Abstract
Ewing sarcoma (ES) is a malignant round cell tumour (MRCT) that usually involves bone and soft tissue of young and paediatric populations. ES of the head and neck region is uncommon. Adamantinoma-like Ewing sarcoma (ALES) is a rare variant of ES that shows complex epithelial differentiation on histopathology and immunohistochemistry (IHC). It demonstrates a t(11;22) translocation and EWSR1- FLI1 fusion. Most documented cases of ALES of the head and neck region were initially misdiagnosed as epithelial tumours. We present a rare case of ALES of the nasal cavity in a young female. The patient subsequently underwent chemotherapy and showed an excellent response. Awareness of this entity is important for pathologists and oncologists due to its distinct therapeutic and prognostic implications. We propose performing upfront NKX2.2 and CD99 IHC studies, as well as other lineage specific IHC markers, in any poorly differentiated MRCT of head and neck region.
Keywords: Adamantinoma-like Ewing sarcoma, Ewing sarcoma, Immunohistochemistry, Malignant round cell tumour, Nasal, NKX2.2
Introduction
The Ewing sarcoma (ES) family of tumours are rare sarcomatous malignancies that affect bone and soft tissue with a predilection for young adult and paediatric populations [1, 2]. Ewing sarcoma with classical histopathologic features rarely involves the head and neck region [3–5].
Adamantinoma-like Ewing sarcoma [ALES] is a rare malignant round cell tumour (MRCT) and incompletely understood variant of ES that was first described in 1999 by Bridge et al. [6].
ALES shows complex epithelial differentiation on histomorphology and/or immunohistochemistry (IHC), along with a t(11;22) translocation and EWSR1- FLI1 fusion, a characteristic diagnostic criteria for ES at the molecular level. Most ALES cases reported in the literature were initially misdiagnosed as various types of carcinomas, especially when involving head and neck region. Given the distinct therapeutic implication of ALES, an accurate diagnosis is imperative.
We describe a rare case of ALES of the nasal cavity in a young female to contribute to the diagnostic and therapeutic knowledge in the existing literature.
Case Report
A 23-year-old female with no significant past medical or family history presented with complaints of nasal blockage and intermittent epistaxis of 10 months duration.
A CT (computed tomography)-PNS (paranasal sinus) scan revealed a mass centered in the right nasal cavity. A biopsy showed features of poorly differentiated carcinoma. The patient was referred to our institute, a tertiary cancer care center, for further management.
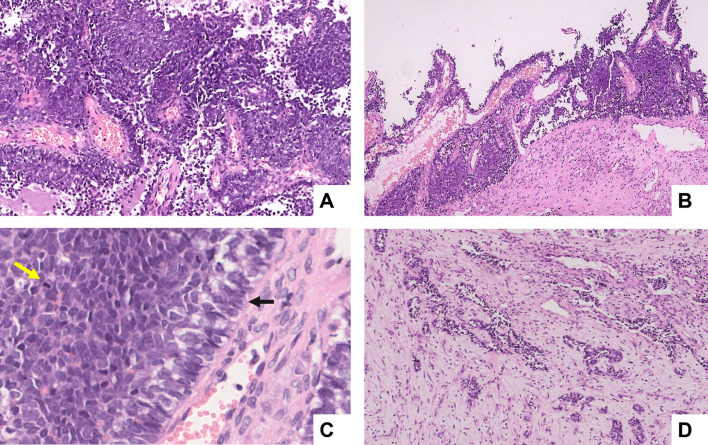
MRI (magnetic resonance imaging) revealed a mass lesion in the superior anterior nasal cavity extending to the anterior ethmoid sinus. It eroded the cribriform plate and crista galli. No significant lymphadenopathy was evident. Histopathologic examination revealed nests and sheets of poorly differentiated MRCT. A few nests showed basaloid features with peripheral palisading. Focal areas demonstrated pseudopapillary and pseudoglandular architecture (Fig. 1).
Fig. 1.
Histologic findings of ALES (haematoxylin and eosin stain). A A round blue cell tumour infiltrating in solid sheets, lobules (× 200), and B pseudopapillary pattern (× 100). C The neoplastic cells are hyperchromatic and demonstrate brisk mitotic activity (yellow arrow) and peripheral palisading (black arrow) (× 400). D Focal areas show pseudoglandular and tubular arrangements embedded in a fibrous stroma, indicating epithelial differentiation (× 100)
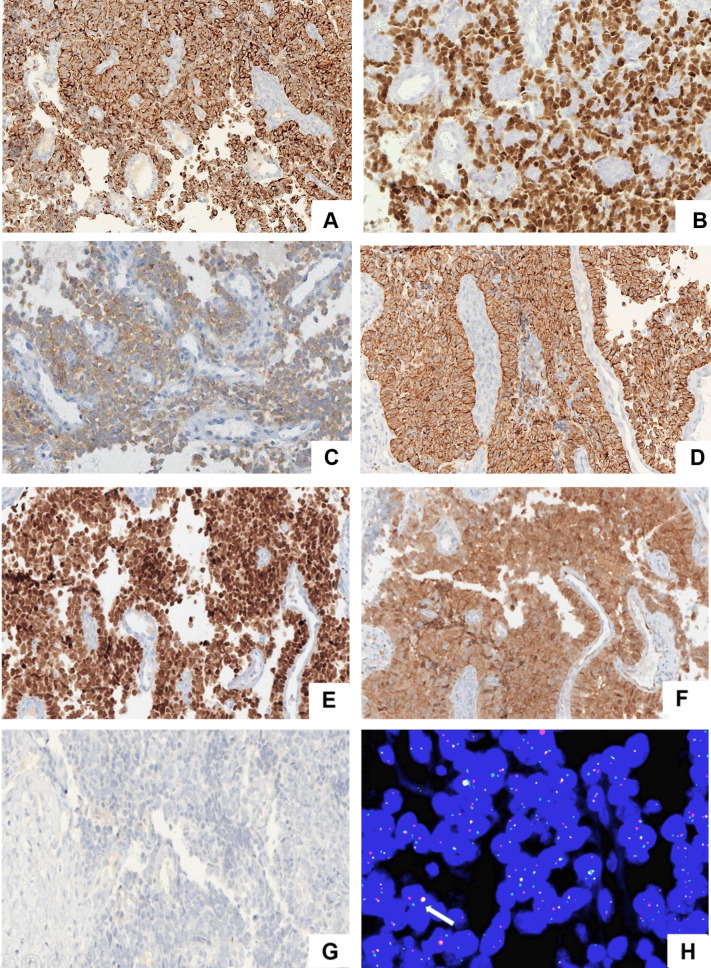
In consideration of the clinical profile, the morphologic differential diagnosis included non-Hodgkin lymphoma (NHL), rhabdomyosarcoma (RMS), poorly differentiated carcinoma/sinonasal undifferentiated carcinoma (SNUC), olfactory neuroblastoma (ONB), and ES. A primary IHC panel revealed strong and diffuse positivity for CK, P40, CD99, NKX2.2, and synaptophysin. The tumor was negative for myogenin, MyoD1, and LCA. On further IHC analysis, tumour cells were negative for CK5/6 and chromogranin. Immunoexpression of p16 was predominantly cytoplasmic (Fig. 2 A–G).
Fig. 2.
Immunohistochemical profile of ALES. Tumour cells are diffusely positive for A CK. B p40, C and weak expression for synaptophysin. D Membranous CD99 expression. E Strong nuclear expression for NKX2.2. F Diffuse cytoplasmic and variable nuclear expression for p16. G) negative expression for CK5/6 in tumour cells. H EWSR1 rearrangement on break apart FISH with separated red and green signal and one fused yellow signal (arrow). [Images are magnified at × 200]
In view of the histopathology and IHC features, a diagnosis of ALES was favoured over poorly differentiated squamous cell carcinoma. Subsequent positivity for the EWSR-1 rearrangement, tested by break apart FISH analysis, confirmed the diagnosis (Fig. 2H).
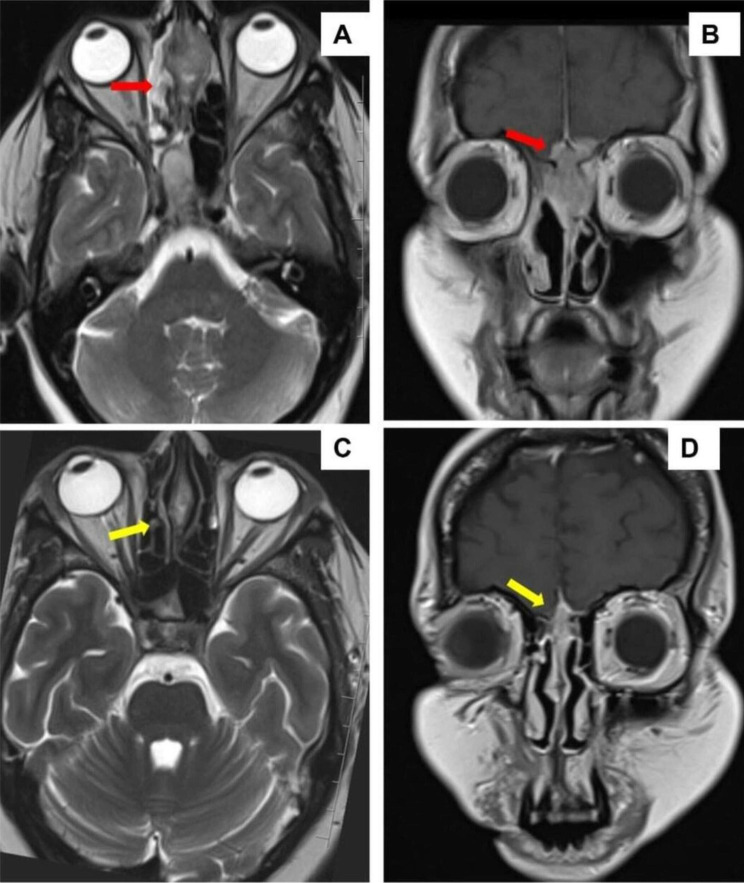
The case was discussed in multispeciality tumour board and the patient was started on VAC-IE (vincristine, Adriamycin, cyclophosphamide—ifosfamide and etoposide) based neoadjuvant chemotherapy. After four cycles of chemotherapy, an interim CT-PNS with contrast was performed which showed excellent response (Fig. 3). PET-MRI head & neck also showed a marked response to chemotherapy.
Fig. 3.
MR imaging findings. A and B Pre-chemotherapy axial and coronal MR imaging showing a lesion of the nasal cavity with infiltration of the cribriform plate and intradural extension respectively (red arrow). C and D Post-chemotherapy MR imaging shows marked response in the lesion (yellow arrow)
The patient then underwent endoscopic craniofacial surgical resection and reconstruction. After extensive sampling, the specimen showed a single microscopic residual focus of tumour which suggested a near complete response to chemotherapy (< 1% residual viable tumour). The surgical margins were free of tumor. Currently, the patient is undergoing continuation of the VAC-IE protocol along with adjuvant local radiation therapy in view of pre-chemotherapy findings of erosion of the cribriform plate and crista galli.
Discussion
ES is a well-recognized, highly malignant tumour of bone and soft tissues characterized by a balanced translocation of the EWSR1 gene. The fusion partner in approximately 90% of the cases is FLI-1, followed by ERG-1 [7, 8].
ALES is an enigmatic variant of ES that demonstrates the recurrent t(11;22) EWSR-FLI1 translocation as well as complex epithelial differentiation including cytokeratin, P40, and CK5 positivity. These IHC findings could prompt an erroneous diagnosis of poorly differentiated squamous cell carcinoma, especially in the head and neck region. Although ALES was initially described in long bones and thorax, the recent literature shows a proclivity for head and neck sites [9, 10].
A poorly differentiated MRCT or basaloid tumour of the sinonasal tract requires a lineage-specific IHC panel for initial work-up. Strong CK and P40 positivity, especially when associated with basaloid nests or squamoid differentiation, is highly suggestive of a poorly differentiated squamous cell carcinoma or basaloid squamous cell carcinoma. These are more common malignancies of the head and neck.
Rooper et al. described ten cases of ALES involving salivary glands that mimicked basaloid salivary carcinomas [9]. Nine cases were received in consultation and initially incorrectly diagnosed as various epithelial neoplasms, most commonly as poorly differentiated carcinoma (five cases). The mean age was 52 years (range: 32–77 years). The authors reported that ALES involving salivary glands showed a predilection for an older population.
Rooper and Bishop reviewed 23 cases of ALES involving head and neck region with an age range from 7 to 77 years (mean age 37 years) [10]. Only 17.4% of the cases (four cases) involved the sinonasal tract and all were younger subjects (age range of 18–41 years).
The most common histomorphologic differential diagnoses of ALES of the sinonasal tract are poorly differentiated squamous cell carcinoma (non-keratinizing), high-grade neuroendocrine carcinoma (HGNEC), NUT midline carcinoma (NMC), NHL, ONB, RMS, and SMARCB-1 deficient sinonasal carcinoma. Therefore, a broad, judicious, and algorithm-based IHC panel is imperative to demonstrate lineage-specific differentiation when confronted with a poorly differentiated malignancy/MRCT of the sinonasal tract.
In the presented case, the primary panel showed CK, P40, NKX2.2, synaptophysin, and CD99 positivity. LCA, myogenin, and MyoD1 were negative which ruled out NHL and RMS.
NKX2.2 has been described as a specific marker for ES in the appropriate clinicoradiologic context; however, positivity has also been described in ONB. Strong and diffuse positivity for both CK and CD99 ruled out a diagnosis of ONB. Combined CD99 and NKX 2.2 positivity is highly specific for Ewing sarcoma [7]. CK, synaptophysin, and NKX2.2 positivity have been described in HGNEC also, although strong and diffuse P40 expression is rare for both HGNEC & ES. EWSR1 rearrangement was helpful to confirm the diagnosis of ALES.
The presented case showed an excellent response to ES specific CT: VAC/IE regimen, as shown in the interim radiological evaluation (Fig. 3). Rooper and Bishop demonstrated favorable outcomes of ALES with surgery, adjuvant chemotherapy, and radiation [10]. However, the limited number of cases and confounding differences in tumour behaviour across various sites has been an impediment in the precise classification and management of this entity [9, 10].
In conclusion, ALES is a rare MRCT variant of ES that can be erroneously diagnosed if a limited IHC panel is performed. A diligent histopathologic assessment is essential for correct diagnosis to ensure that ALES patients receive an appropriate chemotherapy regimen.
We propose the necessity to include NKX2.2 and CD99 IHC in an initial IHC panel, as well as other lineage specific markers, in a poorly differentiated MRCT. Molecular confirmation should also be obtained when required. Increased awareness of this entity for both clinicians and pathologists will help expedite the diagnosis of ALES and establish standardized guidelines for management.
Acknowledgements
The authors would like to acknowledge Ms Sangeeta Arora, and Mr Arvind Bhuker for their technical assistance in performing IHC stains.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
Not required.
Informed Consent
A general informed consent was taken from the patient regarding sharing of clinical data for research purpose. All the patients information in the manuscript is anonymised and only de-identified data is used.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fujii H, Honoki K, Enomoto Y, Kasai T, Kido A, Amano I, Kumamoto M, Morishita T, Mii Y, Nonomura A, Takakura Y. Adamantinoma-like Ewing’s sarcoma with EWS-FLI1 fusion gene: a case report. Virchows Arch. 2006;449:579–584. doi: 10.1007/s00428-006-0279-1. [DOI] [PubMed] [Google Scholar]
- 2.Mahadevan P, Ramkumar S, Gangadharan VP. Adamantinoma-Like Ewing’s family tumour of the sinonasal region: a case report and a brief review of literature. Case Rep Pathol. 2019. [DOI] [PMC free article] [PubMed]
- 3.Alexiev BA, Tumer Y, Bishop JA. Sinonasal adamantinoma-like Ewing sarcoma: a case report. Pathol Res Pract. 2017;213:422–426. doi: 10.1016/j.prp.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafezi S, Seethala RR, Stelow EB, Mills SE, Leong IT, MacDuff E, Hunt JL, Perez-Ordoñez B, Weinreb I. Ewing’s family of tumors of the sinonasal tract and maxillary bone. Head Neck Pathol. 2011;5:8–16. doi: 10.1007/s12105-010-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge JA, Fidler ME, Neff JR, Degenhardt J, Wang M, Walker C, Dorfman HD, Baker KS, Seemayer TA. Adamantinoma-like Ewing's sarcoma: genomic confirmation, phenotypic drift. Am J Surg Pathol. 1999;23:159–165. doi: 10.1097/00000478-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2. 2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605. doi: 10.1007/s00428-014-1627-1. [DOI] [PubMed] [Google Scholar]
- 8.Alnuaim H, Alzahrani M, Ghandurah S, Dibaba M. Adamantinoma-Like Ewing Sarcoma of the Parotid Gland: Report of Two Cases and Review of Literature. Cureus. 2020;12. [DOI] [PMC free article] [PubMed]
- 9.Rooper LM, Jo VY, Antonescu CR, Nose V, Westra WH, Seethala RR, Bishop JA. Adamantinoma-like Ewing sarcoma of the salivary glands: a newly recognized mimicker of basaloid salivary carcinomas. Am J Surg Pathol. 2019;43:187. doi: 10.1097/PAS.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooper LM, Bishop JA. Soft tissue special issue: adamantinoma-like Ewing sarcoma of the head and neck: a practical review of a challenging emerging entity. Head Neck Pathol. 2020;14:59–69. doi: 10.1007/s12105-019-01098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.