Abstract
This report describes two cases of oral localized amyloidosis (LA). In case 1, a 52-year-old man appeared with painful slightly, yellowish multiple nodules located on the dorsum of the tongue, of unknown duration. Incisional biopsy was performed, and the histopathologic analysis revealed a homogeneous, eosinophilic, and extracellular material. Congo red stain showed salmon pink coloration at light microscopy and apple-green birefringence at polarized light. In case 2, a 74-year-old man presented asymptomatic nodular lesions on the labial commissures with duration of several months. An excisional biopsy was performed in both lesions, and microscopically the specimen demonstrated the same histopathologic features of the case 1. Furthermore, amyloidosis with systemic involvement was excluded after investigations for both patients. Thus, the final diagnosis for both cases was LA. The patient 1 refused the surgical excision of the residual lesion, and in both cases, no signs of clinical and systemic progression were observed after 24 and 84 months of follow up. Although it is rare, LA should be considered in the differential diagnosis of multiple or single yellowish nodules on the oral cavity.
Keywords: Amyloidosis, Mouth, Tongue
HISTORY AND CLINICAL FINDINGS
Case 1
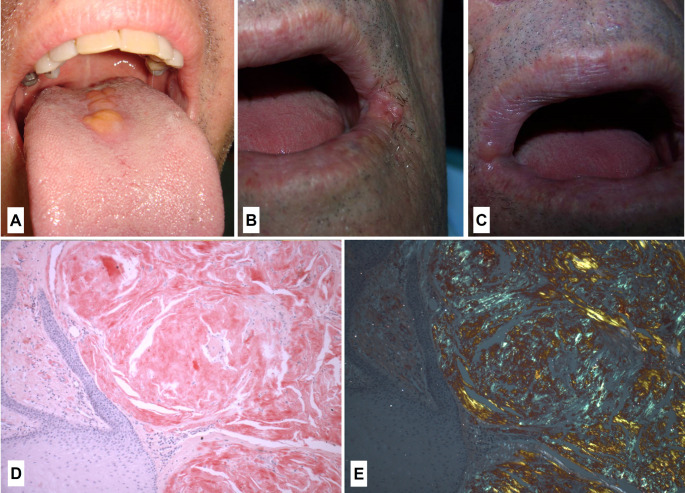
A 52-year-old man presented with a chief complaint of multiple nodules on the tongue of unknown duration. Medical history was not contributory. Intraoral examination revealed painful slightly, well-circumscribed, yellowish multiple nodules located on the dorsal tongue (Fig. 1), with a firm consistency. Choristoma, ectopic lymphoid tissue and amyloidosis were the main clinical diagnoses. Under local anesthesia, an incisional biopsy was performed.
Fig. 1.
– A: Well-circumscribed and yellowish multiple nodules located on the dorsal tongue. B-C: Oral examination revealed nodules with smooth surface and firm consistency located on the left (B) and right (C) labial commissures. D: Abundant amount of eosinophilic, amorphous, and extracellular material adjacent to a hyperplastic epithelium, showing salmon pink coloration (Congo red stain, original magnification, x100). E: Amorphous and extracellular material exhibiting apple-green birefringence at polarized light (Congo red stain at polarized light, original magnification, x100)
Case 2
A 74-year-old man was referred for evaluation of lesions on the labial commissures with duration of several months. The patient was asymptomatic. His past personal and family histories were not contributory. These lesions improved with antifungal treatment but did not disappear. Oral examination revealed nodules with smooth surface and firm consistency, located on the right and left labial commissures (Fig. 1). The main hypothesis of diagnosis was a reactive/inflammatory lesion. Under local anesthesia, an excisional biopsy was performed in both nodules.
DIAGNOSIS AND TREATMENT
Microscopically, in both cases, it was observed abundant amount of eosinophilic, amorphous, and extracellular material in the subepithelial region. The epithelium was hyperplastic (Fig. 1). Congo red stain showed salmon pink coloration at light microscopy and apple-green birefringence at polarized light (Fig. 1). According to microscopical features, the diagnosis of amyloidosis was established. In both cases, hemogram, VSG, biochemistry, serum and urine protein electrophoresis, liver function tests and bone marrow aspiration were normal. Thus, the final diagnosis was LA.
In case 1, it was proposed the surgical excision of the residual lesion, but the patient refused the treatment. The patient is under follow-up and the lesion remains unaltered after 24 months of diagnosis. No signs of systemic amyloidosis were also observed. In case 2, after an 84-month follow up, no signs of recurrences or clinical and systemic changes have been observed.
DISCUSSION
Amyloidosis represents a heterogeneous group of disorders characterized by abnormal extracellular deposition of various insoluble fibrillar proteinaceous materials [1, 2]. There are three forms of amyloidosis defined by the presence or absence of systemic disease: primary systemic amyloidosis (PSA), secondary systemic amyloidosis (SSA), and localized amyloidosis (LA) [3].
PSA is a condition with unknown underlying cause, different from the SSA, which occurs associated with other known diseases, such as tuberculosis, rheumatoid arthritis, and mainly multiple myeloma [4]. LA consists in a nodular mass of amyloid deposit without association with a systemic disease [5–7]. Head and neck region is affected about 12–90% of the cases, typically with involvement of the larynx and tongue [8]. However, involvement of the tongue is almost always resulting of a systemic disease [9].
Oral amyloidosis tends to involve the tongue, buccal mucosa, and gingivae [7, 10]. Head and neck LA is a rare and benign disease [5, 8]. The most reported features of oral LA are multiple soft nodules accompanied by yellowish, red, blue, or purple color changes of the mucous membrane [5, 6, 11]. To the best of our knowledge, only 53 well-documented cases of LA in the oral cavity have been previously described in the English-language literature [1, 6, 7, 10, 11–38]. Thus, this article describes the clinicopathological features of two rare cases of oral LA and review of the literature. Considering all oral LA, the case 2 seems to be the 4th LA case on the labial commissures published in the English-language literature [1, 6, 7, 10, 11–38].
Amyloidosis most commonly affects individuals between 50 and 70 years of age, with a male-female predominance of 3:1 to 3:2 [17]. The mean age of the patients with oral LA is 57.5 years (range 10–90 years), with a female predilection. Among 55 cases of oral LA, including the current cases, 28 (51%) were localized in the tongue, 10 (18.2%) in the palate, 4 (7.3%) in the labial commissure, 1 (1.8%) in the maxilla, 1 (1.8%) in the floor of the mouth, 2 (3.6%) in maxillary alveolar ridge, 3 (5.5%) in the gingiva, 2 (3.6%) in the lower lip. In addition, 1 (1.8%) case involved the tongue, lower lip, and buccal mucosa, 1 (1.8%) the buccal mucosa and tongue, and 2 (3.6%) were in unspecified sites of the oral cavity [1, 6, 7, 10, 11–38].
Oral LA does not usually progress to systemic disease [10]. Although involvement of the tongue may be linked to systemic disease, the case 1 does not have systemic involvement. To date, the patients have not developed clinical or laboratory evidence of systemic amyloidosis or multiple myeloma for 24 and 84 months of follow-up.
The most common head and neck presentations are hoarseness, nasal congestion, odynophagia, articulation problems, mandibular deformities, dysphagia, airway obstruction, speech disorders and hypogeusia [26]. Manifestations of oral amyloid deposits may include macroglossia, xerostomia, and gingival involvement with swelling and hemorrhage [27]. Amyloidosis in the tongue typically results in macroglossia, manifested by increased tongue volume, tongue protrusion beyond the alveolar ridge, speech impairment, and dysphagia [26]. Yellow nodules or raised white lesions occurring predominately along the lateral border are also common. Clinically, the mucosal surface is usually intact, and the underlying lesion may be nodular or flat, with a yellow, pink, or bluish hue [26, 39]. In the case 1, it appeared as a painful, yellowish well-circumscribed multiple nodules located on the dorsum of the tongue and the lesions presented a firm consistency. In case 2, LA appeared as asymptomatic nodular lesions on the labial commissures.
Histologically, amyloid is an eosinophilic amorphous extracellular protein deposit. The diagnosis is based on amyloid binding of Congo red stain, yielding apple-green birefringence under polarized microscopy [33].
The investigation of organ involvement, underlying diseases, and amyloid protein subtypes is important for differentiating between systemic and localized amyloidosis. Underlying diseases are investigated by screening for abnormal proteins in serum and urine, and a bone marrow biopsy to analyze plasma cells. Immunohistochemical staining using anti-λ or κ immunoglobulin light chain antibodies, anti-SSA antibodies, anti-TTR antibodies, and mass spectrometry may be useful to identify amyloid protein subtypes [40]. In the current cases, no signs of systemic diseases were evidenced. Additionally, the microscopic analysis confirmed the amyloid deposition and after long-term follow-up, no evidence of systemic diseases was observed. These findings confirmed the diagnosis of LA.
There is still no consensus regarding the management of oral LA, although numerous therapies have been proposed, including surgical excision and pharmacological treatment. However, lesions often persist or recur. The prognosis is uncertain, owing to the rarity of the condition, requiring regular follow-up and monitoring [31]. In the present cases, no further treatment was performed, and the lesions remained stable after 24 and 84 months of follow-up.
In summary, oral LA is rare. Despite of this, LA should be considered in the differential diagnosis of multiple or single yellowish nodules on the oral cavity.
Authors’ contributions
Conceptualization: Danyel Elias da Cruz Perez; José Divaldo Prado; Rafael Segura Saint-Gerons. Supervision: Danyel Elias da Cruz Perez; José Divaldo Prado. Visualization: Elaine Judite de Amorim Carvalho. Writing – original draft: Hélen Kaline Farias Bezerra; Talita Ribeiro Tenório de França. Writing – review & editing: Danyel Elias da Cruz Perez; Elaine Judite de Amorim Carvalho; Rafael Segura Saint-Gerons.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The study was performed following the 1964 Helsinki Declaration standards.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fahrner KS, Black CC, Gosselin BJ. Localized amyloidosis of the tongue: a review. Am J Otolaryngol. 2004;25(3):186–9. doi: 10.1016/j.amjoto.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Cengiz MI, Wang HL, Yıldız L. Oral involvement in a case of AA amyloidosis: a case report. J Med Case Rep. 2010;4:200. doi: 10.1186/1752-1947-4-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen AS, Calkins E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959;183(4669):1202–3. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- 4.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 5.Pentenero M, Davico Bonino L, Tomasini C, Conrotto D, Gandolfo S. Localized oral amyloidosis of the palate. Amyloid. 2006;13(1):42–6. doi: 10.1080/13506120500537343. [DOI] [PubMed] [Google Scholar]
- 6.Balatsouras DG, Eliopoulos P, Assimakopoulos D, Korres S. Primary local amyloidosis of the palate. Otolaryngol Head Neck Surg. 2007;137(2):348–9. doi: 10.1016/j.otohns.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Aono J, Yamagata K, Yoshida H. Local amyloidosis in the hard palate: a case report. Oral Maxillofac Surg. 2009;13(2):119–22. doi: 10.1007/s10006-009-0158-4. [DOI] [PubMed] [Google Scholar]
- 8.Pang KP, Chee LWJ, Busmanis I. Amyloidoma of the nose in a pediatric Patient: a case report. Am J Otolaryngol. 2001;22(2):138–41. doi: 10.1053/ajot.2001.22576. [DOI] [PubMed] [Google Scholar]
- 9.Babburi S, B R, Rv S, Srivastava VA G. Amyloidosis of the tongue – report of a rare case. J Clin Diagn Res. 2013;7(12):3094-5. Doi: 10.7860/JCDR/2013/7028.3865. [DOI] [PMC free article] [PubMed]
- 10.Stoor P, Suuronen R, Lindqvist C, Hietanen J, Laine P. Local primary (AL) amyloidosis in the palate. A case report. Int J Oral Maxillofac Surg. 2004;33(4):402–3. doi: 10.1016/j.ijom.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Yamagata K, Bukawa H. Oral localized amyloidosis. In: Güvenç IA ed. Amyloidosis-an insight to disease of systems and novel therapies. London: IntechOpen; 2011. pp. 141–52. doi:10.5772/1785.
- 12.Haraguchi H, Ohashi K, Yamada M, Hasegawa M, Maeda S, Komatsuzaki A. Primary localized nodular tongue amyloidosis associated with Sjögren’s syndrome. J Otorhinolaryngol Relat Spec. 1997;59(1):60–3. doi: 10.1159/00027690. [DOI] [PubMed] [Google Scholar]
- 13.Koren R, Veltman V, Halpern M, Szabo R, Gal R. Localized amyloid tumor of the tongue. A case report and review of the literature. Rom J Morphol Embryol. 1998;44(1–4):179–82. [PubMed] [Google Scholar]
- 14.Asaumi J, Yanagi Y, Hisatomi M, Konouchi H, Kishi K. CT and MR imaging of localized amyloidosis. Eur J Radiol. 2001;39(2):83–7. doi: 10.1016/s0720-048x(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Haq M, Gautam P, Gautam D, Handa AC, Handa KK. Clinical Profile of Patients with Head and Neck Amyloidosis: A Single-Institution Retrospective Chart Review. Int Arch Otorhinolaryngol. 2020;24(4):e450–6. doi: 10.1055/s-0039-3402494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Reilly A, D’Souza A, Lust J, Price D. Localized tongue amyloidosis: a single institutional case series. Otolaryngol Head Neck Surg. 2013;149(2):240–4. doi: 10.1177/0194599813490896. [DOI] [PubMed] [Google Scholar]
- 17.McAlpine JC, Fuller AP. Localized laryngeal amyloidosis, a report of a case with a review of the literature. J Laryngol Otol. 1964;78:296–314. doi: 10.1017/s0022215100062113. [DOI] [PubMed] [Google Scholar]
- 18.Yamoaka Y, Suzuki A, Hatakeyama S, Noda M, Hiraga M, Sekiyama S. Median rhomboid glossitis associated with amyloid deposition. Act Pathol Jpn. 1978;23:319–23. doi: 10.1111/j.1440-1827.1978.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Zhou P, Hua H. Characteristics of Orofacial Amyloidosis: A Case Series. Ann Clin Case Rep. 2016;1:1133. [Google Scholar]
- 20.Noguchi T, Jinbu, Sakurai S. Kusama. Primary Localized Amyloidosis of the Angle of Mouth: Report of a case. Oral Med Pathol. 2003;8(1):27–30. doi: 10.3353/omp.8.27. [DOI] [Google Scholar]
- 21.Silva WPP, Wastner B, Bohn JC, Jung JE, Schussel JL, Sassi LM. Unusual presentation of oral amyloidosis. Contemp Clin Dent. 2015;6(1):282-4. doi: 10.4103/0976-237X.166814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timoşca G, Gavriliţă L. Primary localized amyloidosis of the palate. Oral Surg. 1977;44(1):76–83. doi: 10.1016/0030-4220(77)90247-x. [DOI] [PubMed] [Google Scholar]
- 23.Rennie JS, Critchlow HA. Solitary oral amyloid. A report of two cases. Int J Oral Surg. 1982;11(1):73–6. doi: 10.1016/s0300-9785(82)80053-7. [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, Sekiyama S, Suzuki A, Hirose H. Localized oral amyloidosis: ultrastructural and immunohistochemical study. J Oral Pathology. 1987;16(5):278–81. doi: 10.1111/j.1600-0714.1987.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 25.Madani M, Harwick RD, Chen SY, Miller AS. Amyloidosis of the oral cavity: report of five cases. Compendium. 1991;12(5):336. [PubMed] [Google Scholar]
- 26.Kerner MM, Wand MD, Angier G, Calcaterra TC, Ward PH. Amyloidosis of the head and neck—a clinicopathologic study of the UCLA experience. Arch Otolaryngol Head Neck Surg. 1995;121(7):778–82. doi: 10.1001/archotol.1995.01890070064014. [DOI] [PubMed] [Google Scholar]
- 27.Alvi A, Goldstein MN. Amyloidosis of the palate. Otolaryngol Head Neck Surg. 1999;120(2):287. doi: 10.1016/S0194-5998(99)70424-9. [DOI] [PubMed] [Google Scholar]
- 28.Penner CR, Muller S. Head and neck amyloidosis: a clinicopathologic study of 15 cases. Oral Oncol. 2006;42(4):421–9. doi: 10.1016/j.oraloncology.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Henley E, Houghton N, Bucknall R, Triantafyllou A, Field EA. Localized amyloidosis of the palate. Clin Exp Dermatol. 2008;33(1):100–1. doi: 10.1111/j.1365-2230.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 30.Akyildiz S, Doganavsargil B, Göde S, Veral A. Solitary amyloid tumor of the tongue base. Int J Otolaryngol. 2009;2009:515068. doi: 10.1155/2009/515068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angiero F, Seramondi R, Magistro S, Crippa R, Benedicenti S, Rizzardi C, Cattoretti G. Amyloid deposition in the tongue: clinical and histopathological profile. Anticancer Res. 2010;30(7):3009–14. [PubMed] [Google Scholar]
- 32.Andreadis D, Poulopoulos A, Papadopoulos P, Epivatianos A. Localized tongue amyloidosis in a patient with neurofibromatosis type II. Head Neck Pathol. 2011;5(3):302–5. doi: 10.1007/s12105-011-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouvêa AF, Ribeiro ACP, Léon JE, Carlos R, De Almeida OP, Lopes MA. Head and neck amyloidosis: clinicopathological features and immunohistochemical analysis of 14 cases. J Oral Pathol Med. 2012;41(2):178–85. doi: 10.1111/j.1600-0714.2011.01073.x. [DOI] [PubMed] [Google Scholar]
- 34.Bucci T, Bucci E, Rullan AMP, Bucci P, Nuzzolo Localized amyloidosis of the upper gingiva: a case report. J Med Case Rep. 2014;8:198. doi: 10.1186/1752-1947-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkard SS, Gibbs SD, Shah KA, Dhariwak DK. A rare case of localized oral amyloid of the labial mucosa. Br J Oral Maxillofac Surg. 2014;52(4):e24–5. doi: 10.1016/j.bjoms.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo FS, De Paulo LFB, Servato JPS, De Faria PR, Cardoso SV, Loyola AM. Involvement of oral tissue by AL amyloidosis: a literature review and report of eight new cases. Clin Oral Invest. 2016;20(8):1913–20. doi: 10.1007/s00784-015-1649-3. [DOI] [PubMed] [Google Scholar]
- 37.Adamo D, Gasparro R, Marenzi G, Mascolo M, Cervasio M, Cerciello G, De Novellis D, Mignogna MD. Amyloidoma of the tongue: case report, surgical management, and review of the literature. J Oral Maxillofac Surg. 2020;78(9):1572–82. doi: 10.1016/j.joms.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Morelato L, Smojver I, Seiwerth S, Gabric D. Extremely rare intraoral presentation of localized amyloidosis. Case Rep Dent. 2021;2021:5541320. doi: 10.1155/2021/5541320. [DOI] [PMC free article] [PubMed]
- 39.Thompson LDR, Deringer GA, Wenig BM. Amyloidosis of the larynx: a clinicopathologic study of 11 cases. Mod Pathol. 2000;13(5):528–35. doi: 10.1038/modpathol.3880092. [DOI] [PubMed] [Google Scholar]
- 40.Deng J, Chen Q, Ji P, Zeng X, Jin X. Oral amyloidosis: A strategy to differentiate systemic amyloidosis involving the oral cavity and localized amyloidosis. Oral Dis. 2019;25(3):670–5. doi: 10.1111/odi.12870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.