Abstract
Small-diameter vascular substitutes remain necessary, especially in the absence of an available autologous vein. Using a completely autologous approach termed “in-body tissue architecture,” a small-diameter, long, tissue-engineered vascular graft, a “Biotube,” was developed. A below-the-knee distal bypass using the Biotube as a composite with expanded polytetrafluoroethylene grafts was performed to treat a patient with chronic limb-threatening ischemia without a venous graft available. The wound on the foot had completely healed 3 months after the bypass surgery, and limb salvage and walking without claudication were achieved. At the 1-year postoperative follow-up examination, duplex ultrasound scans demonstrated graft patency without thrombus or stenosis.
Keywords: Chronic limb-threatening ischemia, In-body tissue architecture, Small diameter vascular conduit, Peripheral artery bypass surgery, Tissue-engineered vascular graft
Below-the-knee bypass surgery to treat chronic limb-threatening ischemia (CLTI) using nonautologous conduits, such as expanded polytetrafluoroethylene (ePTFE) or polyethylene terephthalate (Dacron) grafts, has remained insufficiently effective.1, 2, 3, 4 Furthermore, the availability of good quality autologous vein conduits is key to the success of bypass surgery. However, the use of autologous vein as a conduit can be limited by the presence of varicose veins, narrowed veins, and/or a lack of available vein because these veins could have been used during previous procedures, such as for coronary and peripheral artery bypass grafts. Thus, a considerable need exists for alternatives to autologous veins for bypass surgery in the lower limb.
Recently, a clinically applicable, small-diameter, long, tissue-engineered vascular graft was developed using a completely autologous approach termed “in-body tissue architecture” (iBTA) technology.5 In the present report, we have described the case of a man who had undergone distal bypass surgery using the “Biotube”, an iBTA-induced autologous collagenous tube as a vascular bypass conduit for CLTI. The ethics committee of Yokohama General Hospital approved this procedure. The patient provided written informed consent for the report of his case details and imaging studies.
Case report
A 72-year-old man had been referred to our Wound Care Center of Yokohama General Hospital with sustained severe left foot pain and a nonhealing wound after amputation of the first, second, and fourth toes because of gangrene. At 3 years before the referral to our hospital, he had undergone left common femoral artery to left below-the-knee popliteal artery bypass using a distal right great saphenous vein for CLTI with a second toe ulcer. At 1.5 years after the bypass surgery, the bypass graft had become occluded and the second toe ulcer had reappeared. A second operation was performed using a composite graft of a 6-mm internal diameter (ID) ePTFE graft anastomosed end to end to the left great saphenous vein. The proximal end of the ePTFE graft was anastomosed to the left external iliac artery, and the distal end of the saphenous vein graft was anastomosed to the left posterior tibial artery. In the infragenicular portion, the saphenous vein graft was anastomosed side to side to the left tibioperoneal trunk. At 1 year after the second bypass surgery, the ePTFE graft had become infected and was removed. Although the saphenous vein graft between the left tibioperoneal trunk and posterior tibial arteries was patent, an ischemic ulcer and gangrene of the left foot had developed. Therefore, the third operation, which involved a left common femoral artery to left posterior tibial artery bypass using a composite of a 6-mm ID ePTFE graft with a residual right great saphenous vein, was performed, followed by the amputation of the three toes. However, the third bypass graft had become occluded by 3 months postoperatively and the wound had not healed (Fig 1). Also, the patient had developed severe pain causing difficulty in walking.
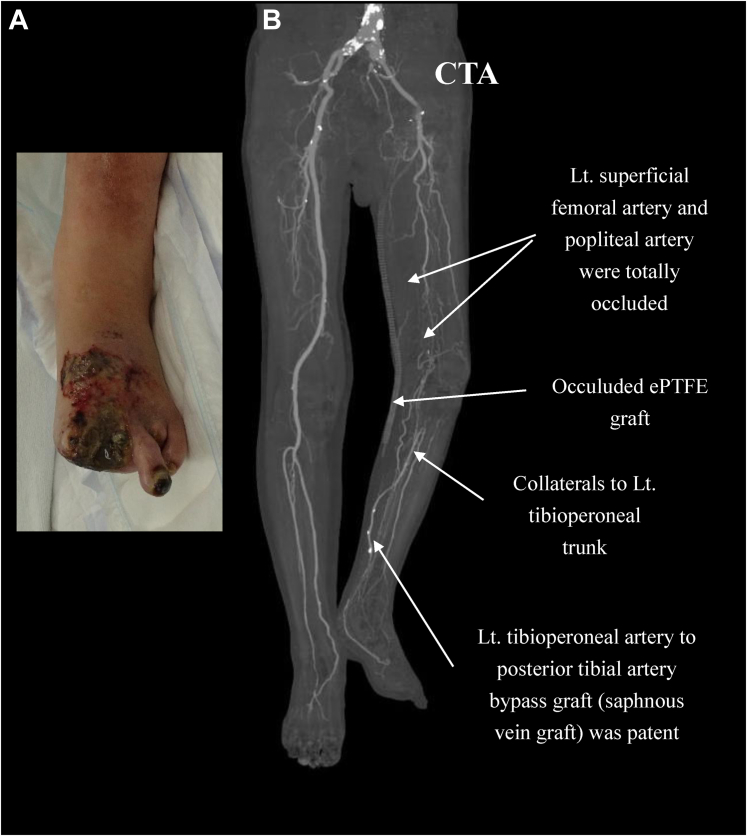
Fig 1.
A, Preoperative photograph of the left foot showing that the first, second, and fourth toes had been amputated and that a huge ulcer and gangrene had not healed. B, Computed tomography angiogram (CTA) demonstrating total occlusion of both left superficial femoral and popliteal arteries. The left (Lt.) common femoral artery to posterior tibial artery bypass with an expanded polytetrafluoroethylene (ePTFE) graft was occluded. However, the tibioperoneal trunk to posterior tibial artery bypass with a great saphenous vein graft was patent.
The left ankle brachial index was 0.46, and enhanced computed tomography angiography demonstrated that the left superficial femoral artery and popliteal artery were totally occluded. However, the saphenous vein bypass graft between the tibioperoneal trunk and posterior tibial artery was patent (Fig 1). Duplex ultrasound scanning showed no available vein to use as a conduit for bypass surgery. Therefore, the patient was electively scheduled for revision of the revascularization with a Biotube graft.
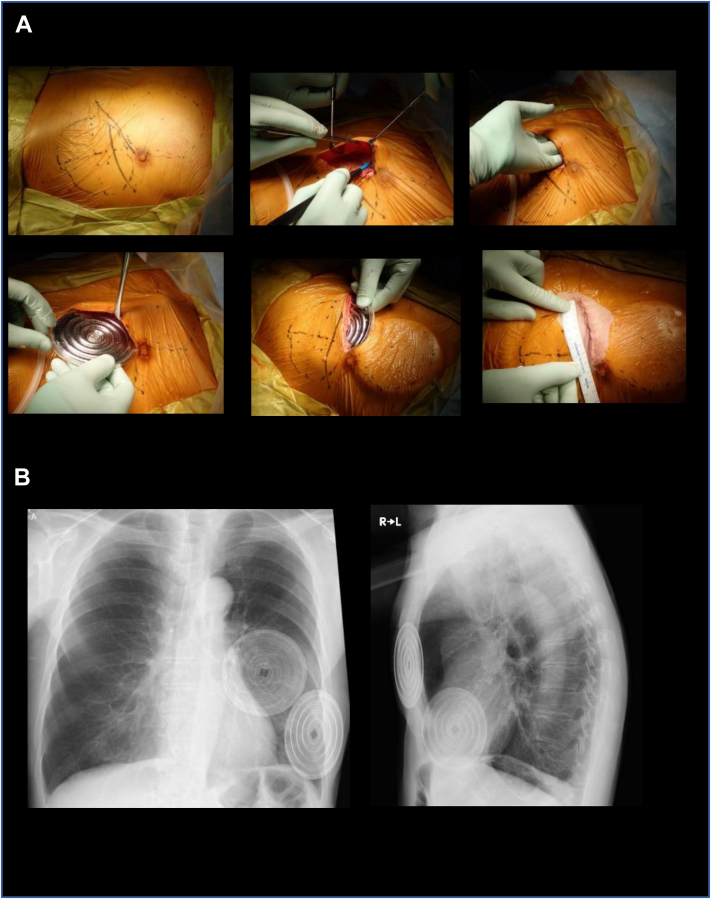
In accordance with previous reports,5,6 a Biotube graft was prepared using spiral molds (Biotube Maker; Biotube Co, Ltd, Tokyo, Japan). With the patient under general anesthesia, two Biotube Makers were subcutaneously embedded at the left anterolateral portion of the chest with a 10-cm incision (Fig 2). At 2 months after the embedding procedure, the Biotube Makers were harvested, and the Biotube was obtained by removing the Biotube Makers. The Biotube was placed in a 70% ethanol solution for 30 minutes and then kept in a saline solution (Fig 3, A).
Fig 2.
Preparation of the Biotube. A, Two spiral molds designed to form a Biotube with an internal diameter (ID) of 4 mm, length of 50 cm, and thickness of 0.85 mm were subcutaneously embedded into the left anterolateral portion of the patient. B, Chest radiographs after embedding the molds. L, Left; R, right.
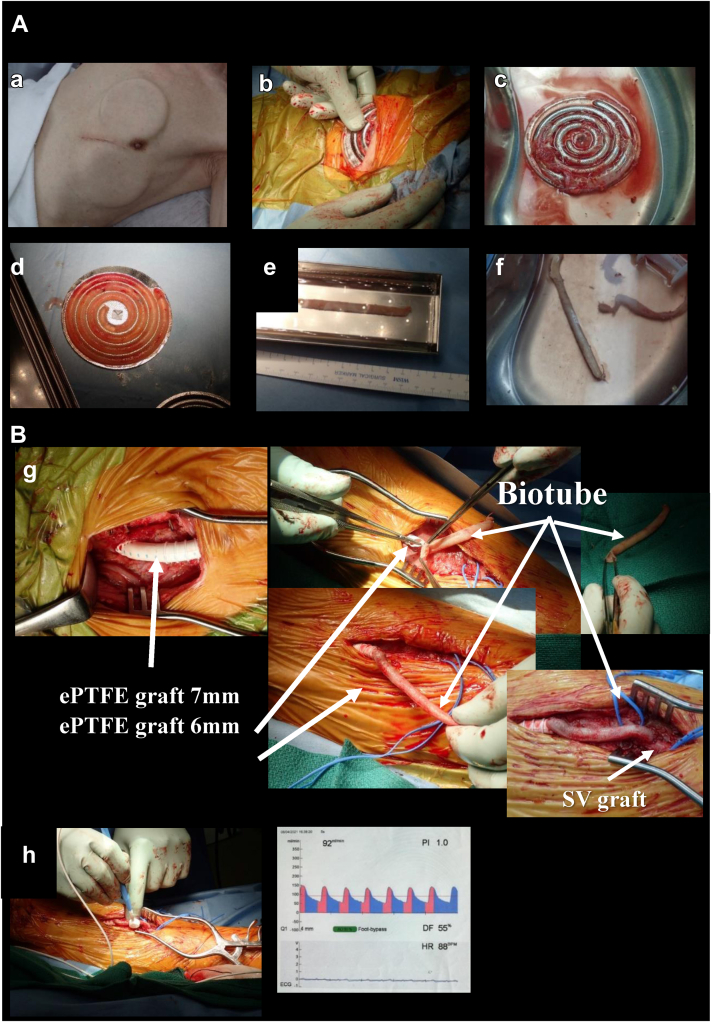
Fig 3.
Intraoperative findings. A, a, Body incubation by placing the mold under the skin for 2 months. b, Extraction of the mold. c, Removal of redundant connective tissue around the mold. d, Harvesting the Biotube by disassembling the mold. e, Shape correction of the Biotube by insertion of the orthodontic rod and immersion in a 70% alcohol solution for 30 minutes. f, Preparation of the Biotube in saline solution. B, g, Left common femoral artery to saphenous vein (SV) graft (tibioperoneal trunk to posterior tibial artery) bypass. h, Measurement of graft flow. bpm, Beats per minute; DF, dominant frequency; ECG, electrocardiogram; ePTFE, expanded polytetrafluoroethylene; HR, heart rate.
The fourth revascularization was performed using the Biotube (ID, 4 mm; length, 10 cm) as a composite with two ePTFE grafts (ID, 7 mm; length, 30 cm; and ID, 6 mm; length, 10 cm). The proximal site of the ePTFE graft was anastomosed to the left common femoral artery, and the distal site of the graft (Biotube) was anastomosed to the saphenous vein bypass graft (Fig 3, B). Satisfactory graft flow (92 mL/min) was obtained. Clopidogrel 75 mg/d and warfarin 3 to 3.5 mg/d, with adjustment to maintain a prothrombin time/international normalized ratio of 1.6 to 2.5, were used from the first postoperative day and throughout the follow-up period.
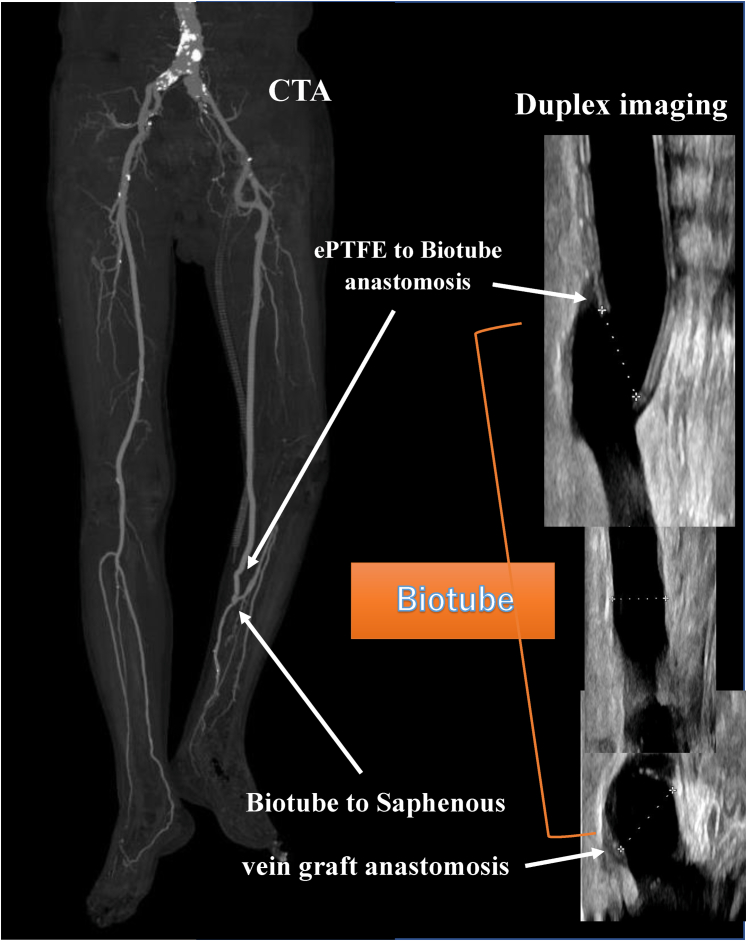
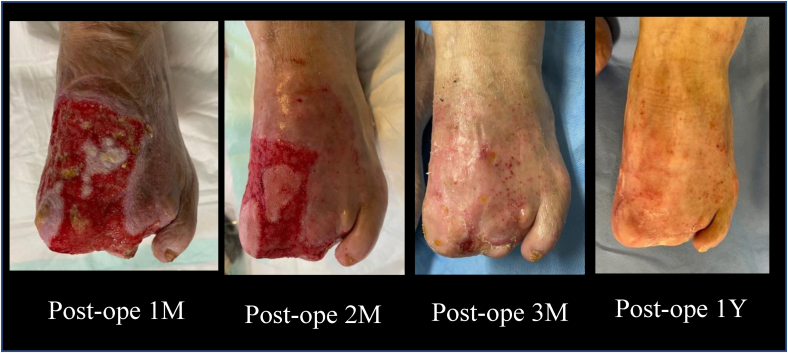
The postoperative ankle brachial index was 0.99, and duplex ultrasound and enhanced computed tomography demonstrated a patent bypass graft (Fig 4). The caliber of the implanted Biotube had self-adjusted to a diameter of 6.6 mm within 1 week. The 3-month, 6-month, and 1-year postoperative follow-up visits with duplex ultrasound scanning demonstrated graft patency without thrombus and stenosis. Moreover, the Biotube had not dilated further. The wound of the left foot had completely healed by 3 months after the bypass surgery (Fig 5). He had presented for his 1-year follow-up examination walking without claudication or pain.
Fig 4.
Postoperative computed tomography angiogram (CTA) showing a patent graft and duplex ultrasound scan showing the Biotube without stenosis, thrombosis, or aneurysmal deformity. ePTFE, Expanded polytetrafluoroethylene.
Fig 5.
Wound healing course of the left foot ulcer. M, Month; Post-ope, postoperative; Y, year.
Discussion
Although CLTI patients with below-the-knee arterial disease require good quality applicable autologous vein for the best mid- and long-term patency and limb salvage outcomes, their availability can be limited. Therefore, a very important need has existed to develop innovative technology to create small-caliber grafts to replace the necessity of using veins. Tissue engineering has been challenged to make ideal grafts with immune acceptance, the requisite tissue mechanics, low thrombogenicity, and immediate availability. The use of iBTA can produce autologous implantable tissues with the desired shape by simply subcutaneously embedding a specially designed mold. Because the body itself works as a bioreactor, iBTA-induced Biotubes can be produced without complex steps or largescale factories.
It has been previously reported that iBTA-induced autologous Biotubes (ID, 6 mm; length, 7 cm) were implanted for internal shunt restoration for hemodialysis patients.7 To the best of our knowledge, we have described the first case of lower extremity bypass using a small-diameter Biotube as an in-human implant. Because no rigorous method has been developed to assess the quality of the Biotube in the operating room, we tested it with reference to the availability of veins and chose to use the 10-cm parts that seemed to be of high quality. His Biotube has worked well for 1 year as a conduit without any complications such as stenosis, aneurysm formation, or rupture.
In previous nonclinical studies of goats, endothelial cells derived from living anastomotic sites had extended onto the luminal surface of the Biotube.8 Also, vascular endothelial progenitor cells in the blood had attached to the luminal surface in the middle part of the Biotube, causing endothelialization. Similarly, autologous vascularization, including a smooth muscle cell layer, was observed in rats,9 rabbits,10 and dogs5 within a few months after Biotube implantation. These results have suggested that the collagenous scaffolds of the Biotubes had metabolically resolved and been replaced by autologous vascular tissue. Thus, it is possible that the Biotube implanted during this treatment will also have regenerated into an autologous blood vessel within ≥1 year. We are planning a first investigator-initiated clinical trial using long Biotubes to perform below-the-knee artery bypass surgery for patients with CLTI.
Conclusions
In the present study, the Biotube showed satisfactory 1-year patency without stenosis or aneurysmal changes on the lower limb graft. Thus, the Biotube could be a potential alternative blood vessel to venous grafts.
Footnotes
Author conflict of interest: R.H. is a director and stockholder of Biotube Co, Ltd. M.M., M.O., and N.I. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almasri J., Adusumalli J., Asi N., Lakis S., Alsawas M., Prokop L.J., et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. 2018;68:624–633. doi: 10.1016/j.jvs.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury A.W., Adam D.J., Bell J., Forbes J.F., Fowkes F.G.R., Gillespie I., et al. BASIL Trial Participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51(Suppl):5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury A.W., Adam D.J., Bell J., Forbes J.F., Fowkes F.G.R., Gillespie I., et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51(Suppl):18S–31S. doi: 10.1016/j.jvs.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama Y., Furukoshi M., Terazawa T., Iwai R. Development of long in vivo tissue-engineered “Biotube” vascular grafts. Biomaterials. 2018;185:232–239. doi: 10.1016/j.biomaterials.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama Y., Higashita R., Shiraishi Y., Umeno T., Tajikawa T., Yamada A., et al. iBTA-induced Biotube® blood vessels: 2020 update. Kidney Dial. 2021;1:3–13. [Google Scholar]
- 7.Nakayama Y., Kaneko Y., Okumura N., Terazawa T. Initial 3-year results of first human use of an in-body tissue-engineered autologous “Biotube” vascular graft for hemodialysis. J Vasc Access. 2020;21:110–115. doi: 10.1177/1129729819852550. [DOI] [PubMed] [Google Scholar]
- 8.Higashita R., Nakayama Y., Shiraishi Y., Iwai R., Inoue Y., Yamada A., et al. Acute phase pilot evaluation of small diameter long iBTA induced vascular graft “Biotube” in a goat model. EJVES Vasc Forum. 2022;54:27–35. doi: 10.1016/j.ejvsvf.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii D., Enmi J.I., Iwai R., Kurisu K., Tatsumi E., Nakayama Y. One year rat study of iBTA-induced “microbiotube” microvascular grafts with an ultra-small diameter of 0.6 mm. Eur J Vasc Endovasc Surg. 2018;55:882–887. doi: 10.1016/j.ejvs.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T., Kanda K., Yamanami M., Ishibashi-Ueda H., Yaku H., Nakayama Y. Long-term animal implantation study of biotube-autologous small-caliber vascular graft fabricated by in-body tissue architecture. J Biomed Mater Res B Appl Biomater. 2011;98:120–126. doi: 10.1002/jbm.b.31841. [DOI] [PubMed] [Google Scholar]