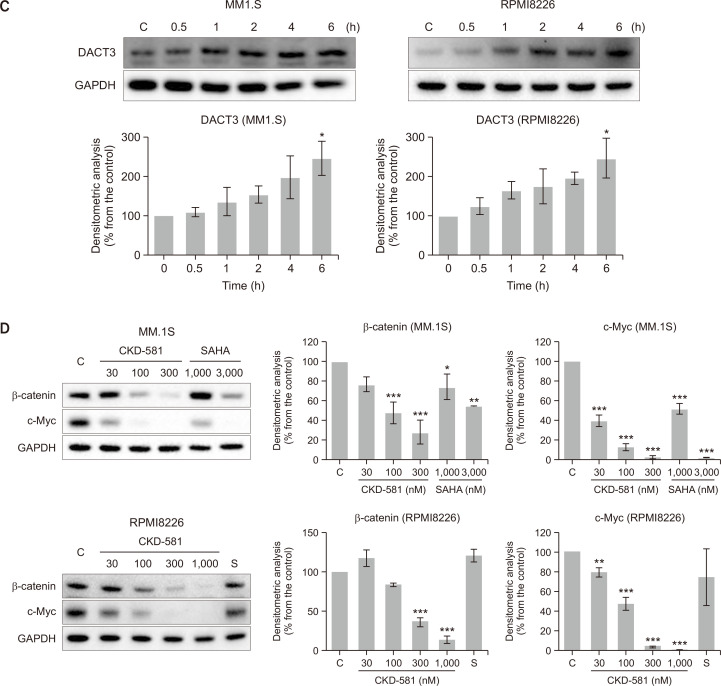
CKD-581 down-regulates Wnt/β-catenin pathway. (A) Heatmap analysis of gene expression profiles in hematologic cancer cells. HH and MJ cells were treated with 100 nM CKD-581, and Total RNA samples were purified. Expression level of mRNA was analyzed with the Infinium® HumanHT-12 BeadChip. (B) Effects of HDAC inhibitors on DACT family expression. MM.1S and RPMI8226 cells were treated with the indicated concentrations of CKD-581 or SAHA for 24 h, and total cell lysates were subjected to immunoblottings. (C) DACT3 expression induced by CKD-581. MM.1S and RPMI8226 cells were treated with 300 nM CKD-581 in a time-dependent manner. (D) MM.1S and RPMI8226 cells were treated with various concentrations of CKD-581 or SAHA for 24 h. And total cell lysates were analyzed by immunoblottings for β-catenin and c-Myc. Data represent mean ± SEM (significant vs. control; *p<0.05, **p<0.01, ***p<0.001). C, control; S, 1000 nM SAHA.
Genes of which the expression levels increased or decreased by CKD-581 more than 4-fold relative to vehicle-treated control samples were selected from cDNA microarray data. A total of 19 genes were identified as down-regulated and 9 genes as up-regulated by CKD-581 in HH cells, at 3 time points commonly. In the case of MJ cells, there was no commonly down-regulated gene, and only one gene, DACT3, was identified as up-regulated by CKD-581 treatment at the three different time points. Therefore, DACT3 was the only gene modulated by CKD-581 in both cell types and at all time points (Supplementary Fig. 1A).
DACT3 is known to be an epigenetic regulator of Wnt/β-catenin signaling in colorectal cancer (Jiang et al., 2008) and non-small cell lung cancer (NSCLC) (Zhao et al., 2017). It has been reported that DACT3 expression is regulated by histone modifications (Jiang et al., 2008). To further confirm whether CKD-581 induces DACT3 in MM cell lines, the protein expression levels of the DACT family were determined in CKD-581- or SAHA-treated MM.1S cells. The protein expression of DACT3 was significantly enhanced by 300 nM CKD-581 or 3000 nM SAHA (Fig. 2B), and the protein expression of DACT3 was increased in a time-dependent manner (Fig. 2C). Notably, the protein expression of the other two DACT family members, DACT1 and DACT2, was not altered by CKD-581 or SAHA in MM.1S cells. We also found that, in RPMI8226 cells, DACT3 was induced by 300 and 1000 nM CKD-581 but not by 1000 nM SAHA (Fig. 2B).
We then tested the effects of CKD-581 on the expression of β-catenin and c-Myc, which are representative downstream target genes of the Wnt/β-catenin pathway in MM cells. As expected, CKD-581 markedly decreased the protein levels of β-catenin and c-Myc in MM.1S and RPMI8226 cells, and its inhibition intensity was stronger than that of SAHA (Fig. 2D). These results suggest that CKD-581 inactivates the Wnt/β-catenin pathway, presumably via DACT3 upregulation in MM cells.
Downregulation of cyclin D1/CDK4 and apoptosis induction by CKD-581
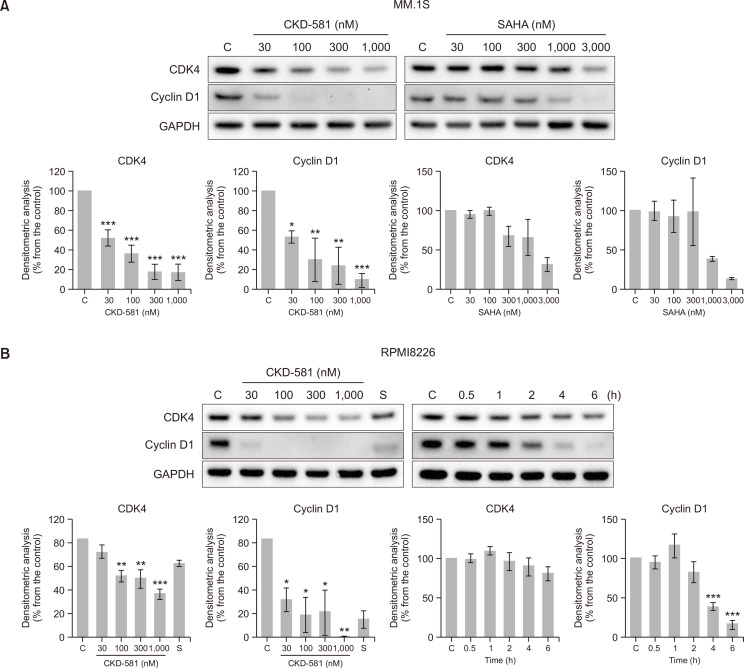
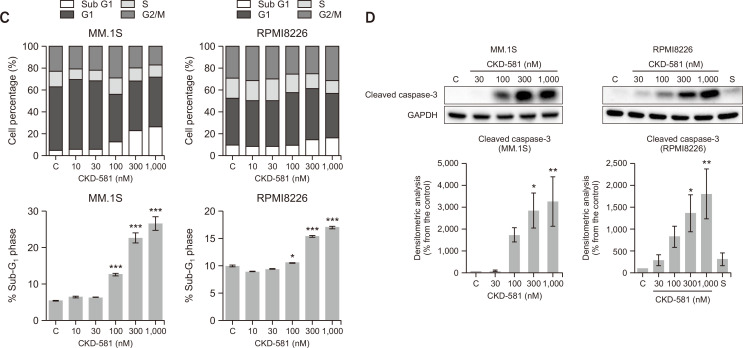
Overexpression of β-catenin is known to increase the expression of several target oncogenes such as CCND1 and c-myc (Xi and Chen, 2014). Cyclin D1 is a key regulator of the cell cycle for stimulation of S-phase entry of cells from the G1 phase in human cancer (Musgrove et al., 2011; VanArsdale et al., 2015; Qie and Diehl, 2016). D cyclins form active complex with either CDK4 or CDK6, which drives the G1-to-S-phase transition (Musgrove et al., 2011; Qie and Diehl, 2016). Immunoblotting results showed that treatment of the MM cell lines (MM.1S and RPMI8226) with CKD-581 decreased the levels of cyclin D1 and CDK4 in a concentration- and time-dependent manners (Fig. 3A, 3B). SAHA also reduced the expression level of CDK4 and Cyclin D1 at higher concentration ranges in the MM.1S cells. However, when we performed cell-cycle analyses in MM.1S and RPMI8226 cells incubated with CKD-581 for 24 h, we could not determine G1/S arrest in either cell type (Fig. 3C). On the contrary, CKD-581 significantly increased the sub-G1 portion in concentration-dependent manners in both MM.1S and RPMI8226 cells (Fig. 3C). Relative to the vehicle-treated control cells, the sub-G1 population increased from 5.45 ± 0.16% to 22.65 ± 1.43 and 26.61 ± 1.80% in MM.1S cells following exposure to 300 and 1000 nM CKD-581 for 24 h, respectively (p<0.001). We further found that CKD-581 concentration-dependently increased the formation of cleaved caspase-3 after 24 h incubation in MM.1S and RPMI8226 cells (Fig. 3D). However, 1000 nM SAHA only marginally increased the cleaved caspase-3 in RPMI8226 cells (Fig. 3D). The data imply that CKD-581 may cause apoptosis via suppression of the Wnt/β-catenin pathway in hematologic cancer cells.
Fig. 3.
CKD-581 decreases the expression of cell cycle related proteins and induces apoptosis in MM. (A, B) Effects of CKD-581 and SAHA on CDK4 and cyclin D1 expression. (A) 30-1000 nM CKD-581 or 30-3000 nM SAHA was treated on MM.1S cells for 24 h. (B) CKD-581 (30-1000 nM) or 1000 nM SAHA was treated on RPMI8226 for 24 h (left). RPMI8226 cells were treated with 300 nM CKD-581 according to time course (right). After treatment, total cell lysates were analyzed by immunoblotting for CDK4 and cyclin D1. (C) Flow-cytometry analysis. MM.1S and RPMI8226 cells were treated with CKD-581 for 24 h. And cell cycle populations were determined using flow-cytometry. (D) Cleaved caspase-3 was detected by immunoblotting. Data represent mean ± SEM (significant vs. control; *p<0.05, **p<0.01, ***p<0.001). C, control; S, 1000 nM SAHA.
p53/p21 activation and BCL-xL inactivation by CKD-581 in MM cells
Because β-catenin and c-Myc are related to regulation of p53 phosphorylation, inhibition of the Wnt/β-catenin pathway induces apoptotic cell death in cancer cells (Riascos-Bernal et al., 2016; Kwak et al., 2018). To determine whether the increased sub-G1 population mainly results from the activation of the p53 pathway, p53 and downstream effectors were analyzed by immunoblottings. Ser15 phosphorylation of p53 is critical to proper functioning of p53 as a transcription factor, and p53 activation controls the diverse proteins related to DNA repair and apoptosis, including p21 and the BCL-2 family (Vousden, 2000). Among them, p21 plays a crucial role in the induction of cell-cycle arrest by acting as an inhibitor of CDKs. As shown in Fig. 4A, CKD-581 increased the level of p21 in MM cells. The expression of p21 was correlated with increased Ser15 phosphorylation of p53 (Fig. 4A). Under the transcriptional regulation of p53, BCL-2 family proteins also are involved in control of apoptosis (Burger et al., 1998). BCL-xL protein expression was downregulated by CKD-581 in both MM.1S and RPMI8226 cells (Fig. 4B). However, BCL-2 was only marginally affected by CKD-581 (Fig. 4B). It supportedthat CKD-581 increased the expression of tumor suppressor genes, which led in turn to MM cell death via apoptosis.
Fig. 4.
CKD-581 activates tumor suppressors and reduces the expression of BCL-2 family in MM. (A) MM.1S and RPMI8226 cells were treated with 300 nM CKD-581 according to time course. And total cell lysates were subjected to immunoblottings for Ser15-phosphorylated p53, p53 and p21. (B) MM.1S and RPMI8226 cells were treated with CKD-581 or SAHA for 24 h. BCL-2 and BCL-xL were detected by immunoblottings. Data represent mean ± SEM (significant vs. control; *p<0.05, **p<0.01, ***p<0.001). C, control; S, 1000 nM SAHA.
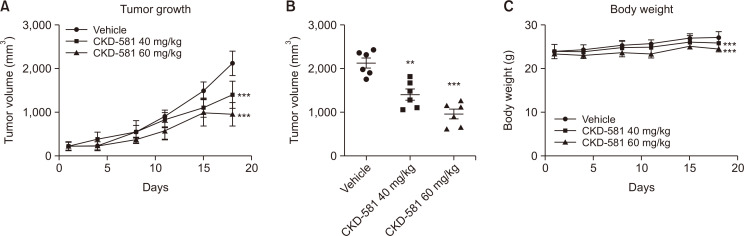
Anti-cancer effect of CKD-581 in MM xenograft model
SCID mice bearing MM.1S cells were used to assess the in vivo anti-cancer effect of CKD-581 on MM. CKD-581 (40 and 60 mg/kg) or vehicle (normal saline) were intraperitoneally injected twice per week. As depicted in Fig. 5A and 5B, the tumor sizes of the CKD-581-treated groups were significantly diminished relative to the vehicle-treated group (p<0.001). Although the CKD-581 groups showed slightly smaller body-weight gains (Fig. 5C), there was no symptomatic toxicity. Thus, we assumed that the smaller body weight gains by CKD-581 treatment may have resulted from the reduced tumor sizes.
Fig. 5.
Tumor growth is delayed by CKD-581 in MM.1S xenograft model. (A) NOD.CB17 SCID mice were used for MM.1S (1×106 cells/mouse) implantation. After tumors reached to about 100 mm3, the mice were treated with vehicle or CKD-581 (40 and 60 mg/kg) two times a week. (B) Dot graph showed individual tumor size. (C) Body weight change. Data represent mean ± SEM (significant vs. vehicle group; **p<0.01, ***p<0.001).
DISCUSSION
CKD-581, a novel HDAC inhibitor, is an efficacious anti-cancer agent in patients suffering from lymphoma or MM (Cho et al., 2018). Currently, two clinical trials with MM patients are ongoing (NCT03051841, NCT03150316). Although we have reported that CKD-581 showed anti-cancer activity against diffuse large B cell lymphoma (DLBCL) (Kim et al., 2020), the pharmacological mechanism of CKD-581-induced cancer cell death has yet to be studied in MM. In this study, we showed that CKD-581 induced apoptosis via suppression of the Wnt/β-catenin pathway in MM cells. Several studies have suggested that the Wnt/β-catenin pathway could be a possible therapeutic target for inhibition of cancer progression, metastasis, chemo-resistance and immunosurveillance evasion (Xi and Chen, 2014; Yuan et al., 2020). The Wnt/β-catenin pathway also plays a crucial role in the progression of hematologic cancers (Spaan et al., 2018). In MM, β-catenin silencing caused induction of apoptosis as well as recovery of lenalidomide sensitivity (Bjorklund et al., 2011; Su et al., 2016). However, the physiological role of Wnt/β-catenin signaling would differ by cancer type. Unlike most cancer types, wherein Wnt/β-catenin is crucial to tumorigenesis, the opposite aspects have been shown in several cancer types. For instance, low β-catenin levels are related to poor prognosis in melanoma and pancreatic ductal adenocarcinoma (PDAC) patients (Chien et al., 2009; Saukkonen et al., 2016). HDAC inhibitors, such as sodium butyrate, trichostatin A, suberoyl bis-hydroxamic acid and valproic acid, induced apoptosis via hyperactivation of Wnt/β-catenin in solid or hematologic cancer (Bordonaro et al., 2007; Shao et al., 2012). Although the pathological functions on the Wnt/β-catenin pathway remain controversial, HDAC inhibitors show anti-cancer activity regardless of cancer type. This phenomenon may reflect the multiple functions of the HDAC family and/or the variable mutations involved in the Wnt/β-catenin pathway. For instance, diverse targets regulating proliferation, differentiation and cell death are simultaneously affected by HDACs (Gong et al., 2019). Thus, the therapeutic effects of HDAC inhibitors could be the sum of complicated factors on various pathways. Additionally, mutations in adenomatous polyposis coli (APC) and β-catenin induce constitutive activation of canonical Wnt/β-catenin signaling in colon cancer (Munemitsu et al., 1995; Morin et al., 1997), which may cause HDAC-independent activity changes in the signaling pathway.
The DACT family, consisting of DACT1, DACT2 and DACT3, is known to be a functional suppressor group for the Wnt/β-catenin pathway during embryonic development and carcinogenesis (Fisher et al., 2006; Jiang et al., 2008; Mandal and Waxman, 2014; Zhao et al., 2017). Especially, DACT3 expression can be regulated by histone modification such as acetylation or methylation (Jiang et al., 2008). However, up to now, nothing is known about the function of HDAC-inhibitor-mediated DACT3 regulation in MM. In these pages, we have reported that CKD-581 and SAHA suppressed the Wnt/β-catenin pathway by selective induction of DACT3. Hence, it is plausible that the Wnt/β-catenin signaling pathway is regulated by HDAC inhibitors via DACT3 overexpression in MM. However, the precise regulation mechanism of interaction between DACT3 and HDAC needs to be clarified in future studies.
In the present study, we determined that CKD-581 induced apoptosis in the tested MM cell lines, though cell-cycle arrest was not seen up to 1 µM CKD-581. c-Myc, a representative target gene of the Wnt/β-catenin pathway, is known to be a potent oncogene (Gabay et al., 2014; Rennoll and Yochum, 2015). In fact, several HDAC inhibitors reduce c-Myc expression and increase apoptotic cell death in cancer cells (Nebbioso et al., 2017; Shieh et al., 2017). For example, it was reported that c-Myc increases Bcl-xL expression through lncRNA H19 and STAT5 activation in leukemia (Guo et al., 2014), and that loss of Bcl-xL prevents c-Myc-induced lymphomatogenesis (Kelly et al., 2011). Therefore, Bcl-xL upregulation coinciding with limited apoptosis may be actively involved in c-Myc-driven tumor progression. In this study, HDAC inhibitors (CKD-581 and SAHA) efficiently decreased the expression of c-Myc and BCL-xL in MM cells (Fig. 2D, 4B).
Wnt/β-catenin activation suppresses p53 function. Active β-catenin abrogated the transcriptional activity of p53, and inactivation of β-catenin by XAV939 increased the p53 acetylation and transcriptional activity in smooth muscle cells (Riascos-Bernal et al., 2016). Also, it is known that the Wnt/β-catenin signaling pathway inhibits expression of p53 via miR-552 in colorectal cancers (Kwak et al., 2018). Therefore, it seems certain that inhibition of the Wnt/β-catenin axis by CKD-581 affects p53, a representative tumor suppressor gene. In fact, the protein level of Ser15-phosphorylated p53 was enhanced by CKD-581 without change to total p53 expression (Fig. 4B). Both Ser15-phosphorylated p53 and p21 expression are known to be good prognostic markers in cancer (Kao et al., 2007; Yang et al., 2019). Thus, the pharmacological features of CKD-581 for activation of tumor suppressor genes p53 and p21 would be involved in its anti-cancer effects against MM.
The AKT/mTOR signaling pathway is one of the well-known cancer-related pathways that regulate cell proliferation, survival, metabolism and immunity (Leal et al., 2013; Hua et al., 2019), and crosstalk between the AKT/mTOR and Wnt/β-catenin pathway has been reported (Daisy Precilla et al., 2022). In addition, mTOR is believed as a therapeutic target for anti-cancer therapy. Because HDAC inhibitors suppress the AKT/mTOR signaling pathway in cancer cells (Zhang et al., 2015), we tested whether CKD-581 affects the AKT/mTOR pathway. CKD-581 decreased phosphorylation of mTOR and 70 kDa ribosomal protein S6 kinase (p70S6K) in MM cell lines (Supplementary Fig. 1B), indicating that mTOR pathway inhibition is related with anti-cancer effects of CKD-581 in MM.
The intensity of tubulin acetylation induced by CKD-581 or SAHA was comparable (Fig. 1B). Tubulins are subject to various post-translational modifications (PTMs), such as detyrosination, polyglutamylation, polyglycylation and acetylation (Janke and Bulinski, 2011). It has been known that paclitaxel induces acetylation at Lys40 on the luminal surface of microtubules (Howes et al., 2014). Paclitaxel stimulates Lys40 acetylation of tubulin and stabilizes myeloid cell leukemia-1 (MCL-1), an anti-apoptotic protein, which is related with chemoresistance (Wattanathamsan et al., 2021). MCL-1 overexpression has been reported in various cancers, and down-regulation of MCL-1 increase the sensitivity to chemotherapies (Xiang et al., 2018).
HDAC enzymes are able to remove the acetyl groups from various proteins. Whereas HDAC inhibitors increase the acetylation of the target proteins (Kim and Bae, 2011). Although tubulin acetylation at Lys40 was increased by HDAC6 inhibition, MCL-1 and other anti-apoptotic proteins were down-regulated by pan- or class I HDAC inhibition (He et al., 2013; Ramakrishnan et al., 2019; Kim et al., 2020). Hence, class I- or pan-HDAC inhibitors, including CKD-581 would increase the sensitivity to chemotherapy through MCL-1 down-regulation.
In comparison with SAHA, CKD-581 more potently induced acetylation of histone proteins. These results imply that CKD-581 more efficiently acetylates target proteins in class I HDACs. Because dysregulation of class I HDAC is correlated with poor prognosis in MM patients (Mithraprabhu et al., 2014), and MM cell death is more prominent by class I HDAC inhibitors than class II HDAC inhibitors (Mithraprabhu et al., 2013), CKD-581 is expected to be more effective than SAHA for treatment of MM. Safety is an important issue in new-drug development, particularly since combination therapy is considered to be a means of improving treatment efficacy for diverse cancer types. Thus far in clinical trials, CKD-581 has had an acceptable safety profile (Cho et al., 2018).
In summary, CKD-581 is a potent HDAC inhibitor targeting types I and II HDACs. CKD-581 is highly effective in inhibiting proliferation of hematologic cancer cells including MM and T cell lymphoma. cDNA microarray and immunoblot analyses revealed that DACT3 overexpression-related downregulation of the Wnt/β-catenin pathway is correlated with CKD-581-induced cell death in hematologic cancer. Certainly, CKD-581 could be a useful HDAC inhibitor against hematologic cancers. Our present findings, furthermore, are meaningful in explicating the mechanistic basis of HDAC inhibitors’ anti-cancer effects.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by Korean Government (2021R1A4A1021787).
Footnotes
CONFLICT OF INTEREST
Soo Jin Kim and U Ji Kim works in CKD Research Institution, Chong Kun Dang Pharmaceutical Corporation.
REFERENCES
- Beltran A. S., Russo A., Lara H., Fan C., Lizardi P. M., Blancafort P. Suppression of breast tumor growth and metastasis by an engineered transcription factor. PLoS ONE. 2011;6:e24595. doi: 10.1371/journal.pone.0024595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel P. L., Kuehl W. M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Bjorklund C. C., Ma W., Wang Z. Q., Davis R. E., Kuhn D. J., Kornblau S. M., Wang M., Shah J. J., Orlowski R. Z. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. J. Biol. Chem. 2011;286:11009–11020. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordonaro M., Lazarova D. L., Sartorelli A. C. The activation of beta-catenin by Wnt signaling mediates the effects of histone deacetylase inhibitors. Exp. Cell Res. 2007;313:1652–1666. doi: 10.1016/j.yexcr.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., Nooter K., Boersma A. W., Kortland C. J., Stoter G. Expression of p53, Bcl-2 and Bax in cisplatin-induced apoptosis in testicular germ cell tumour cell lines. Br. J. Cancer. 1998;77:1562–1567. doi: 10.1038/bjc.1998.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A. J., Moore E. C., Lonsdorf A. S., Kulikauskas R. M., Rothberg B. G., Berger A. J., Major M. B., Hwang S. T., Rimm D. L., Moon R. T. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Yoon D. H., Kim K. P., Bae K. S., Kim W. S., Eom H. S., Kim J. S., Hong J. Y., Kim S. J., Lee H., Kim S. J., Suh C. Phase I study of CKD-581, a pan-histone deacetylase inhibitor, in patients with lymphoma or multiple myeloma refractory to standard therapy. Invest. New Drugs. 2018;36:877–885. doi: 10.1007/s10637-018-0582-0. [DOI] [PubMed] [Google Scholar]
- Daisy Precilla S., Biswas I., Kuduvalli S. S., Anitha T. S. Crosstalk between PI3K/AKT/mTOR and WNT/beta-Catenin signaling in GBM - could combination therapy checkmate the collusion? Cell. Signal. 2022;95:110350. doi: 10.1016/j.cellsig.2022.110350. [DOI] [PubMed] [Google Scholar]
- Dawson M. A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- de la Puente P., Muz B., Azab F., Luderer M., Azab A. K. Molecularly targeted therapies in multiple myeloma. Leuk. Res. Treatment. 2014;2014:976567. doi: 10.1155/2014/976567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh S. E., Green A. R., Rakha E. A., Powe D. G., Ahmed R. A., Collins H. M., Soria D., Garibaldi J. M., Paish C. E., Ammar A. A., Grainge M. J., Ball G. R., Abdelghany M. K., Martinez-Pomares L., Heery D. M., Ellis I. O. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009;69:3802–3809. doi: 10.1158/0008-5472.CAN-08-3907. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Kivimae S., Hoshino J., Suriben R., Martin P. M., Baxter N., Cheyette B. N. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev. Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- Fraga M. F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G., Bonaldi T., Haydon C., Ropero S., Petrie K., Iyer N. G., Perez-Rosado A., Calvo E., Lopez J. A., Cano A., Calasanz M. J., Colomer D., Piris M. A., Ahn N., Imhof A., Caldas C., Jenuwein T., Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- Gabay M., Li Y., Felsher D. W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 2014;4:a014241. doi: 10.1101/cshperspect.a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N., Accardi F., Marchica V., Dalla Palma B., Storti P., Toscani D., Vicario E., Malavasi F. Novel targets for the treatment of relapsing multiple myeloma. Expert Rev. Hematol. 2019;12:481–496. doi: 10.1080/17474086.2019.1624158. [DOI] [PubMed] [Google Scholar]
- Gong P., Wang Y., Jing Y. Apoptosis induction byHistone deacetylase inhibitors in cancer cells: role of Ku70. Int. J. Mol. Sci. 2019;20:1601. doi: 10.3390/ijms20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., Kang Q., Chen Q., Chen Z., Wang J., Tan L., Chen J. L. High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett. 2014;588:1780–1786. doi: 10.1016/j.febslet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- He L., Torres-Lockhart K., Forster N., Ramakrishnan S., Greninger P., Garnett M. J., McDermott U., Rothenberg S. M., Benes C. H., Ellisen L. W. Mcl-1 and FBW7 control a dominant survival pathway underlying HDAC and Bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013;3:324–337. doi: 10.1158/2159-8290.CD-12-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes S. C., Alushin G. M., Shida T., Nachury M. V., Nogales E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol. Biol. Cell. 2014;25:257–266. doi: 10.1091/mbc.e13-07-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H., Kong Q., Zhang H., Wang J., Luo T., Jiang Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019;12:71. doi: 10.1186/s13045-019-0754-1.b2f26c6c535c4e95a373b46bd7052153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Hirano M., Kobayashi M., Futami M., Tojo A. HDAC inhibitors exert anti-myeloma effects through multiple modes of action. Cancers (Basel) 2019;11:475. doi: 10.3390/cancers11040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C., Bulinski J. C. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Jiang X., Tan J., Li J., Kivimae S., Yang X., Zhuang L., Lee P. L., Chan M. T., Stanton L. W., Liu E. T., Cheyette B. N., Yu Q. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. T., Chuah S. K., Huang C. C., Chen C. L., Wang C. C., Hung C. H., Chen C. H., Wang J. H., Lu S. N., Lee C. M., Changchien C. S., Hu T. H. P21/WAF1 is an independent survival prognostic factor for patients with hepatocellular carcinoma after resection. Liver Int. 2007;27:772–781. doi: 10.1111/j.1478-3231.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- Kelly P. N., Grabow S., Delbridge A. R., Strasser A., Adams J. M. Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood. 2011;118:6380–6386. doi: 10.1182/blood-2011-07-367672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Bae S. C. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Transl. Res. 2011;3:166–179. [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Kim U. J., Yoo H. Y., Choi Y. J., Kang K. W. Anti-cancer effects of CKD-581, a potent histone deacetylase inhibitor against diffuse large B-cell lymphoma. Int. J. Mol. Sci. 2020;21:4377. doi: 10.3390/ijms21124377.2c866506cdc143d58a8762de96824724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Reifenberger G., Lu D., Endo T., Carson D. A., Gast S. M., Meschenmoser K., Nowak M., Schmidt-Wolf I. G. Influencing the Wnt signaling pathway in multiple myeloma. Anticancer Res. 2011;31:725–730. [PubMed] [Google Scholar]
- Kwak B., Kim D. U., Kim T. O., Kim H. S., Kim S. W. MicroRNA-552 links Wnt signaling to p53 tumor suppressor in colorectal cancer. Int. J. Oncol. 2018;53:1800–1808. doi: 10.3892/ijo.2018.4505. [DOI] [PubMed] [Google Scholar]
- Leal P., Garcia P., Sandoval A., Buchegger K., Weber H., Tapia O., Roa J. C. AKT/mTOR substrate P70S6K is frequently phosphorylated in gallbladder cancer tissue and cell lines. Onco Targets Ther. 2013;6:1373–1384. doi: 10.2147/OTT.S46897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016;6:a026831. doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A., Waxman J. Retinoic acid negatively regulates dact3b expression in the hindbrain of zebrafish embryos. Gene Expr. Patterns. 2014;16:122–129. doi: 10.1016/j.gep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C. K., Kim K., Kim S. J., Yoon D. H., Park S.-S., Han S. A phase 1, open-label, multi-center study of alteminostat (CKD-581) in combination with lenalidomide and dexamethasone in patients with previously treated multiple myeloma (MM) Blood. 2019;134:1847. doi: 10.1182/blood-2019-127643. [DOI] [Google Scholar]
- Mithraprabhu S., Kalff A., Chow A., Khong T., Spencer A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics. 2014;9:1511–1520. doi: 10.4161/15592294.2014.983367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithraprabhu S., Khong T., Jones S. S., Spencer A. Histone deacetylase (HDAC) inhibitors as single agents induce multiple myeloma cell death principally through the inhibition of class I HDAC. Br. J. Haematol. 2013;162:559–562. doi: 10.1111/bjh.12388. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Caldon C. E., Barraclough J., Stone A., Sutherland R. L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- Nebbioso A., Carafa V., Conte M., Tambaro F. P., Abbondanza C., Martens J., Nees M., Benedetti R., Pallavicini I., Minucci S., Garcia-Manero G., Iovino F., Lania G., Ingenito C., Belsito Petrizzi V., Stunnenberg H. G., Altucci L. c-Myc modulation and acetylation is a key HDAC inhibitor target in cancer. Clin. Cancer Res. 2017;23:2542–2555. doi: 10.1158/1078-0432.CCR-15-2388. [DOI] [PubMed] [Google Scholar]
- Qie S., Diehl J. A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar S. V., Dimopoulos M. A., Palumbo A., Blade J., Merlini G., Mateos M. V., Kumar S., Hillengass J., Kastritis E., Richardson P., Landgren O., Paiva B., Dispenzieri A., Weiss B., LeLeu X., Zweegman S., Lonial S., Rosinol L., Zamagni E., Jagannath S., Sezer O., Kristinsson S. Y., Caers J., Usmani S. Z., Lahuerta J. J., Johnsen H. E., Beksac M., Cavo M., Goldschmidt H., Terpos E., Kyle R. A., Anderson K. C., Durie B. G., Miguel J. F. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V. G., Miller K. C., Macon E. P., Kimlinger T. K., Haug J., Kumar S., Gonsalves W. I., Rajkumar S. V., Kumar S. K. Histone deacetylase inhibition in combination with MEK or BCL-2 inhibition in multiple myeloma. Haematologica. 2019;104:2061–2074. doi: 10.3324/haematol.2018.211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Chen Y., Liang X., Lu Y., Pan W., Yang M. MiRNA-638 promotes autophagy and malignant phenotypes of cancer cells via directly suppressing DACT3. Cancer Lett. 2017;390:126–136. doi: 10.1016/j.canlet.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Rennoll S., Yochum G. Regulation of MYC gene expression by aberrant Wnt/beta-catenin signaling in colorectal cancer. World J. Biol. Chem. 2015;6:290–300. doi: 10.4331/wjbc.v6.i4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riascos-Bernal D. F., Chinnasamy P., Cao L. L., Dunaway C. M., Valenta T., Basler K., Sibinga N. E. beta-Catenin C-terminal signals suppress p53 and are essential for artery formation. Nat. Commun. 2016;7:12389. doi: 10.1038/ncomms12389.714f114658344539a951b80874578064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E., Shen J., Steinberg J., Li M., Wang C., Bonavida B., Chen H., Li Z.-W., Berenson J. R. The histone deacetylase inhibitor LBH589 enhances the anti-myeloma effects of chemotherapy in vitro and in vivo. Leuk. Res. 2011;35:373–379. doi: 10.1016/j.leukres.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Hagstrom J., Mustonen H., Juuti A., Nordling S., Kallio P., Alitalo K., Seppanen H., Haglund C. PROX1 and beta-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer. 2016;16:472. doi: 10.1186/s12885-016-2497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N., Zou J., Li J., Chen F., Dai J., Qu X., Sun X., Ma D., Ji C. Hyper-activation of WNT/beta-catenin signaling pathway mediates anti-tumor effects of histone deacetylase inhibitors in acute T lymphoblastic leukemia. Leuk. Lymphoma. 2012;53:1769–1778. doi: 10.3109/10428194.2012.663085. [DOI] [PubMed] [Google Scholar]
- Shieh J. M., Tang Y. A., Hu F. H., Huang W. J., Wang Y. J., Jen J., Liao S. Y., Lu Y. H., Yeh Y. L., Wang T. W., Lin P., Wang Y. C. A histone deacetylase inhibitor enhances expression of genes inhibiting Wnt pathway and augments activity of DNA demethylation reagent against nonsmall-cell lung cancer. Int. J. Cancer. 2017;140:2375–2386. doi: 10.1002/ijc.30664. [DOI] [PubMed] [Google Scholar]
- Singh A. K., Bishayee A., Pandey A. K. Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients. 2018;10:731. doi: 10.3390/nu10060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraj D., Green M. M., Gasparetto C. Panobinostat for the management of multiple myeloma. Future Oncol. 2017;13:477–488. doi: 10.2217/fon-2016-0329. [DOI] [PubMed] [Google Scholar]
- Spaan I., Raymakers R. A., van de Stolpe A., Peperzak V. Wnt signaling in multiple myeloma: a central player in disease with therapeutic potential. J. Hematol. Oncol. 2018;11:67. doi: 10.1186/s13045-018-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N., Wang P., Li Y. Role of Wnt/beta-catenin pathway in inducing autophagy and apoptosis in multiple myeloma cells. Oncol. Lett. 2016;12:4623–4629. doi: 10.3892/ol.2016.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanArsdale T., Boshoff C., Arndt K. T., Abraham R. T. Molecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin. Cancer Res. 2015;21:2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- Vousden K. H. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/S0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Zhang J. H., Gu Z. W. Wnt/beta-catenin signal pathway and malignant hematological disease -- review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:234–237. [PubMed] [Google Scholar]
- Wattanathamsan O., Thararattanobon R., Rodsiri R., Chanvorachote P., Vinayanuwattikun C., Pongrakhananon V. Tubulin acetylation enhances lung cancer resistance to paclitaxel-induced cell death through Mcl-1 stabilization. Cell Death Discov. 2021;7:67. doi: 10.1038/s41420-021-00453-9.48077c0dc21e4bcbaf7f8ffd8d001e3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W., Denkert C., Noske A., Darb-Esfahani S., Dietel M., Kalloger S. E., Huntsman D. G., Kobel M. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008a;10:1021–1027. doi: 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W., Roske A., Gekeler V., Beckers T., Ebert M. P., Pross M., Dietel M., Denkert C., Rocken C. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008b;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- Xi S., Yang M., Tao Y., Xu H., Shan J., Inchauste S., Zhang M., Mercedes L., Hong J. A., Rao M., Schrump D. S. Cigarette smoke induces C/EBP-beta-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS ONE. 2010;5:e13764. doi: 10.1371/journal.pone.0013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Chen Y. Wnt signaling pathway: implications for therapy in lung cancer and bone metastasis. Cancer Lett. 2014;353:8–16. doi: 10.1016/j.canlet.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Xiang W., Yang C. Y., Bai L. MCL-1 inhibition in cancer treatment. Onco Targets Ther. 2018;11:7301–7314. doi: 10.2147/OTT.S146228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Choi Y., Joh J. W., Cho S. K., Kim D. S., Park S. G. Phosphorylation of p53 serine 15 is a predictor of survival for patients with hepatocellular carcinoma. Can. J. Gastroenterol. Hepatol. 2019;2019:9015453. doi: 10.1155/2019/9015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Tao F., Zhang X., Zhang Y., Sun X., Wu D. Role of Wnt/beta-catenin signaling in the chemoresistance modulation of colorectal cancer. Biomed. Res. Int. 2020;2020:9390878. doi: 10.1155/2020/9390878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X. G., Huang Y. R., Yu T., Jiang H. W., Xu Y., Zhao X. Y. Chidamide, a histone deacetylase inhibitor, induces growth arrest and apoptosis in multiple myeloma cells in a caspase-dependent manner. Oncol. Lett. 2019;18:411–419. doi: 10.3892/ol.2019.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Guo Z., Wu Y., Hu R., Du J., He X., Jiao X., Zhu X. Histone deacetylase inhibitors inhibit the proliferation of gallbladder carcinoma cells by suppressing AKT/mTOR signaling. PLoS ONE. 2015;10:e0136193. doi: 10.1371/journal.pone.0136193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang L., Han Y., Li H., Ling Z., Wang Y., Wang E., Wu G. Dact3 inhibits the malignant phenotype of non-small cell lung cancer through downregulation of c-Myb. Int. J. Clin. Exp. Pathol. 2017;10:11580–11587. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.