Abstract
Ghost cell odontogenic carcinoma (GCOC) is a rare malignant tumor of odontogenic origin, with only about 50 cases reported in the English literature so far. Histologically, it is characterized by ghost cells, dentinoid deposits, high grade malignant cellular features, and areas of necrosis and invasion. Having common histological features with other odontogenic ghost cell lesions (OGCL) like calcifying odontogenic cyst (COC) and dentinogenic ghost cell tumors, it is crucial to recognize GCOC malignant features, as it can be destructive and invasive, sometimes showing distant metastases and high recurrence rate. For this reason, it may entail more aggressive surgical approach and multimodal therapeutic regimen. Here we present a case report of GCOC arising in a previous COC, treated with surgical excision that showed persistence and recurrence after two years. The clinical and histological features of this rare occurrence are presented, in addition to the surgical approach, and a summary of literature review of OGCL.
Keywords: Ghost cell odontogenic carcinoma (GCOC), Malignant cellular features and invasion, Calcifying odontogenic cyst (COC), Dentinogenic ghost cell tumors (DGCT), Odontogenic ghost cell lesions (OGCL)
Case Report/Introduction
History and Clinical Findings
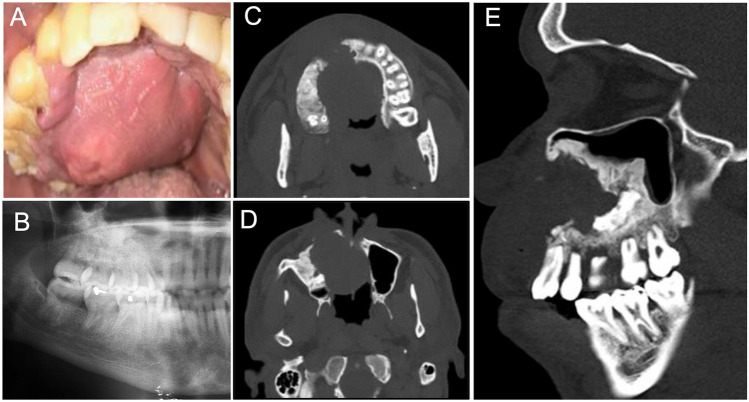
A 36-year-old African American male presented to the Oral and Maxillofacial Surgery Clinic with a long-standing history of right sided facial swelling, difficulty with speech and right nasal obstruction. Two and a half years prior, after a biopsy was taken and proven to be a calcifying odontogenic cyst (COC), he was treated initially with a decompression tube placed in the right posterior maxilla. He described that the decompression tube had recently fallen out and swelling had increased. The patient did not report any fever, chills, drainage, or other constitutional symptoms. He reported a history of tobacco, marijuana, and alcohol use. Physical exam revealed a large intra-oral mass, that was soft to palpation, encompassing the right maxilla with significant palatal expansion crossing the mid-line (Fig. 1A). Multiple loose teeth and ulcerated palatal mucosa were noted. The mass was appreciated within the nasal cavity on speculum exam as well. There was no palpable cervical adenopathy.
Fig. 1.
Clinical and radiographic presentation: A Intra-oral examination showed diffuse right sided palatal mass crossing the midline involving the hard and soft palate; B Panoramic radiograph. An ill-defined, predominantly radiopaque lesion with areas of radiolucency extending from the right anterior maxillary area and involving the right maxillary jawbone, right maxillary sinus, lateral nasal wall, and extending backwards towards the maxillary tuberosity; C-E Computed tomography scan. Bone window; C & D, Axial-bone view showed expansion of the tumor mass crossing the midline; E Sagittal view—the tumor mass filling the right maxillary sinus and into nasal cavity with areas of hyperdense calcific deposits
Radiologic Findings
The panoramic radiograph (Fig. 1B) showed an ill-defined predominately radiopaque lesion of the right maxilla extending from the maxillary incisor/canine area to the right maxillary tuberosity and into the maxillary sinus, lateral nasal wall and nasal cavity with appreciable growth and bone destruction. The computed tomography (CT) scan axial view bone window (Fig. 1C & D) showed expansion of the tumor mass crossing the midline. The sagittal view (Fig. 1E) showed the tumor occupying the right maxillary sinus with erosion of the anterior borders and expansion into the nasal cavity with areas of hyperdense calcific deposits.
Diagnosis and Treatment
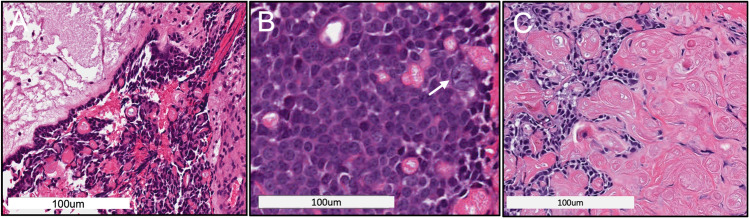
A new intra-oral maxillary incisional biopsy was performed. The gross examination of the submitted specimens revealed multiple pink-tan irregular fragments of soft tissue. The histological examination revealed surface epithelium with underlying fibrous connective tissue stroma containing proliferation of anastomosing cords and strands of hyperchromatic basaloid cells, some of which appeared to be lining a cystic space (Fig. 2A). Some areas showed larger polygonal cells with scattered ghost cell keratinization, atypical nuclei, and mitotic figures (Fig. 2B), while other areas showed sheets of ghost cells and some calcifications (Fig. 2C). The histological findings of the incisional biopsy led to a diagnosis of Ghost Cell Odontogenic Tumor (GCOT) with uncertain biological potential, with a recommendation for complete surgical excision and close follow up. The patient subsequently underwent a right subtotal maxillectomy via Weber-Ferguson facial splitting approach with intra-operative frozen section analysis. Simultaneous reconstruction of the maxillary defect with a fibula-osteocutaneous free flap was also performed.
Fig. 2.
Incisional biopsy (H&E* staining): A H&E-stained soft tissue section showed tumor islands with basaloid hyperchromatic cells mixed with ghost cells; B Hyperchromatic atypical cells and abnormal mitotic figure (arrow); C Sheets of ghost cell keratinization (*Hematoxylin and eosin)
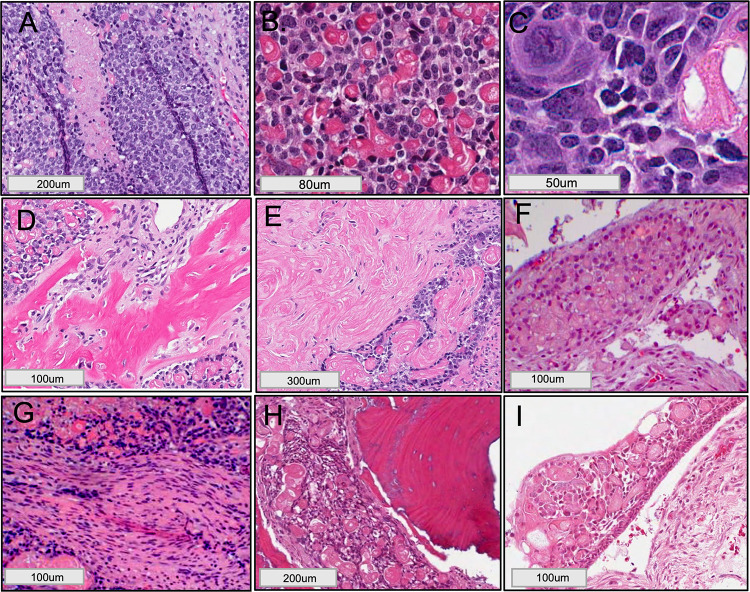
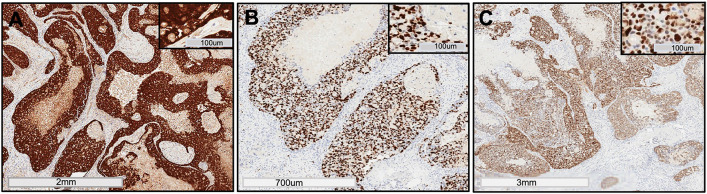
The histological examination of the excisional biopsy revealed proliferating cells in the form of broad sheets and strands. The tumor cells demonstrated malignant features such as pleomorphism, foci of comedo-necrosis (Fig. 3A), hyperchromatism, abnormal mitotic figures, and bizarre looking cells, intermixed with ghost cell keratinization (Fig. 3B & C). Areas of dentinoid deposits (Fig. 3D), and sheets of ghost cell keratinization (Fig. 3E) were noted, as well as intravascular ghost cells (Fig. 3F) and encroachment of tumor cells on nerve fibers (Fig. 3G). The medial margin showed evidence of tumor invasion into the maxillary alveolar bone and nasal septum (Fig. 3H). Cystic spaces lined by delicate odontogenic epithelium consisting of ameloblast-like cells and an overlying stellate reticulum-like layer with ghost cell keratinization, findings consistent with calcifying odontogenic cyst (COC), noted towards the Schneiderian membrane of the maxillary sinus (Fig. 3I). The immunohistochemical (IHC) stain of beta-catenin demonstrated strong diffuse positivity in the tumor islands (Fig. 4A). Ki67 was strongly positive with proliferative index of more than 75% (Fig. 4B). p53 stain was also strongly positive in the tumor cells (Fig. 4C), revealing focal areas of diffuse nuclear staining. A final diagnosis of GCOC arising in a previous COC was determined based on the examined H&E sections and IHC profile.
Fig. 3.
Excisional biopsy and GCOC histological features (H&E* staining): A High power showed malignant cellular features with areas of necrosis surrounded by large pleomorphic cells demonstrating vesicular nuclei; B & C Large bizarre looking cells and abnormal mitotic figures; D areas of dentinoid deposits; E sheets of ghost cell keratinization; F intravascular invasion; G tumor cells encroaching on nerve fiber. H The medial margin was positive for tumor islands invasion into the nasal septum; I COC-like area of cystic space lined with delicate odontogenic epithelium formed of basal ameloblast-like cells and an upper layer of stellate reticulum-like cells with ghost cell keratinization. (*Hematoxylin and eosin)
Fig. 4.
Immunohistochemical profile: Tumor cells showed strong positive expression for A Beta-catenin (β-Catenin antibody pre-diluted, clone 14, Cell Marque, VENTANA, Rocklin, CA); B Ki67 (Ki67 antibody pre-diluted, clone 30–9 VENTANA, Tucson, AZ); C p53 (p53 antibody pre-diluted, clone DO-7, VENTANA, Tucson, AZ)
The patient was then presented to the multidisciplinary head and neck tumor board with a recommendation for re-resection due to the positive medial margin, and adjuvant radiotherapy. The patient declined the proposed treatment plan and continued with follow-up for approximately 4 months. Surveillance PET/CT imaging at 3 months demonstrated no evidence of disease. The patient was non-compliant with follow-up appointments and returned approximately 2 years later with new onset swelling and a mass in the left maxilla. Biopsies performed along with repeat PET/CT imaging (Fig. 5) were consistent with persistent or recurrent GCOC. The patient underwent complete left hemi-maxillectomy, with simultaneous fibula flap reconstruction and adjuvant proton radiotherapy. He is currently 10 months from completion of therapy without evidence of disease.
Fig. 5.
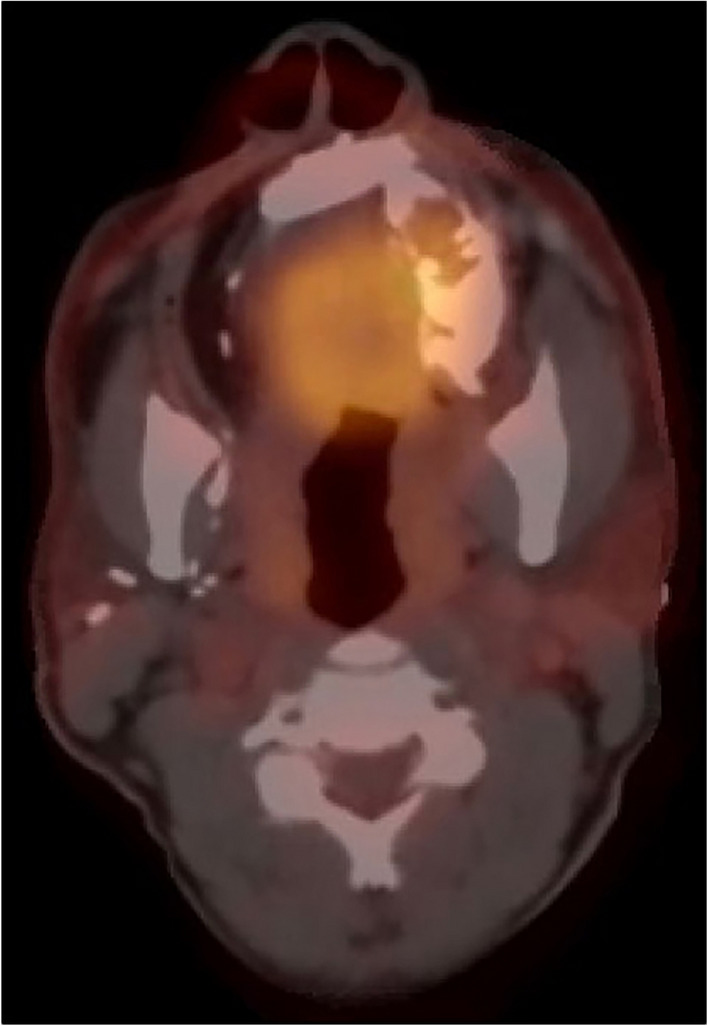
Two years follow up: PET/CT* imaging revealed evidence of disease in the left maxilla after two years from the excisional biopsy. (*Positron Emission Tomography—Computed Tomography)
Discussion
The GCOC is a very rare malignant tumor of odontogenic origin, accounting for approximately 0.23% of all odontogenic tumors and less than 3% of all ghost cell lesions [1]. About 50 cases have been reported in the English literature, with a higher prevalence seen in Asian population, with almost 38% of the cases being reported in this group [1–3]. It is most commonly seen in the maxilla with a higher predilection for males (M:F 3.4:1), often affecting patients over the 4th decade of life [1].
GCOC was first briefly described and presented in the International Histological Classification of Odontogenic Tumors by the WHO in 1971 [4, 5]. In 1975 another case of GCOC arising from COC was published in abstract form, in Japanese [6]. However, GCOC was first described as a malignant tumor in 1985 by Ikemura et al., where the previously described two cases also were referenced [7].
Over the years, different names were used to describe GCOC: malignant calcifying odontogenic cyst, carcinoma arising in a calcifying odontogenic cyst, calcifying ghost cell odontogenic carcinoma, malignant epithelial odontogenic ghost cell tumor, aggressive epithelial ghost cell odontogenic tumor, and malignant calcifying ghost cell odontogenic tumor [3, 8].
The term “ghost cells” describes pale, eosinophilic epithelial cells, anucleated or with a very faint shadow of the nuclei that are mainly seen in COCs or GCOTs, though they also can be seen in other odontogenic tumors such as odontomas, ameloblastic fibro-odontomas, or even in some nonodontogenic entities like craniopharyngiomas and pilomatricomas [3]. In two recently published papers, two cases of a new salivary gland malignancy with a prominent ghost cell population were described and a new terminology of “salivary ghost cell carcinoma” was proposed and introduced by Ihrler et al. in 2020 [9, 10]. The ghost cells might be a form of apoptosis in odontogenic epithelium, or an aberrant form of keratinization [11].
In 2005, Barnes et al. in the WHO International Histological Classification of Odontogenic Tumors guideline included the calcified cyst odontogenic tumors (CCOT), old terminology for the COC, along with the DGCT and the GCOT under the category of “GCOT” [12]. In the 2017 edition of the WHO book, the GCOC was classified under the malignant odontogenic tumors and the DGCT was included in the benign mixed epithelial and mesenchymal odontogenic tumors, whereas the COC was redefined as a developmental cyst, including it under the odontogenic and non-odontogenic developmental cysts category [3].
Etiologically, GCOC can arise either “de novo”, or from a previous COC, or in a previous DGCT [13]. Clinically, GCOC could present as a slow growing tumor, with swelling of the jaw, ulceration, pain, paresthesia, tooth mobility and root resorption or root displacement in the area, along with possible invasion of the tumor into the surrounding soft tissue [14].
Radiologically, it is characterized as an ill-defined radiolucent lesion, or mixed radiolucent/radiopaque lesion, with the opacity caused by dentinoid formation or mineralization of the ghost cells.
The radiographic differential diagnosis for GCOC can vary considerably according to the stage of calcification and due to the ill-defined nature of the lesion. Macroscopically, the tumor could be solid or multicystic, with a gritty consistency noted upon sectioning [15]. Histologically, GCOC presents with the following criteria: (i) an infiltrative growth pattern associated with ghost cell keratinization, (ii) areas of dentinoid formation may be found, and (iii) evidence of malignant transformation including pleomorphism, hyperchromatism, abnormal mitotic figures, and areas of necrosis [16]. It could present also with vascular or perineural invasion, like what was seen in our case (Fig. 3 F & G).
GCOC has overlapping microscopic features with COC and DGCT. The demographic and clinical features of COC, DGCT and GCOC are represented in the Table 1 [14, 17, 18]. Histologically, the COC (a.k.a. Gorlin cyst/or CCOT) accounts for less than 1% of all odontogenic cysts, and histopathologically presents as a simple cyst lined with lower ameloblastoma-like cells, and upper layers of stellate reticulum-like cells, containing focal ghost cells that can be calcified [3, 19]. This entity was described for the first time by Thoma in 1917 (the first radiological evidence of CCOT with odontoma) [20]. Later, in 1922 a CCOT radiograph was published for the first time, but in 1962 Gorlin et al. named it COC [21]. DGCT is a benign neoplasm of odontogenic epithelial origin that presents with ameloblastomatous proliferation, ghost cells and dentinoid deposits. Some lesions have sheets of basaloid hyperchromatic and isomorphic cells. Some authors consider it as being the solid type of COC [22]. DGCT can be locally destructive, with a recurrence rate of 33% for cases treated with radical surgery and 73% for cases treated with conservative surgery [3]. Approximately 40% of cases are peripheral lesions, mostly occurring in older patients, presenting as a painless firm nodule often with underlying “cup-shaped” cortical bone erosion [18].
Table 1.
Comparison of different features of the three GCOL: calcifying odontogenic cyst (COC), dentinogenic ghost cell tumor (DGCT) and ghost cell odontogenic carcinoma (GCOC)
| OGCL | COC | DGCT (only about 130 casesa) | GCOC (only about 50 casesa) |
|---|---|---|---|
| Decade mostly affected/(mean age) | 2nd (30.7 y/o) | 4th (45 y/o) | 5th (42.5 y/o) |
| Male to female ratio | 1: 1 | 1.5: 1 | 3.4: 1 |
| Main anatomical location affected | - Mandible (especially posterior) (51.7%) | - Mandible (especially posterior) (61.5%) | - Maxilla (especially anterior) (62.5%) |
| - Maxilla | - Mandible | ||
| - Maxillary sinus | - Maxillary sinus | ||
| - Ethmoid sinus | - Zygomatic region | ||
| Centrally or peripherally located | - Mostly central | - Mostly central | - Central lesions |
| - 10% peripheral (especially located in the anterior region of both jaws) | - 40% peripheral (mostly elderly pts.) | ||
| Signs and symptoms | - Asymptomatic (89.2%) | - Swelling/enlargement (73.8%) | - Swelling/enlargement (79.2%) |
| - Swelling | - Discomfort | - Pain | |
| - Pain | - Pain | - Mobility and /or displacement of teeth | |
| - Absence of tooth eruption | - Mobility and /or displacement of teeth | - Root resorption | |
| - Paresthesia | - Root resorption | - Paresthesia | |
| - Dental sensitivity | - Pus discharge | - Bleeding | |
| - Tooth displacement | - Lymphadenopathy | - Lymphadenopathy | |
| Radiologically | - Well-defined margins (87%) | - Well-defined margins (34.6%) | - Mostly with ill-defined borders (56.3%) |
| - Unilocular (81.9%) | - Unilocular (23.1%) or multilocular (13.8%) | - Unilocular (22.9%) or multilocular (14.6%) | |
| - Mixed RL/RO (61.1%) | - Mixed RL/RO (36.2%); RL (26.2%); RO (2.6%) | - Mixed RL/RO (47.9%); RL (31.3%); RO (5.4%)—irregular locularity (69.7%) | |
| - Could present with bone expansion, root displacement, root resorption or cortical erosion | - Could present with: root resorption, tooth displacement | ||
| Treatment | - Enucleation (69.9%) | - Conservative surgery (38.5%) | - Conservative surgery (2.1%—1 pt.) |
| - Radical surgery (32.3%) | - Conservative surgery + radiotherapy (2.1%—1pt.) | ||
| - Surgical removal or excisional biopsy (18.5%) | - Radical surgery + radiotherapy (0.8%, 1 pt.) | - Radical surgery (54.1%) | |
| - A two-stage approach (4%): decompression/marsupialization + removal | - Radical surgery + radiotherapy + chemotherapy (0.8%—1 pt.) | - Radical surgery + concomitant radiotherapy and/or chemotherapy and/or immunotherapy, including neoadjuvant therapy (29.3%) | |
| Recurrence/metastases | - No recurrence (96%) | - No recurrence (45.4%) | - No recurrence (43.8%) |
| - High recurrence rate | |||
| - Destructive and aggressive behavior | |||
| - Death was end point (in 9 pts.) | |||
| - Regional lymph node metastases (2 pts.) | |||
| - Distant metastasis (14.6%) to: lungs (5 pts.), cranium/brain (2 pts.) | |||
| - Recurrence | |||
| Cases related to other odontogenic lesions | - 28.6% of total no. of COCs occurred with: O (82%); AF (5%); DC (4%); AOT (3%); A (2%); AFO (2%); OKC (1%); OKOC (1%); A and AOT (1%) | - De novo (45.8%) | |
| - Precursor lesions: COC (25%) or DGCT (8.3%) | |||
| - COC arising in DF of impacted 3rd molars (radiolucency) (2%) | - History of previous: A or CEOT |
a- number of cases reported in the English literature up to date, A ameloblastoma, AF ameloblastic fibroma, AFO ameloblastic fibro-odontoma, AOT adenomatoid odontogenic tumor, COC calcifying odontogenic cyst, CEOT calcifying epithelial odontogenic tumor, DC dentigerous cyst, DF dental follicle, DGCT dentinogenic ghost cell tumor, GCOC ghost cell odontogenic carcinoma, O odontoma, OGCL odontogenic ghost cell lesions, OKC odontogenic kerato-cyst, OKOC orthokeratinized odontogenic cyst, pt./pts. patient/patients, RL radiolucent, RO radiopaque [14, 18]
In terms of IHC profile, the diagnosis of GCOC versus GCOT, as illustrated in our case, is favored by the p53 positive stain present in 2/3 of cases, and a high Ki67 proliferation marker. Positive beta-catenin stain, has been described in GCOC, as well as in several other benign and malignant tumors [3]. Other positive stains in GCOC are p63, pan-cytokeratin AE1/ AE3, and CAM5.2 [8]. In the ghost cells was also found a high expression of AE13, a hair cortex keratin [23].
In 2015, Bose et al. presented the first case of GCOC with genomic and exome sequencing [24]. The analysis revealed multiple alterations in the SHH signaling pathway, a deleted exon in the UBR5 gene, which has an important role in cell differentiation and proliferation. UBR5 was described before as being disrupted in different other cancer types, for instance the UBR5 gene fusion has also been reported in head and neck squamous cell carcinoma [25,24].
In 2017, Youkimori et al. showed that mutations in CTNNB1, the gene that encodes beta-catenin, are involved in the formation of the ghost cells and are found in COCs [26]. In 2018, the same group identified the same CTNNB1 mutation in a lesion that was diagnosed between GCOC and DGCT in a 44-year-old Japanese male patient that presented with left maxillary swelling and a previous history of maxillary cyst persistent for 25 years [23]. The IHC also showed strong beta-catenin nuclear positivity for this tumor.
The GCOC tumors could either be slowly growing and locally invasive carcinoma (the large majority of lesions), or rapidly growing and very aggressive with metastases and local recurrence. In our case, recurrence might have been due to the positive medial margin and persistence of the tumor cells. In terms of treatment, a wide surgical resection is recommended, as this approach was successful in 2/3 of reported cases.
In a 2017 case report, Gomez et al. emphasized the importance of 3-dimensional (3D) imaging for a successful and precise surgical resection because using a volumetric analysis helps in determining the depth and the contour of the tumor [27]. A few cases have been treated with adjuvant radiotherapy, but in some patients this treatment was not effective [15].
In 2015, Ahmed et al. reported the first case treated successfully with aggressive multimodal therapy in a 10 year old patient with regional lymph node metastasis that included surgery, adjuvant chemoradiation, and adjuvant immunotherapy [28].
GCOC has a high recurrence rate of approximately 63%, with destructive and aggressive behavior and distant metastases documented in three cases (one to the cranium and two to the lungs) out of 25 cases [29]. Death was the end point documented in 9 patients [14]. The overall 5-year survival rate was 73% for the first 16 reported cases in the literature [18].
Conclusion
GCOC has an unpredictable prognosis, due to the wide variety of growth patterns, the high recurrence rate, and the very limited number of cases reported in literature. It is important to report more cases in order to advance our understanding about this uncommon GCOL. Early recognition of this disease is very important to avoid possible underdiagnoses since it is an extremely rare tumor that may arise in otherwise innocuous COC or DGCT, and it could exhibit aggressive behavior with an uncertain outcome. For all these reasons, long-term follow-up for these patients is highly recommended.
Acknowledgements
None.
Authors Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Funding
Department of Oncology and Diagnostic Sciences, School of Dentistry and Department of Pathology, School of Medicine, University of Maryland Baltimore.
Data Availability
Department of Oncology and Diagnostic Sciences, University of Maryland, School of Dentistry, 650 West Baltimore Street, Baltimore, MD 21201, USA and Department of Pathology, School of Medicine, North Hospital UMMC NBW71, Baltimore, MD 21201.
Code Availability
Not Applicable.
Declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical Approval
Ethics approval was exempt in view of the retrospective nature of the study and the fact that all procedures were part of the routine care, based on institutional regulations.
Research Involving Humans and/or Animals
Not applicable.
Consent to Participate
No informed consent was obtained due to the retrospective nature of the case and since only intra-oral images and pathology slides were used.
Consent for Publication
No consent was obtained from the participant due to the retrospective nature of the case and since only intra-oral images and pathology slides were used.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park SY, Park J, Kwon DH, Jeon JH, Kim SM, Myoung H, Lee JH. Ghost cell odontogenic carcinoma on right mandible and its respective surgical reconstruction: a case report. J Korean Assoc Oral Maxillofac Surg. 2017;43(6):415–422. doi: 10.5125/jkaoms.2017.43.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumors: II. Calcifying epithelial odontogenic tumor versus ghost cell odontogenic tumors derived from calcifying odontogenic cyst. Korean J Pathol. 2014;48(3):175–187. doi: 10.4132/KoreanJPathol.2014.48.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization Classification of Head and Neck Tumors 4th ed. IARC/ WHO; 2017.
- 4.Pindborg, JJ. Histological typing of odontogenic tumours, jaw cysts, and allied lesions. International histological classification of tumors. Geneva : World Health Organization; 1971: 1–44.
- 5.Remya K, Sudha S, Nair RG, Jyothi H. An unusual presentation of ghost cell odontogenic carcinoma: a case report with review of literature. Indian J Dent Res. 2018;29(2):238–243. doi: 10.4103/ijdr.IJDR_442_17. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Takigawa T, O.M.M.M.F.K. Malignant transformation of calcifying odontogenic cyst: A case report. Jap J Oral Sirrg, 1975; 21:664 (in Japanese).
- 7.Ikemura K, Horie A, Tashiro H, Nandate M. Simultaneous occurrence of a calcifying odontogenic cyst and its malignant transformation. Cancer. 1985;56(12):2861–2864. doi: 10.1002/1097-0142(19851215)56:12<2861::aid-cncr2820561224>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Magliocca K, Mandible MA, & maxilla Odontogenic carcinoma Ghost cell odontogenic carcinoma. PathologyOutlines.com. 2016 February 12, 2021; https://www.pathologyoutlines.com/topic/mandiblemaxillaghostcellodontogeniccarcinoma.html.
- 9.Harada H, Sato MP, Otsuki N, Kawamura M, Kurose A, Satou T. A novel parotid carcinoma with a prominent ghost cell population: a masquerading tumor or "salivary ghost cellcarcinoma"? Med Mol Morphol. 2021 doi: 10.1007/s00795-021-00302-9. [DOI] [PubMed] [Google Scholar]
- 10.Ihrler S, Mollenhauer M, Weitmayr B, Haas CJ. Salivary ghost cell carcinoma: case report and proposal of a new entity. Virchows Arch. 2020;476(3):465–468. doi: 10.1007/s00428-019-02657-y. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Lee EH, Yook JI, Han JY, Yoon JH, Ellis GL. Odontogenic ghost cell carcinoma: a case report with reference to the relation between apoptosis and ghost cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(5):630–635. doi: 10.1067/moe.2000.109016. [DOI] [PubMed] [Google Scholar]
- 12.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology & Genetics. Head and Neck Tumours. Tumours of the oral cavity and oropharynx. 3rd ed. IARC/ WHO. 2005; pp 168–175.
- 13.Li BB, Gao Y. Ghost cell odontogenic carcinoma transformed from a dentinogenic ghost cell tumor of maxilla after multiple recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):691–695. doi: 10.1016/j.tripleo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.de Arruda JAA, Monteiro JLGC, Abreu LG, de Oliveira Silva LV, Schuch LF, de Noronha MS, Callou G, Moreno A, Mesquita RA. Calcifying odontogenic cyst, dentinogenic ghost cell tumor, and ghost cell odontogenic carcinoma: a systematic review. J Oral Pathol Med. 2018;47(8):721–730. doi: 10.1111/jop.12727. [DOI] [PubMed] [Google Scholar]
- 15.Ali EA, Ali Karrar M, El-Siddig AA, Gafer N, Abdel Satir A. Ghost cell odontogenic carcinoma of the maxilla: a case report with a literature review. Pan Afr Med J. 2015 doi: 10.11604/pamj.2015.21.260.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neville BW, Damm DD, Allen CM, Chi AC, Oral and Maxillofacial Pathology 4th ed. Elsevier; 2016.
- 17.Ledesma-Montes C, Gorlin RJ, Shear M, Prae Torius F, Mosqueda-Taylor A, Altini M, Unni K, Paes de Almeida O, Carlos-Bregni R, Romero de León E, Phillips V, Delgado-Azañero W, Meneses-García A. International collaborative study on ghost cell odontogenic tumours: calcifying cystic odontogenic tumour, dentinogenic ghost cell tumour and ghost cell odontogenic carcinoma. J Oral Pathol Med. 2008;37(5):302–308. doi: 10.1111/j.1600-0714.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- 18.de Souza VG, de Pinho MP, Rozza-de-Menezes RE, Cunha KSG, Conde DC. Comparative analysis between dentinogenic ghost cell tumor and ghost cell odontogenic carcinoma: a systematic review. Head Neck Pathol. 2021;15(4):1265–1283. doi: 10.1007/s12105-021-01347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ide F, Kikuchi K, Miyazaki Y, Kusama K, Saito I, Muramatsu T. The early history of odontogenic ghost cell lesions: from Thoma to Gorlin. Head Neck Pathol. 2015;9(1):74–78. doi: 10.1007/s12105-014-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoma KH. Oral roentgenology. A roentgen study of theanatomy and pathology of the oral cavity. Boston: Ritter; 1917; pp. 136–137.
- 21.Gorlin RJ, Pindbourg JJ, Clausen FP, Vickers RA. The calcifying odontogenic cyst - a possible analogue of the cutaneous calcifying epithelioma of Malherbe. An analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. 1962;15:1235–1243. doi: 10.1016/0030-4220(62)90159-7. [DOI] [PubMed] [Google Scholar]
- 22.Ravi B, Kamath G, Srivathsa S, Babshet M, Dayanarayana U. Dentinogenic ghost cell tumor - a rare case report. J Stomatol Oral Maxillofac Surg. 2020;121(2):186–188. doi: 10.1016/j.jormas.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Ohata Y, Kayamori K, Yukimori A, Sumikura K, Ohsako T, Harada H, Sakamoto K, Ikeda T. A lesion categorized between ghost cell odontogenic carcinoma and dentinogenic ghost cell tumor with CTNNB1 mutation. Pathol Int. 2018;68(5):307–312. doi: 10.1111/pin.12659. [DOI] [PubMed] [Google Scholar]
- 24.Bose P, Pleasance ED, Jones M, Shen Y, Ch'ng C, Reisle C, Schein JE, Mungall AJ, Moore R, Ma Y, Sheffield BS, Thomson T, Rasmussen S, Ng T, Yip S, Lee CW, Ho C, Laskin J, Marra MA, Jones SJ. Integrative genomic analysis of ghost cell odontogenic carcinoma. Oral Oncol. 2015;51(9):e71–e75. doi: 10.1016/j.oraloncology.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Chung GT, Lung RW, Hui AB, Yip KY, Woo JK, Chow C, Tong CY, Lee SD, Yuen JW, Lun SW, Tso KK, Wong N, Tsao SW, Yip TT, Busson P, Kim H, Seo JS, O'Sullivan B, Liu FF, To KF, Lo KW. Identification of a recurrent transforming UBR5-ZNF423 fusion gene in EBV-associated nasopharyngeal carcinoma. J Pathol. 2013;231(2):158–167. doi: 10.1002/path.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yukimori A, Oikawa Y, Morita KI, Nguyen CTK, Harada H, Yamaguchi S, Kayamori K, Yamaguchi A, Ikeda T, Sakamoto K. Genetic basis of calcifying cystic odontogenic tumors. PLoS ONE. 2017;12(6):e0180224. doi: 10.1371/journal.pone.0180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes JP, Costa AL, Chone CT, Altemani AM, Altemani JM, Lima CS. Three-dimensional volumetric analysis of ghost cell odontogenic carcinoma using 3-D reconstruction software: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(5):e170–e175. doi: 10.1016/j.oooo.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed SK, Watanabe M, deMello DE, Daniels TB. Pediatric metastatic odontogenic ghost cell carcinoma: a multimodal treatment approach. Rare Tumors. 2015;7(2):5855. doi: 10.4081/rt.2015.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namana M, Majumdar S, Uppala D, Avv A, Rao AK. Ghost cell odontogenic carcinoma arising denovo with distant metastasis: a case report and review of literature. J Clin Diagn Res. 2017;11(8):01–03. doi: 10.7860/JCDR/2017/28143.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Department of Oncology and Diagnostic Sciences, University of Maryland, School of Dentistry, 650 West Baltimore Street, Baltimore, MD 21201, USA and Department of Pathology, School of Medicine, North Hospital UMMC NBW71, Baltimore, MD 21201.
Not Applicable.