Abstract
The past decade has seen a dramatic increase in the number of new head and neck tumor entities, most of which are genetically defined. DEK::AFF2 carcinoma is one of the most recently defined neoplasms; it shows a non-keratinizing squamous morphology and occurs in the sinonasal region. We present an unusual neoplasm that was found to harbor a novel fusion involving AFF2. The case was encountered in our clinical practice. Immunohistochemistry was performed along with targeted next generation sequencing (NGS). The case presented as a metastasis to a cervical lymph node from an unknown primary, in a 49-year-old man. The tumor consisted of sheets of primitive round cells which were strongly positive for synaptophysin and chromogranin but negative for cytokeratins, S-100 protein, WT-1, desmin, and many other markers. NGS uncovered CHD4::AFF2. We found a CHD4::AFF2 fusion in a high-grade neuroendocrine tumor. Although it is just a single case, the presence of a novel fusion in a neoplasm that is otherwise not classifiable suggests that it could be a distinct entity within a possible family of AFF2-rearranged tumors. Molecular analysis should be considered for any unclassified round cell tumor in the head and neck, as additional cases will be needed to further elucidate this area.
Keywords: CHD4::AFF2, Neuroendocrine tumor, DEK::AFF2
Introduction
Head and neck small round blue cell tumors (SRBCTs) represent a diagnostically challenging group of phenotypically and genetically heterogenous clinical entities. The cytomorphologic and histopathologic characterization of SRBCTs is simply a monotonous tumor cell population with small to medium sized nuclei and minimal cytoplasm. However, there are considerable diagnostic challenges when it comes to definitively classifying SRBCTs in small tissue biopsies or cytologic preparations due to overlapping morphologic features, limited material, and the requirement of a careful and algorithmic utilization of ancillary studies. Additionally, with increased utilization of molecular technologies such as RNA sequencing, new entities characterized by recurrent clonal translations are still being defined. Here, we report a case of a metastatic high-grade SRBCT showing no overt histopathologic differentiation with strong expression of neuroendocrine markers, negative cytokeratins, and no expression of S-100 protein by immunohistochemistry, which was found to harbor a novel gene fusion.
Case Description
Clinical and Radiological Findings
A 49-year-old man with a history of chronic sinus infections presented to ENT clinic in the fall of 2020 after a 10-day course of antibiotics with no improvement. His symptoms included facial pain in the left cheek and behind the left eye. On rigid nasal endoscopy, the sinuses appeared slightly edematous with no masses seen. He was advised to continue nasal irrigations and nasal steroid spray. However, over the following few weeks, he continued to have pain behind his left eye and left side of face, as well as a more pronounced swelling along his jaw. This prompted the ordering of facial and brain MRIs, and a facial bone CT exam. The facial MR images showed no abnormalities within the nasal cavity, paranasal sinuses, nasopharynx, oropharynx, or hypopharynx. A concurrent brain MRI showed no intracranial, orbit, periorbital, skull base, or calvarium abnormalities. However there was a 2.5 × 1.7 cm round T2 hyperintense nodule along the margin of the left submandibular gland with a thin rim of post gadolinium enhancement. This was further elucidated on follow-up CT showing a 2.5 cm left submandibular area mass with mostly solid consistency and possible cystic or necrotic changes. On a follow-up clinic visit, there was a palpable mass adjacent to mandible and submandibular gland, with no overlying skin change. He initially noticed the submandibular swelling a few months prior, which was small but noticeable at first, then grew slightly and had stabilized in size over the prior 6 + weeks.
Otherwise, his medical and surgical history included allergic rhinitis status post sinus surgery in December 2019 and nasolacrimal duct dysfunction, but was otherwise unremarkable. He was a former one pack-per-week smoker for 15 years but quit in 2017. He grew up in an area with a tributary with an extensive history of hazardous-waste and radioactive material problems. His maternal grandparents both had lung cancer, as did his mother.
Pathologic Findings
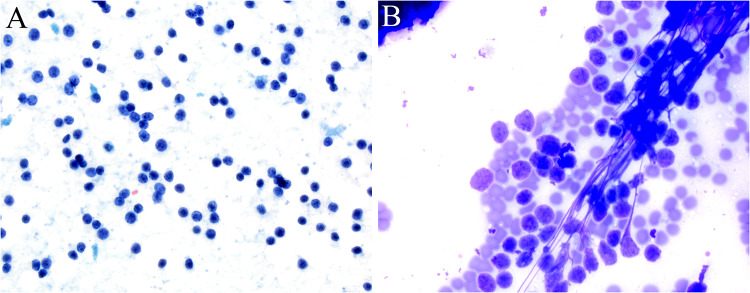
In March, the patient underwent palpation fine needle aspiration in clinic which showed a monotonous population of dyscohesive small round blue cells with scant cytoplasm (Fig. 1). A few clusters of more cohesive cells but no necrosis, molding, or apoptotic bodies were noted so the aspirate was initially signed out as a benign lymphocytic population.
Fig. 1.
Fine needle aspiration with Pap A and Diff-Quik B stains showed a monotonous population of dyscohesive small round, lymphocyte-like cells with scant cytoplasm
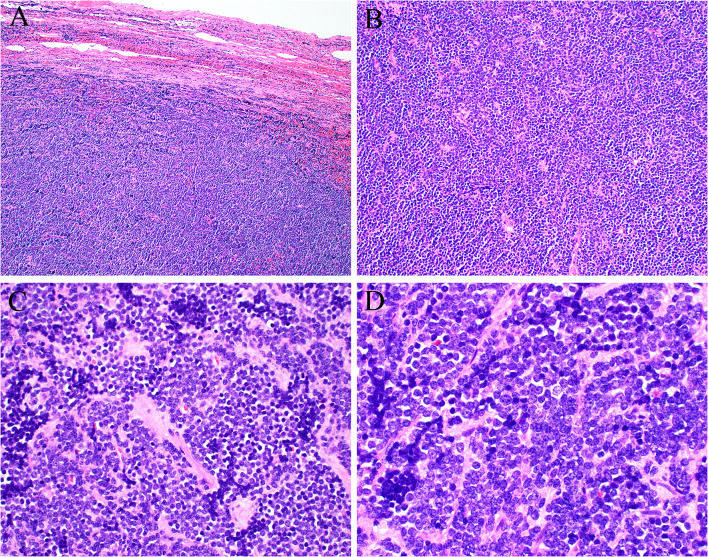
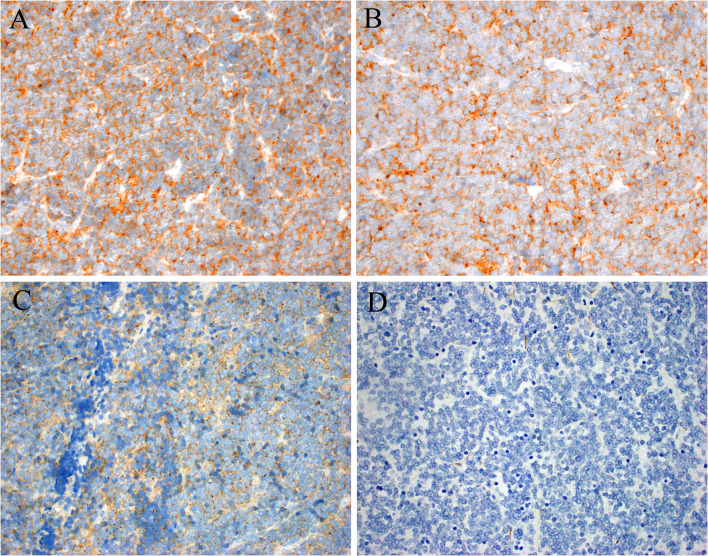
In April, he underwent excision of a mass in the left level IB area, deep and superior to the submandibular gland. Grossly, the tumor was a pink-tan ovoid nodule measuring 3.0 cm which was sectioned and submitted entirely. Histologic sections demonstrated a well-circumscribed lymph node that was almost completely effaced by sheets of primitive-appearing small round cells with scant to absent cytoplasm and round to oval shaped nuclei with irregular borders (Fig. 2). The chromatin appeared coarsely clumped with variably prominent chromocenters. The background stroma was delicate, eosinophilic and vaguely hyalinized with prominent anastomosing blood vessels, but the tumor cells did not show any architectural features indicating a specific line of differentiation. By immunohistochemistry (Fig. 3) the tumor cells were strongly positive for synaptophysin, chromogranin, CD56 and BCL2; weakly positive for p63, SOX-11 and c-MYC; focally positive for CD99 (mostly paranuclear dot-like with minimal membranous); while negative for pankeratin, Cam5.2, HMWCK (34-beta), S-100 protein, CK5/6, CK7, CK20, CDX2, CD45, cyclin D1, CD3, CD10, CD20, CD30, CD23, CD5, MUM-1, BCL6, TTF-1, p16, p40, WT-1, desmin, beta catenin, calretinin, NKX2.2, and Merkel cell polyomavirus. Immunostains showed intact/normal expression of SMARCB1 and SMARCA4, and in situ hybridization for HR-HPV RNA was negative. PD-L1 was negative in tumor cells and lymphocytes. Mitotic figures were conspicuous with 5–7 crisply identifiable mitotic figures per one (1) mm2, and the Ki-67 proliferation index was between 30–40%.
Fig. 2.
Excisional biopsy showed a lymph node with residual capsule and subcapsular sinus (A) otherwise obliterated by sheets of primitive round cells showing no signs of specific differentiation (B). The tumor cells had scant amounts of pale cytoplasm and uniform round nuclei, with small amounts of fibrotic stroma (C). The tumor nuclei were round to oval shaped with coarsely clumped chromatin (D)
Fig. 3.
By immunohistochemistry the tumor was strongly positive for synaptophysin (A) and chromogranin (B). CD99 showed weak cytoplasmic staining with paranuclear dots (C). Cam5.2 was negative (D), along with essentially all other markers of differentiation (e.g., desmin, SOX10, S100, NKX2.2, etc.)
The tumor was diagnosed simply as metastatic high-grade neuroendocrine tumor. To further classify the tumor, clinical and radiological correlation was strongly advised.
Following this diagnosis, the fine needle aspiration underwent intradepartmental review. And although the aspirates showed a small round blue cell morphology, some of the clusters appeared more cohesive than would be expected in germinal centers or reactive lymphoid tissue. Additionally, the chromatin was coarsely clumped and lymphoglandular bodies were inconspicuous. Although the cell block was limited, the cells of interest were present and showed strong expression of synaptophysin, chromogranin, and CD56 with virtually absent immunostaining for CD45. An amendment was issued correcting the diagnosis.
Next Generation Sequencing (NGS)
To address the possibility of a Ewing-like round cell sarcoma or other fusion-driven tumor, targeted NGS was performed as previously described [1]. A CHD4::AFF2 fusion was found with breakpoints of exons CHD4 exon 10 and AFF2 exon 6. No mutations were identified, and the tumor mutational burden was low at 2.6 somatic mutations per megabase, consistent with a fusion-driven tumor. To the authors’ knowledge, CHD4::AFF2 fusions have not been described previously in any human neoplasm.
Clinical Follow-up and Treatment
Following the patient’s diagnosis, F-18 FDG PET/CT imaging showed FDG avid sub-centimeter left level II cervical lymph nodes with SUV max of 2.7, concerning for metastatic disease and no other significant uptake, asymmetries, or abnormalities in the head and neck, chest, abdomen, or pelvis, including no hypermetabolic or enlarged abdominal or pelvic lymphadenopathy. Serum chromogranin, serotonin, and 5-HIAA were within normal limits. A Cu-64 mCi Cu-64 DOTATATE PET/CT exam including the skull vertex to the proximal thighs, was negative for any suspicious DOTATATE avidity, even within the cervical nodes described on the FDG imaging. However, the patient underwent a left neck dissection including levels I—IV, which showed additional metastatic neuroendocrine tumor involving three (3) of thirty-three (33) lymph nodes (levels I & III). The patient was staged as pathologic stage IVB (pT0, pN3b, cM0) and began adjuvant treatment with radiation combined with cisplatin. Treatment related toxicities, however, led to the discontinuation of cisplatin in favor of carboplatin. A primary site for his tumor has not been identified. The patient’s prior routine endoscopic sinus pathology was re-reviewed and confirmed to be negative for tumor.
Discussion
In the past decade, there have been several genetically-defined groups of head and neck tumors elucidated. The ever growing list includes secretory carcinoma (ETV6::NTRK3), NUT carcinoma (rearrangement of NUT, most commonly BRD4::NUT), SWI/SNF tumors (SMARCB1-deficient carcinoma and SMARCA4-deficient sinonasal carcinoma), adamantinoma-like Ewing sarcoma (EWSR1::FLI1), PAX3 rearrangement in biphenotypic sinonasal sarcoma, IDH-mutant sinonasal undifferentiated carcinoma, as well as non-keratinizing squamous cell carcinoma of the sinonasal region [2–13]. Importantly, some of the previously mentioned groups of tumors have both diagnostic and therapeutic implications with NTRK-inhibitors, histone deacetylase and bromodomain inhibitors, agents targeting mediators of DNA damage repair, and EFT-specific chemotherapy under active clinical investigation, or even front-line usage [14–19].
Head and neck tumors with DEK::AFF2 are histologically characterized by non-keratinizing basaloid squamous differentiation [4]. The series of tumors described have been positive for squamous differentiation markers by IHC, such has CK5, p40, and HMWCKs. Because one of the patients with a DEK::AFF2 responded exceptionally well to anti-PDL1 therapy, determining if this fusion is a biomarker of therapeutic response to immunotherapy is an active area of investigation.
We describe a case of a metastatic tumor harboring CHD4::AFF2, with the same AFF2 breakpoint seen in DEK::AFF2. Although it is just a single case without a known site of tumor origin, the novel fusion coupled with features that made the tumor otherwise unclassifiable suggest the possibility that this could be a new tumor type in what may be a family of AFF2-rearranged malignancies. The lack of epithelial differentiation by morphology and immunohistochemistry combined with negative S-100 protein and calretinin ruled out classification of the tumor as a neuroendocrine carcinoma, olfactory neuroblastoma, or paraganglioma. The negative immunostain for NKX2.2, absence of membranous CD99 and positive immunostain for chromogranin argued against Ewing sarcoma. The absence of any fusion associated with a list of “Ewing-like” sarcomas argues against that designation. The additional markers excluded lymphoma, melanoma, and traditional round cell sarcomas such as rhabdomyosarcoma or poorly differentiated synovial sarcoma. Indeed, neuroendocrine positivity in a head and neck tumor that is otherwise unclassifiable might be a clue to a CHD4::AFF2 tumor.
AFF2 is located on the long arm of the X chromosome (Xq28) and belongs to the AFF (AF4/FMR2) family of nuclear transcriptional activators which includes AFF1/AF4, AFF2/FMR2, AFF3/LAF4 and AFF4/AF5q31. Mutations in AFF2 are a known cause of X-linked intellectual disability with relatively mild to borderline phenotypes possibly suggesting functional redundancy among the AFF family members [20]. While AFF2 rearrangements have only recently been described in the setting of carcinomas involving the sinonasal/middle ear/skull base region, the other three members are known partners in KMT2A (MLL)-rearranged leukemias [21]. Until now, the only documented fusion partner gene has been DEK, a chromatin-associated oncoprotein involved in the maintenance of heterochromatin integrity and histone H3.3 deposition, transcriptional regulation, DNA repair damage and susceptibility, and splice site selection during mRNA processing [22].
The DEK protein contains an N-terminal DNA binding domain – the SAP Scaffold attachment factor A/B-Acinus-Pias) box [23]- which is preserved in all DEK::AFF2 fusion transcripts reported to date [5]. The oncogenic mechanism of DEK::AFF2 requires further investigation; however, insights may be gleaned from experimental models of the KMT2A (MLL)-AF4 family fusion oncoproteins, which have been shown to activate Elk-1 through the Ras/MEK/ERK pathway [24] and regulate BCL2 and MYC through combinatorial transcription factor activity [25]. CHD4 is located on 12p13.31 and encodes a chromatin remodeling protein that is part of the nucleosome remodeling and histone deacetylase (NURD) repressor complex, which serves as an epigenetic regulator of gene transcription, DNA repair, and cell cycle progression. The CHD4 (Mi-2β) protein contains paired N-terminal PHD (plant homeodomain) domains, which mediate nucleosome interactions through binding of histone H3 [26, 27] and are preserved in the CHD4::AFF2 fusion transcript. The PHD domains of CHD4 and the SAP domain of DEK likely enable chromatin targeting of their respective fusion products.
In summary, we describe a novel fusion in a metastatic high-grade neuroendocrine neoplasm of unknown origin. Additional cases will be needed to determine if CHD4::AFF2 neuroendocrine tumor is a distinct tumor entity, or if there are other members of a possible family of AFF2-rearranged neoplasms. Fusion analysis should therefore be considered on any primitive round cell head and neck tumor that defies classification.
Author contributions
All authors confirm they have meaningfully contributed to the research and read and approved the final manuscript.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Data availability
Availability of data and material is possible upon reasonable request, deidentified for maintenance of anonymity and compliance with IRB approval.
Code availability
Not applicable.
Declarations
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017–073), which did not require informed consent.
Consent to participate
The IRB-approved study was classified as exempt, which does not require informed consent.
Consent for publication
Consent for publication was obtained from all individual participants for whom identifying information is uniquely included in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rooper LM, Thompson LDR, Gagan J, Oliai BR, Weinreb I, Bishop JA. Salivary intraductal carcinoma arising within intraparotid lymph node: a report of 4 cases with identification of a novel STRN-ALK fusion. Head Neck Pathol. 2021;15(1):179–185. doi: 10.1007/s12105-020-01198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooper LM, Agaimy A, Dickson BC, et al. DEK-AFF2 Carcinoma of the Sinonasal Region and Skull Base: Detailed Clinicopathologic Characterization of a Distinctive Entity. Am J Surg Pathol. 2021;45:1693. doi: 10.1097/PAS.0000000000001741. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JA, Gagan J, Paterson C, McLellan D, Sandison A. Nonkeratinizing squamous cell carcinoma of the sinonasal tract with DEK-AFF2: further solidifying an emerging entity. Am J Surg Pathol. 2021;45(5):718–720. doi: 10.1097/PAS.0000000000001596. [DOI] [PubMed] [Google Scholar]
- 4.Todorovic E, Truong T, Eskander A, et al. Middle ear and temporal bone nonkeratinizing squamous cell carcinomas with DEK-AFF2 fusion: an emerging entity. Am J Surg Pathol. 2020;44(9):1244–1250. doi: 10.1097/PAS.0000000000001498. [DOI] [PubMed] [Google Scholar]
- 5.Kuo YJ, Lewis JS, Zhai C, et al. DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol. 2021;34(10):1820–1830. doi: 10.1038/s41379-021-00846-2. [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A, Bishop JA. SWI/SNF-deficient head and neck neoplasms: an overview. Semin Diagn Pathol. 2021;38(3):175–182. doi: 10.1053/j.semdp.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. OncoTargets Ther. 2019;12:3235–3244. doi: 10.2147/OTT.S173056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCuiston A, Bishop JA. Usefulness of NKX2.2 Immunohistochemistry for Distinguishing Ewing Sarcoma from Other Sinonasal Small Round Blue Cell Tumors. Head Neck Pathol. 2018;12(1):89–94. doi: 10.1007/s12105-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilmette J, Sadow PM. High-grade sinonasal carcinoma: classification through molecular profiling. Arch Pathol Lab Med. 2019;143(11):1416–1419. doi: 10.5858/arpa.2018-0224-RS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Bledsoe KL, Graham RP, et al. Recurrent PAX3-MAML3 fusion in biphenotypic sinonasal sarcoma. Nat Genet. 2014;46(7):666–668. doi: 10.1038/ng.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SC, Ghossein RA, Bishop JA, et al. Novel PAX3-NCOA1 fusions in biphenotypic sinonasal sarcoma with focal rhabdomyoblastic differentiation. Am J Surg Pathol. 2016;40(1):51–59. doi: 10.1097/PAS.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017;30(5):650–659. doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 14.Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma (NMC) of the head and neck (HN) Cancer. 2016;122(23):3632–3640. doi: 10.1002/cncr.30242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French C. NUT midline carcinoma. Nat Rev Cancer. 2014;14(3):149–150. doi: 10.1038/nrc3659. [DOI] [PubMed] [Google Scholar]
- 16.Mittal P, Roberts CWM. The SWI/SNF complex in cancer—biology, biomarkers and therapy. Nat Rev Clin Oncol. 2020;17(7):435–448. doi: 10.1038/s41571-020-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centore RC, Sandoval GJ, Soares LMM, Kadoch C, Chan HM. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends Genet. 2020;36(12):936–950. doi: 10.1016/j.tig.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Bishop JA. Recently described neoplasms of the sinonasal tract. Semin Diagn Pathol. 2016;33(2):62–70. doi: 10.1053/j.semdp.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Melko M, Douguet D, Bensaid M, et al. Functional characterization of the AFF (AF4/FMR2) family of RNA-binding proteins: insights into the molecular pathology of FRAXE intellectual disability. Hum Mol Genet. 2011;20(10):1873–1885. doi: 10.1093/hmg/ddr069. [DOI] [PubMed] [Google Scholar]
- 21.Meyer C, Burmeister T, Gröger D, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32(2):273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandén C, Gullberg U. The DEK oncoprotein and its emerging roles in gene regulation. Leukemia. 2015;29(8):1632–1636. doi: 10.1038/leu.2015.72. [DOI] [PubMed] [Google Scholar]
- 23.Böhm F, Kappes F, Scholten I, et al. The SAF-box domain of chromatin protein DEK. Nucleic Acids Res. 2005;33(3):1101–1110. doi: 10.1093/nar/gki258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng MHJ, Ng RK, Kong CT, Jin DY, Chan LC. Activation of Ras-dependent Elk-1 activity by MLL-AF4 family fusion oncoproteins. Exp Hematol. 2010;38(6):481–488. doi: 10.1016/j.exphem.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Harman JR, Thorne R, Jamilly M, et al. A KMT2A-AFF1 gene regulatory network highlights the role of core transcription factors and reveals the regulatory logic of key downstream target genes. Genome Res. 2021 doi: 10.1101/gr.268490.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield RE, Musselman CA, Kwan AH, et al. Plant homeodomain (PHD) fingers of CHD4 are histone H3-binding modules with preference for unmodified H3K4 and methylated H3K9. J Biol Chem. 2011;286(13):11779–11791. doi: 10.1074/jbc.M110.208207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson AA, Mahajan P, Mertens HDT, et al. The PHD and chromo domains regulate the ATPase activity of the human chromatin remodeler CHD4. J Mol Biol. 2012;422(1):3–17. doi: 10.1016/j.jmb.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material is possible upon reasonable request, deidentified for maintenance of anonymity and compliance with IRB approval.
Not applicable.