Abstract
Phosphaturic mesenchymal tumour (PMT) is a rare tumour that occurs in bone or soft tissue and is associated with production of fibroblast growth factor 23 (FGF23) leading to tumor-induced osteomalacia. We report three cases of PMT involving the head and neck that highlight the broad spectrum of clinical and histologic features of PMT. One of these lesions from the hard palate demonstrated an admixture of epithelial and mesenchymal elements, a feature that can pose a diagnostic challenge. The diagnostic utility of immunohistochemistry including FGF23, somatostatin receptor 2A, SATB2, ERG and CD56 is discussed. The biochemical pathway in the development of PMT associated tumor induced osteomalacia and its role in investigations and management of PMT is also described.
Keywords: Epithelial component in phosphaturic mesenchymal tumor, ERG, FGF23, Phosphaturic mesenchymal tumor, SATB2, SSTR2A, Tumor-induced osteomalacia
Introduction
Phosphaturic mesenchymal tumor (PMT) is a rare tumor that occurs in bone or soft tissue. It is associated with tumor induced osteomalacia (TIO) or hypophosphataemic syndrome (HPS) due to the production of fibroblast growth factor 23 (FGF23) by the neoplastic cells. The WHO Classification of Tumors describes PMT as a bland spindle cell tumor with a distinctive ‘grungy’ calcified osteoid-like matrix and haemangiopericytoma-like vessels [1]. The diagnosis requires clinical evidence of hypophosphataemia and/or osteomalacia that resolves once the tumor is removed. While increased serum FGFR23 or immunohistochemical expression of FGFR23 by the lesional cells augment diagnostic confidence, these are not essential to make the diagnosis of PMT.
Correct identification of PMT is critical as complete resection of the tumor has a curative effect in patients with intractable osteomalacia and other consequences of high FGF23 levels, such as cardiovascular and renal disease. In addition, the tumor typically has an excellent prognosis with rare local recurrence limited to those with an incomplete initial excision. Distant metastases are anecdotally rare.
We present three cases of PMT with an emphasis on the expanding morphologic spectrum of PMT in the head and neck. The importance of clinicopathologic correlation and the role of immunohistochemistry (IHC) including FGFR23, SSTR2A, SATB2 and ERG is discussed.
Materials and Methods
Three cases of PMT involving the head and neck were identified from the Royal Prince Alfred Hospital Department of Tissue Pathology and Diagnostic Oncology. Informed consent was obtained from the patients for case review by their surgeons and study approved by the Sydney Local Health District Health Research Ethics Committee. The methods for FGF23 and SSTR2A immunohistochemistry have previously been described in detail [2]. Briefly for FGF23, an affinity purified goat polyclonal antibody (kindly gifted by Immutopics Inc., San Clemente, California) was used at a dilution of 1:100 and for SSTR2A, a commercially available rabbit monoclonal antibody was used also at a dilution of 1:100 (Clone UMB-1, Epitomics Inc, Burlingame CA).
Detailed clinicopathologic information was obtained for all cases from the surgical and pathology records.
Results
A summary of each case is reported below and in Table 1.
Table 1.
Summary of cases of phosphaturic mesenchymal tumours
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Clinical information | |||
| Sex/age | F/49 | F/13 | F/86 |
| Symptoms/signs | Hard palate mass | Floor of mouth mass | Epistaxis and nasal mass |
| TIO/HPS | Yes | No | HPS |
| Management | Biopsies taken then definitive excision with partial maxillectomy and reconstruction | Biopsies taken constituting most of the mass | Biopsies taken constituting most of the mass followed by definitive excision |
| Histology | |||
| Encapsulation | Well demarcated but not encapsulated | Partial peripheral thick capsule | No demarcation or encapsulation |
| Sclerosis and/or cystic degeneration | Absent | Central cystic degeneration with peripheral sclerosis | Absent |
| Cellular composition | Spindle-shaped cells with indistinct cell membranes and mildly enlarged hyperchromatic nuclei | Spindle-shaped cells with minimal pleomorphism, ill-defined cytoplasm, elongated nuclei and small inconspicuous nucleoli | Spindle-shaped cells with indistinct cell membranes and round to ovoid nuclei |
| Nests of epithelial cells dispersed throughout the lesion | |||
| Multinucleate giant cells present | Multinucleate giant cells present | Multinucleate giant cells absent | |
| Fascicular architecture | Present | Indistinct | Prominent |
| Nuclear pleomorphism/hyperchromasia/ mitoses | Absent | Occasional mitoses. No nuclear pleomorphism | Occasional enlarged nuclei with smudgy hyperchromasia. No mitoses |
| Grungy calcification | Easily identified | Striking amount present | Focal |
| Adipocytic areas | Present | Absent | Present |
| Staghorn vasculature | Present | Hyalinised vessels | Present |
| IHC | |||
| CKAE1/3 | Negative in spindle cells. Strongly positive in epithelial elements | Negative | Negative |
| SATB2 | Strongly positive in spindle cells | Positive | Strongly positive |
| ERG | Weakly positive in spindle cells | Weakly positive | Weakly positive |
| CD56 | Positive in both elements | Not available | Patchy positive |
| p40 | Negative | Not available | Negative |
| Chromogranin | Negative | Not available | Negative |
| Synaptophysin | Negative | Not available | Negative |
| S100 | Negative | Scattered individual positive cells | Negative |
| Sox10 | Negative | Not available | Negative |
| CD34 | Negative | Negative | Negative |
| SMA | Negative | Focally positive | Negative |
| Desmin | Negative | Negative | Negative |
| STAT6 | Negative | Not available | Negative |
| BCL2 | Positive | Positive | Positive |
| Ki67 | < 5% | < 10% | < 5% |
| FGF23 | Strong diffusely positive in both epithelial and mesenchymal elements | Performed 3 years later, negative | Positive in biopsies and technical failure in resection specimen |
| SSTR2A | Strong diffusely positive in both epithelial and mesenchymal elements | Performed 3 years later, positive | Positive |
| PCR | |||
| FGF23 mRNA | Not available | Present | Not available |
| Follow-up | |||
| Narrow complete resection. No complications nor evidence of recurrence at 1 year | Recurrence of tumour after 9 years. Tumour size stable and no TIO/HPS | Approximately 2 years post excision with no complications or signs of recurrence |
Case 1
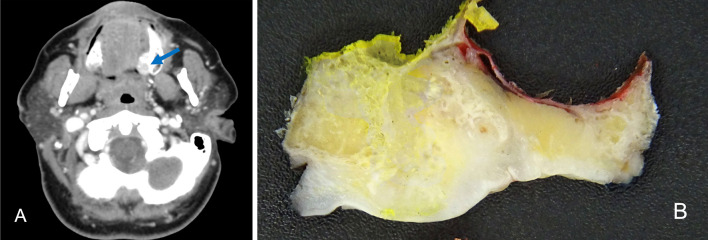
A 49 year old woman presented with significant musculoskeletal pain and multiple fractures of the femur, metacarpal bones and ribs from low force injuries. Serum phosphate was low, and a nuclear medicine bone scan suggested osteomalacia. There was no other significant medical history. As part of her work-up, an asymptomatic mass, approximately 20 mm in diameter, was identified on her hard palate. Computerised tomography (CT) demonstrated the mass within the hard palate abutting the maxilla with osseous remodeling (Fig. 1A). The lesion demonstrated internal and peripheral calcifications. There was no evidence of spread to regional lymph nodes or distant sites. Macroscopic photograph of the surgical resection is shown in Fig. 1B.
Fig. 1.
Representative radiological and macroscopic images from Case 1. A CT demonstrating a mass within the hard palate with internal and peripheral calcification (blue arrow). B Macroscopic photograph of the cut surface of the resected lesion showing a tan lesion with ill-defined margins and areas of calcification
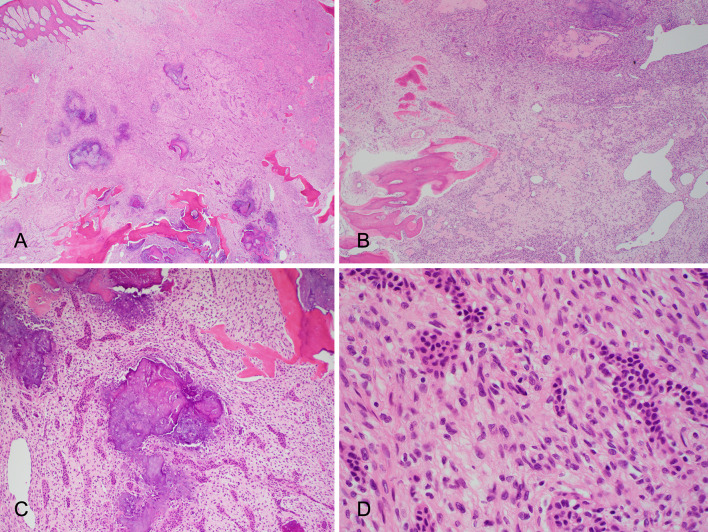
The histologic features are demonstrated in Fig. 2. There was a well demarcated, yet unencapsulated, lesion within the submucosa. The lesion was composed of fascicles of spindle-shaped cells with indistinct cell membranes and mildly enlarged hyperchromatic nuclei. Nests of epithelial cells were dispersed throughout the lesion. The epithelial nests were solid and occasional glandular structures were also identified. These nests were composed of cells with moderate amounts of eosinophilic cytoplasm and round to ovoid hyperchromatic nuclei. Occasional multinucleate foreign-body type giant cells were present. Areas of grungy calcification were observed, as well as foci of osteoid like matrix.
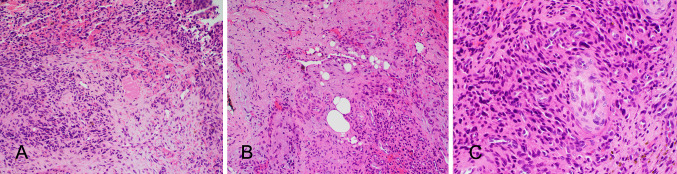
Fig. 2.
Representative H&E photomicrographs from Case 1 showing a lesion within the submucosa composed of fascicles of spindle-shaped cells and grungy calcification (A) and staghorn like vessels (B). Nests of epithelial cells within the lesion with both solid and glandular structures (C). The spindle shaped cells show indistinct cell borders and elongated nuclei, and the epithelial cells show moderate amounts of eosinophilic cytoplasm and relatively uniform round nuclei (D)
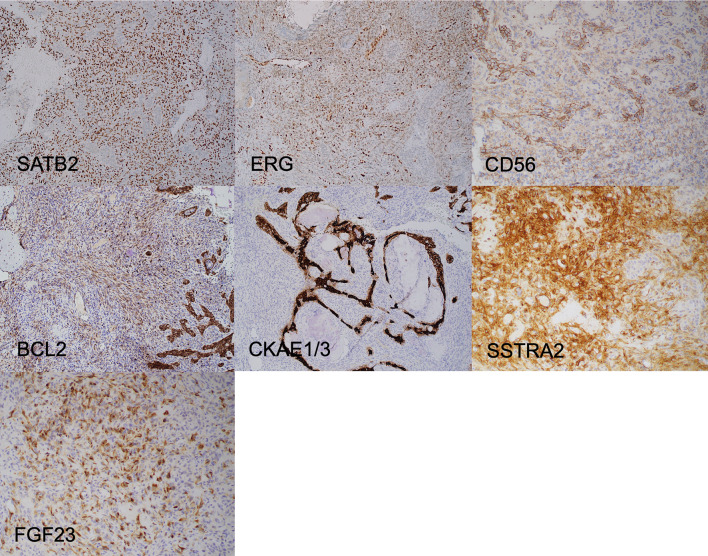
An IHC panel of cytokeratin (CK) AE1/3, SATB2, ERG, CD56, BCL2, FGF23, SSTR2A, CD34, chromogranin, synaptophysin, desmin, STAT6, S100 and SOX10 was performed (representative IHC in Fig. 3). The spindle cell element stained positively FGF23 with characteristic dot-like perinuclear cytoplasmic expression and SSTR2A, SATB2, ERG, CD56 and were negative for cytokeratins. The epithelial elements stained positively for FGF23, SSTR2A CKAE1/3 and CD56. CD56 staining was more prominent in the epithelial elements compared with the spindle cell elements. A diagnosis of PMT was made with reference to a newly proposed subtype of PMT with an admixture of epithelial and mesenchymal elements.
Fig. 3.
Representative IHC from Case 1. The spindle cells show strong diffuse immunostaining with SATB2, while the epithelial elements are negative. Only the spindle cells show diffuse staining with ERG and the nuclear staining in the spindle cells is weaker than that observed in the native endothelial cells. Immunostaining is identified in both the spindle shaped and epithelial elements with CD56 and BCL2, but the epithelial elements show stronger staining. CKAE1/AE3 only stains the epithelial elements, and SSTRA2 and FGF23 stains only the spindle cells. FGF23 immunostaining can be patchy and focal; it characteristically demonstrates dot-like perinuclear expression
Surgical resection included left partial maxillectomy with reconstruction. The curative resection demonstrated similar histologic features as those observed in the biopsy. The lesion was clear of the margins of resection by at least 1 mm. Bone involvement was not identified, and the lymphatic and perineural spaces were within normal limits.
The patient has shown no evidence of local recurrence or metastatic spread at 2 year follow up.
Case 2
A 13 year old girl presented with a 1 month history of a painless mass on the floor of mouth. She was otherwise well. An excisional biopsy was performed.
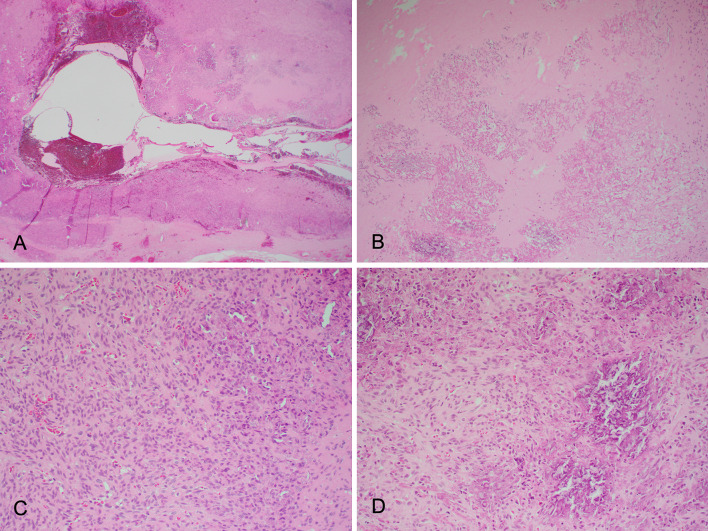
Microscopy demonstrated a variably sclerotic spindle cell tumour with central cystic degeneration and at least partially surrounded by a thick fibrous capsule (Fig. 4). The tumour comprised of spindle cells with minimal pleomorphism, ill-defined cytoplasm, elongated nuclei and small inconspicuous nucleoli. There was a suggestion of fascicular architecture in areas. Scattered mitoses were present, but no atypical mitoses were identified. There was striking grungy calcification both within the sclerotic areas and within more cellular tumor. The calcification had variable density. There were occasional multi-nucleated giant cells in association with the calcification. ‘Staghorn’ vascular pattern was identified and the vessel walls appeared hyalinized. Convincing osteoid matrix, chondroid matrix, glandular structures or adipocytic change were not identified. There was significant perilesional inflammation, with mast cells and eosinophils within the tumor. The tumor extensively involved the inked surgical margins.
Fig. 4.
Representative H&E photomicrographs from Case 2 showing spindle cell tumour with central cystic degeneration (A) and areas of sclerosis (B). The spindle cells show minimal pleomorphism (C). Grungy calcification is present (D)
IHC included CKAE1/3, EMA, S100, CD34, CD99, vimentin, calretinin, SMA, desmin, CD117, BCL2, and Ki67. Tumor cells were negative for cytokeratins, and demonstrated weak and patchy staining with BCL2. At the time, FGF23 and SSTR2A IHC had not yet been developed. FISH for SS18 18q11.2 gene rearrangement was negative.
A provisional diagnosis of a soft tissue spindle cell neoplasm was made raising the possibility of PMT, despite the lack of TIO, and the tissue was referred for further opinion from the Mayo Clinic, Rochester, Minnesota, USA. The expert opinion included testing for FGF23 using real-time polymerase chain reaction (RT-PCR) and, with the positive finding, there was agreement with the originally proposed diagnosis of PMT.
Three years later the FGF23 and SSTR2A IHC staining was available and the original formalin-fixed and embedded tissue demonstrated patchy weak staining with FGF23 with perinuclear dot-like cytoplasmic expression.
The patient presented with a local recurrence of the tumor nine years after the excisional biopsy. At the latest clinical review, the patient has a slightly expanded lingual plate of the left mandible, with the tumor confirmed on magnetic resonance imaging (MRI) at approximately 17 mm. Tumor size has been relatively stable over time and there continues to be no evidence of TIO. After multidisciplinary meeting review, the decision was to continue to manage this patient conservatively and reserve a segmental mandibulectomy if the patient developed TIO or other complications of elevated FGF23.
Case 3
An 86 year old woman presented with recurrent epistaxis on a background of anticoagulation for atrial fibrillation and colon adenocarcinoma. She was found to have a right-sided nasal mass which was biopsied and demonstrated atypical spindle cell proliferation. She required surgical management of her epistaxis at an outside institution where more extensive biopsies were performed. She then underwent an MRI scan which demonstrated an enhancing lesion in the right ethmoid roof extending medially into the olfactory cleft, with some adjacent dural enhancement of the anterior cranial fossa. Positron Emitting Tomography (PET) demonstrated a linear moderate uptake (maximum SUV of 7.6 compared to 3 on the opposite side) in the mucosa of the right ethmoid roof/olfactory cleft. The scan demonstrated no regional or distant metastases. Initial histological analysis performed in an outside laboratory was reported as esthesioneuroblastoma and the patient was referred to our multidisciplinary team meeting for review.
Microscopy demonstrated pseudostratified respiratory mucosa and a spindle cell neoplasm (Fig. 5). The lesion was composed of fascicles of spindle shaped cells with indistinct cell membranes and round to ovoid nuclei. There were several ectatic ‘staghorn’ congested vascular channels. Areas of grungy calcification were identified and focal adipocytic change. Several nuclei appeared enlarged and demonstrated smudgy hyperchromasia generally observed as a part of degenerative process. Mitotic activity was not identified.
Fig. 5.
Representative H&E photomicrographs from Case 3 showing an atypical spindle cell proliferation with indistinct cell membranes with scanty grungy calcification (A). Scanty adipocytes are present (B). Several nuclei appeared enlarged with smudgy hyperchromasia, generally observed as part of a degenerative process (C)
A broad panel of IHC was performed in the initial histological analysis due to the clinical urgency. It included CKAE1/3, SATB2, ERG, CD56, p40, chromogranin, synaptophysin, S100, SOX10, CD34, CD99, FLI1, SMA, desmin, myogenin, MyoD1, STAT6, CD3, and CD5. The tumor cells demonstrated staining with SATB2, ERG, and CD56, and were negative for all other stains. Following review at our institution, FGF23 and SSTR2A were performed. Immunostaining for FGFR23 was weak and focal while immunostaining with SSTR2A was strong and diffuse. A diagnosis of PMT was favoured. The patient was osteopenic but did not have osteomalacia. FGF23 measurements were performed in light of the favored histopathologic diagnosis, nearly 2 weeks after the majority of the tumor was debulked. Serum FGF23 was not elevated at this time. However, on scrutiny, the patient was found to have hypophosphataemia in timed investigations performed for unrelated reasons prior to surgery.
Definitive surgery was then performed with excision of the tumor from the anterior skull base and middle turbinate. Resected specimen demonstrated scanty residual tumor with histologic features as observed in the biopsies.
Approximately two years post-surgery, there is no evidence of local recurrence or metastatic spread.
Discussion
PMT in the head and neck are rare. The broad demographic profile, wide spectrum of clinical presentations and a diverse histological appearance can cause significant diagnostic difficulties [3]. Our study, including a teenage patient, an octogenarian, as well as patients who have not developed symptomatic TIO highlights the broad demographic and clinical profile of PMT. The clinical presentation of PMT varies greatly depending on the secretory function of the tumor and patients may remain asymptomatic despite an increased serum level of FGF23. Also, hypophosphatemia or osteomalacia may be attributed to other causes including age and other malignancies as was observed in one of our patients. Thus, histopathologic evaluation may be the first indication of this condition requiring a high index of suspicion by the pathologists. Correct diagnosis is critical to avoid consequences of untreated raised FGF23 levels such as intractable osteomalacia, as well as cardiovascular and renal disease. Also, PMT are amenable to conservative but complete local resection without nodal dissection, a critical consideration in anatomically complex sites such as the head and neck. Furthermore, adjuvant radiotherapy or chemotherapy are not required.
The tumors involved the sinonasal tract, the mandible, and the maxilla in our patients. In their review of PMT including 222 cases from all parts of the body, Wu et al. describe an age range of 13–75 years and a slightly higher incidence in males (55%) [4]. Most tumors occurred in the trunk or extremities (60%), and approximately 30% were in the head and neck. Within the head and neck, the most common site was the sinonasal area (29/70), followed by the mandible (19/70) and the maxilla (11/70).
It is well established that PMT can demonstrate a diverse histologic appearance [3]. The unifying feature of these tumors is the production of FGF23. Indeed, the identification of FGF23 and its association with TIO was preceded by multiple publications describing and attempting to subclassify TIO associated tumors with a variety of histological findings, such osteoblastoma-like, non-ossifying fibroma-like and ossifying fibroma-like neoplasms. Weidner and Santa Cruz were the first to recognize these as a single disease entity and coin the term phosphaturic mesenchymal tumor [5]. In 2004, Folpe et al. comprehensively reviewed the cases previously described in the literature and additional 32 such tumours and concluded that the majority represented phosphaturic mesenchymal tumour with a broad histologic spectrum [6].
The role of FGF23 in health and disease is complex and still poorly understood. A review by Bär et al. best summarises the current knowledge [7]. FGF23 is mainly secreted by osteoclasts and osteocytes and produced in multiple sites including the thymus, brain and heart. There are multiple regulators of FGF23 including calcitriol (activated vitamin D), parathyroid hormone and phosphate levels. It has been shown to have both paracrine and endocrine effects. Locally, FGF23 affects bone mineralization, and as a hormone it causes renal tubules to decrease reabsorption and increase excretion of phosphate leading to a lower serum phosphate level. With unregulated high FGF23 production, phosphate and calcium wasting occurs, leading to haematologic and musculoskeletal issues including increased osteolysis and excess formation of uncalcified osteoid. FGF23 is an independent risk factor for mortality, cardiovascular disease and renal disease progression.
Nearly 50% of PMT harbor a FN1:FGFR1 or FN1:FGF1 fusion [8, 9]. Both fibronectin (an essential gene matrix protein encoded by the FN1 gene previously implicated in tumourigenesis in fusion-based diseases) and FGF1 are secretory proteins with gene fusion leading to an increased activity of the 3’ end of FGFR1 with increased FGFR1 signaling and FGF23 over-expression [9]. The variation in clinical profile of PMT, depends upon the secretory profile of each individual tumour resultant from the specific genetic alteration. In some instances, the gene product is nonfunctional, and patients may not develop phosphaturia or TIO, as observed in the teenage patient in this study. In these cases, careful histological evaluation is critical, as has also been reported by Uchihashi et al. in 2013 [10] and Sent-Doux et al. in 2018 [11].
PMT generally show admixtures of spindle-shaped cells, variably hyalinized stroma, grungy calcification, osteoid like matrix and haemangiopericytomatous vessels [1]. Areas of adipocytic change and cystic degeneration can be present. The spindle shaped cells are fairly uniform and lack overt pleomorphism. One of the cases included in the current series also demonstrated nuclear smudgy hyperchromasia as is associated with degenerative changes. In addition, PMT in the head and neck may also demonstrate an epithelial component, as was observed in one of the patients in our series and has been described by Wu et al. in 2019. Wu et al. described an admixture of epithelial and mesenchymal elements and proposed the terminology of PMT of mixed epithelial and connective tissue type. Interestingly, PMT with epithelial elements tends to occur in the gnathic bones, is diagnosed in a younger age group (mean of 37 years vs 43 years) and with a male bias (male:female 2.7:1 vs 1.2:1). All 22 patients in the study by Wu et al. presented with TIO and/or HPS suggesting a higher tumour secretory function. Compared to classical PMT, these cases typically show a lower cellularity, less prominent vasculature and the mesenchymal cells demonstrate a more spindled and elongated morphology, resembling fibroblasts or myofibroblasts. In our series, PMT with an admixture of epithelial and mesenchymal elements occurred in a 49 year old female with clinically apparent TIO. The lesion demonstrated extensive grungy calcification and spindle cells with elongated nuclei as described by Wu et al.
The spectrum of demographic profiles, variable availability of information regarding TIO and morphologic appearances identified in PMT in the head and neck generally prompts a wide panel of immunohistochemical (IHC) stains including epithelial markers, muscle markers and vascular markers. As observed in the current study, PMT consistently demonstrate immunostaining with SATB2, ERG, CD56 and BCL2, and lack immunostaining with desmin. Agaimy et al. in their cohort of 22 cases of PMT have shown positive staining for both ERG and SATB2 in 90% of the cases [12]. We observed that the staining for ERG, though diffuse, is weaker than that demonstrated by the native endothelial cells included in the tissues. Wu et al. demonstrated immunostaining for BCL2 in 86% of their 22 cases of PMT with epithelial and mesenchymal elements [4]. While CD56 is generally considered to be a non-specific stain, Tajima and Fukayama, demonstrated CD56 immunostaining in all cases in their cohort [13]. Thus, a diagnosis of PMT should be considered in a spindle cell lesion with grungy calcification, stromal hyalinization and adipocytic change showing positive immunostaining for SATB2, ERG, CD56 and BCL2. Further investigation with either IHC for FGF23 or SSTR2A, and serum FGF23 level estimation can be useful if available.
Identification of FGF23 within tissue samples was initially performed via mRNA expression using real time polymerase chain reaction (RT-PCR) followed by the development of IHC. It must be noted that FGF23 staining can be patchy and focal and requires careful examination to identify the sparse peri-nuclear dot-like cytoplasmic expression. In the seminal validating study by Houang et al. in 2013 it was found that SSTR2A demonstrated diffuse strong expression in PMTs with focal and weak FGF23 staining [2]. Both FGF23 and SSTR2A may rarely show positive staining in synovial sarcoma, haemangioma, aneurysmal bone cyst and osteosarcoma, making the tests highly sensitive but not entirely specific [2]. The IHC for FGF23 and SSTR2A therefore functions as an excellent ‘rule out’ test for PMT. One of the main issues with IHC for FGF23 and SSTR2A is availability of the stains, as it is generally not cost-effective to optimize and validate stains associated with rare diagnoses. Wasserman et al. have also demonstrated the utility of FGFR1 FISH testing in PMT [14]. However, the rate of false negative is high due to the variability of FN1-FGFR1 gene rearrangements, thus limiting its diagnostic utility [9]. The variability is largely due to the type of rearrangement including insertion of FNI gene into FGFR1. Some of these rearrangements may not result in detectable signal splits on FISH testing [9].
Laboratory testing of serum levels of FGF23 also has a very limited availability and will represent a send-away for the vast majority of laboratories. Patient blood sample for FGF23 testing requires stringent transport and preparation conditions, as it needs to be sent chilled within an EDTA container to the laboratory where it is immediately centrifuged, separated and frozen for transport to the testing location. Communication between the clinical team, blood collecting team and the testing laboratories is critical to ensure that the stringent processes are followed. Thus, the pathologists suspecting PMT on morphologic basis in a biopsy, may need to proactively discuss the issues around blood collection and testing of serum FGF23 with the treating teams. Furthermore, an important pitfall to be aware of is that patients with advanced stage chronic renal disease also have high FGF23 levels that increases with disease progression [7]. Increased FGF23 levels may also be identified in a variety of common adenocarcinomas including prostatic, colon and ovarian adenocarcinomas [3].
The broad morphologic spectrum of PMT raises a range of potential differential diagnoses in the head and neck as listed in Table 2. It is possible to exclude some of these, such as odontogenic fibroma/fibrosarcoma, ameloblastic fibroma/fibrosarcoma and synovial sarcoma, on morphologic basis. Odontogenic fibroma/fibrosarcoma show a more collagenous stroma with uniform fibroblasts in comparison to PMT. In ameloblastic fibroma/fibrosarcoma, the epithelial nests show a characteristic nuclear reverse polarity of peripherally palisading cells around stellate reticulum. In contrast to synovial sarcoma (monophasic or biphasic), PMT lacks the high nuclear to cytoplasm ratio and mitotic rate. Synovial sarcoma also shows SS18 rearrangement by IHC or FISH.
Table 2.
Differential diagnoses for PMT in the Head and Neck
| Differential diagnosis for classically PMT | Morphologic differentiating features | Ancillary testing |
|---|---|---|
| Giant cell tumour of bone | Giant cells generally more frequent and associated with haemorrhage | H3.3G34W IHC ( +) |
| Ossifying fibroma | Morphological overlap | ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Solitary fibrous tumour |
Morphologic overlap with spindle shaped cells, staghorn vessels and areas of hyalinisation Lacks grungy calcification or adipocytic differentiation |
STAT6 ( +) SATB2 (−), ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Synovial sarcoma, monophasic type | High N:C ratio and easily identified mitoses | SS18-SSX IHC ( +) or SS18 rearrangement identified with FISH |
| Osteosarcoma | Cytological atypia and easily identified mitoses |
SATB2 ( +) ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Spindle cell rhabdomyosarcoma | Cytological atypia and easily identified mitoses, strap cells |
Desmin ( +), Myogenin ( +), MyoD1 ( +) SATB2 (−), ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Differential diagnosis for PMT with mixed epithelial and mesenchymal elements | Morphologic differentiating features | Ancillary testing |
|---|---|---|
| Odontogenic fibroma/fibrosarcoma | More collagenous stroma with more uniform fibroblasts. Lack of grungy calcification and staghorn vessels | SATB2 (−), ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Ameloblastic fibroma/fibrosarcoma |
Epithelial nests with peripheral pallisading of cells around stellate reticulum Reverse polarity of nuclei in the peripheral columnar cells Lack of grungy calcification and staghorn vessels |
SATB2 (−), ERG (−), CD56 (−), FGF23 (−), SSTR2A (−) |
| Synovial sarcoma, biphasic type | High N:C ratio and easily identified mitoses | SS18-SSX IHC ( +) or SS18 rearrangement identified with FISH |
Presence of giant cells in PMT can raise the differential diagnosis of giant cell tumour and giant cell granuloma. However, in PMT, the giant cells are generally infrequent and associated with haemorrhage. Also, PMT lack H3.3G34W immunostaining, as opposed to giant cell tumors. The presence of osteoid raises the possibility of ossifying fibroma or osteosarcoma. The differential diagnosis of ossifying fibroma is the most difficult to exclude on morphology alone and requires an immunohistochemical panel. Compared to osteosarcoma, PMT lacks the obvious cytological atypia and mitoses.
The prognosis of PMT is generally excellent. Local recurrence is associated with incomplete initial resection and is infrequent. Unfortunately, in our cases, despite the small size of the tumor at identification, one had positive surgical margins and recurred locally, albeit slowly. One of the main goals of surgery is biochemical control. Thus, in the head and neck, balancing the risk of recurrence, aesthetic and biochemical priorities need to be considered. Currently, it is not known whether PMT with mixed epithelial and mesenchymal elements has a different prognosis to classically described PMT. One of the cases in the Wu et al. series had multiple recurrences [4]. Of particular note to pathologists, the subsequent histological specimens demonstrated reduction then disappearance of the epithelial elements. Another published case demonstrated a similar histological progression, culminating in a lung metastasis with an entirely spindle cell morphology [15]. Malignant transformation in PMT is only anecdotally described in the literature [6, 15]. There is a potential role for anti-FGF receptor-1 targeted therapy in recurrent and/or inoperable cases of PMT but these trials are in early phases investigation and have identified unacceptable side effects (for example ClinicalTrials.gov Identifier NCT03510455).
In conclusion, PMT is a rare tumor associated with TIO. Tumor resection can be curative. Over time, the morphological and immunohistochemical properties of these tumors are becoming increasingly understood and the morphologic spectrum is widening with recognition of epithelial elements in the jaws. Awareness of the range of morphologic features identified in PMT, a panel demonstrating immunostaining for SATB2, ERG, BCL2, CD56 and lack of staining with desmin can be helpful in reaching the diagnosis of PMT. Serum evaluation of FGF23 and IHC for FGF23 and SSTR2A aid confirmation. The histopathologic evaluation may often be the first indication of PMT and TIO, thus pathologists should be aware of the practical issues involved in testing for FGF23.
Funding
The authors did not receive support from any organization for the submitted work. The authors have no relevant financial or non-financial interests to disclose.
Data Availability
Data and material used for the manuscript can be made available on request.
Code Availability
Not applicable.
Declarations
Ethical Approval
In accordance with the requirements of the Sydney Local Health District Ethics Review Committee the individual cases gave written informed consent for inclusion in this publication and no further ethical approval is required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Classification of Tumours Soft Tissue and Bone Tumours (5th Ed)
- 2.Houang M, Clarkson A, Sioson L, et al. Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A) Hum Pathol. 2013;44(12):2711–2718. doi: 10.1016/j.humpath.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Folpe AL. Phosphaturic mesenchymal tumors: a review and update. Sem Diag Path. 2019;36:260–268. doi: 10.1053/j.semdp.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Bui MM, Zhou L, Li D, Zhang H, Zhong D. Phosphaturic mesenchymal tumor with an admixture of epithelial and mesenchymal elements in the jaws: clinicopathological and immunohistochemical analysis of 22 cases with literature review. Mod Pathol. 2019;32:189–204. doi: 10.1038/s41379-018-0100-0. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Santa CD. Phosphaturic mesenchymal tumours. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59:1442–1452. doi: 10.1002/1097-0142(19870415)59:8<1442::AID-CNCR2820590810>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most osteomalacia-associated mesenchymal tumours are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28(1):1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bär L, Stournaras C, Lang F, Föller M. Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett. 2019;593:1879–1900. doi: 10.1002/1873-3468.13494. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Su SY, Changou CA, et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumours. Mod Pathol. 2016;29:1335–1346. doi: 10.1038/modpathol.2016.137. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Jeng YM, Su SY, et al. Identification of a novel FN1-FGFr1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol. 2015;235:539–545. doi: 10.1002/path.4465. [DOI] [PubMed] [Google Scholar]
- 10.Uchihashi K, Nishijima-Matsunobu A, Matsuyama A, et al. Phosphaturic mesenchymal tumour, nonphosphaturic variant, causing fatal pulmonary metastasis. Hum Pathol. 2013;44(11):2614–2618. doi: 10.1016/j.humpath.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Sent-Doux KN, Mackinnon C, Lee JC, Folpe AL, Habeeb O. Phosphaturic mesenchymal tumour without osteomalacia; additional confirmation of the "nonphosphaturic" variant, with emphasis on the roles of FGF23 chromogenic in situ hydridization and FN1-FGFR1 fluorescence in situ hydridization. Hum Pathol. 2018;80:94–98. doi: 10.1016/j.humpath.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Agaimy A, Michal M, Chiosea S, et al. Phosphaturic mesenchymal tumours: clinicopathologic, immunohistochemical and molecular analysis of 22 cases expanding their morphologic and immunophenotypic spectrum. Am J Surg Pathol. 2017;41(10):1371–1380. doi: 10.1097/PAS.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 13.Tajima S, Fukayama M. CD56 may be a more useful immunohistochemical marker than somatostatin receptor 2A for the diagnosis of phosphaturic mesenchymal tumors. Int J Clin Exp Pathol. 2015;8(7):8159–8164. [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman JK, Purgina B, Lai CK, et al. Phosphaturic mesenchymal tumour involving the head and neck: a report of five cases with FGFR1 fluorescence in situ hybridization analysis. Head Neck Pathol. 2016;10(3):279–285. doi: 10.1007/s12105-015-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergwitz C, Collins MT, Kamath RS, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 33–2011. A 56-year-old-man with hypophosphataemia. N Engl J Med. 2011;365:1625–1635. doi: 10.1056/NEJMcpc1104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material used for the manuscript can be made available on request.
Not applicable.