Abstract
Sinonasal leiomyosarcoma (LMS) is a rare and aggressive mesenchymal tumor with smooth muscle differentiation. The sinonasal tract is an unusual primary site for LMS, as scant smooth muscle exists in this location, with only 75 cases reported in the English literature including the case presented herein. Sinonasal LMS is considered an aggressive head and neck tumor with significant potential for recurrence and metastasis. Since recurrence is high and the potential for late metastasis exists, lifelong follow-up in these patients would be beneficial, especially among those with previous history of RB.
Keywords: Sinonasal, Nasal cavity, Sinus, Leiomyosarcoma
Introduction
Sinonasal leiomyosarcoma (LMS) is a rare and aggressive mesenchymal tumor with smooth muscle differentiation. LMS is the most common soft tissue sarcoma and accounts for nearly 25% of somatic soft tissue sarcomas [1]. Sinonasal examples are rare and approximately 75 cases have been reported in the English literature, including the case presented herein. We provide this additional case with a comprehensive literature review to characterize the spectrum of clinicopathological features.
Materials and Methods
In addition to our case, published articles of sinonasal LMS in the English literature were identified with PubMed using a combination of the search terms “sinonasal”, “leiomyosarcoma”, “nasal cavity”, “sinus”, “maxillary”, “ethmoid”, “sphenoid”, “nasopharynx”, and retinoblastoma”. Additional articles were noted after reviewing references cited in the relevant reports. All cases of LMS with sufficient details and probable origin in the sinonasal tract and the nasopharynx were included. Cases with suspected origin in the orbit and oral cavity were excluded, though tumor extension from the sinonasal region into these areas were included.
Case Report
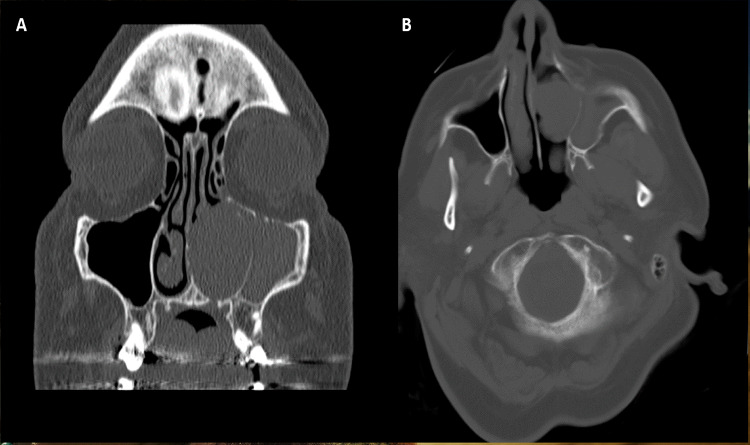
A 64-year-old female presented with a 1-month history of left sided nasal congestion and epistaxis with associated facial pain and otalgia. The epistaxis was present for one month with worsening symptoms. Computed Tomography (CT) imaging revealed a 2.2 × 2.0 cm soft tissue mass in the left nasal cavity extending into the maxillary sinus and causing thinning of the orbital floor (Fig. 1). Upon nasal endoscopy, an expansile mass with increased vascularity was identified arising from the left inferior turbinate causing deviation of the nasal septum. Since the mass extended to the basal lamella, a complete resection, including the inferior and middle turbinates, was performed.
Fig. 1.
Radiologic findings showing tumor infiltrating the maxillary sinus and thinning of the orbital floor. A Coronal image. B Axial image
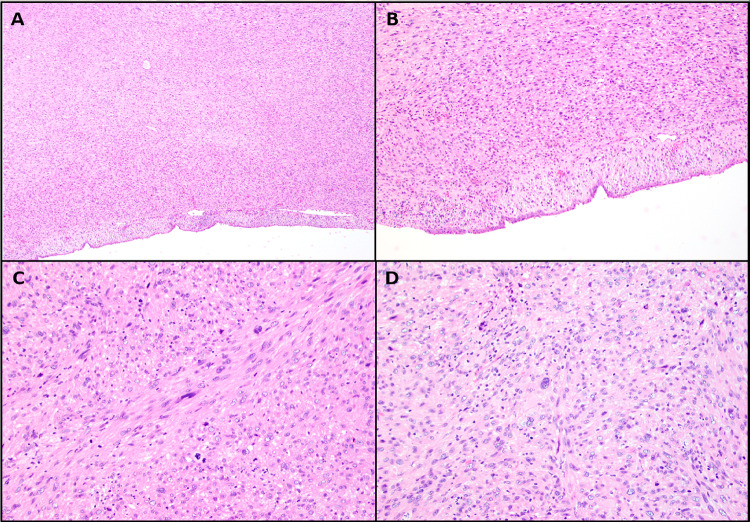
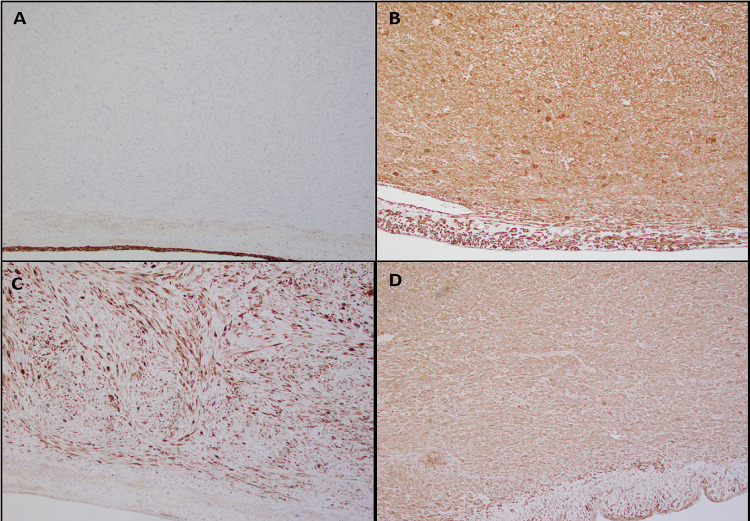
Upon gross examination, a polypoid mass with a rubbery consistency and a white whorled pattern on the cut surface was observed. Microscopic examination revealed a well-circumscribed lesion with overlying intact sinonasal respiratory epithelium. The tumor was composed of spindled and pleomorphic cells organized in intersecting fascicles (Fig. 2A, B). The tumor cells showed centrally located cigar-shaped nuclei with blunt ends, marked nuclear pleomorphism, and high mitotic activity (21 mitoses/10 high-power fields (HPF)) (Fig. 2C, D). Necrosis was not identified. Immunohistochemical stains revealed the tumor cells to be reactive for smooth muscle actin (SMA), muscle specific actin (MSA /HHF35), and desmin (Fig. 3B–D). No reactivity was identified for cytokeratin AE1/AE3 (Fig. 3A), cytokeratin CAM 5.2, epithelial membrane antigen (EMA), SOX10, S100, CD34 and HMB-45. Epstein-Barr virus (EBV)-encoded RNA (EBER) by chromogenic in situ hybridization (CISH) was negative. The morphologic features and immunoprofile supported the diagnosis of sinonasal LMS, Grade 3 by the French Federation of Cancer Centers Sarcoma Group (FNCLCC) Grading System. Post-operative radiation therapy was strongly recommended, but the patient declined further treatment. The patient is presently under close clinical follow-up.
Fig. 2.
Histologic findings of leiomyosarcoma showing. A A subepithelial proliferation without involving the surface epithelium (H&E 4 ×). B Proliferation of spindle cells without involvement of the surface epithelium (H&E 10 ×). C Marked nuclear pleomorphism, hyperchromasia and numerous mitotic figures (H&E 20 ×). D Marked nuclear pleomorphism (H&E 20 ×)
Fig. 3.
Immunohistochemical characteristics of leiomyosarcoma. A Negative cytokeratin AE1/AE3 in the tumor cells with positive expression in the overlying epithelium (10 ×). B Positive desmin expression in the tumor cells (10 ×). C Positive smooth muscle actin (SMA, 10 ×). D Positive muscle specific actin (HHF35, 10 ×)
Discussion
The sinonasal tract is an unusual primary site for LMS, as scant smooth muscle exists in this location. A few potential sources of origin have been described including undifferentiated mesenchyme and smooth muscle from the tunica media of blood vessel walls [2–4]. Derivation from blood vessels is probable, as this is the only source of smooth muscle in the sinonasal tract, and the malignant cells are often found in close association with endothelial cells microscopically [3, 5]. Moreover, compared to the paranasal sinuses, the nasal cavity is more consistently involved in sinonasal LMS. This may be accounted for by the substantial vascular network that supplies this region [3].
In review of the 75 cases of sinonasal LMS, the age range was from 10 to 86 years and the mean age of incidence was 49.1 years (Table 1) [2–45]. Most patients in the first and second decade had a history of retinoblastoma (RB) and radiation therapy (80%); therefore, these cases are likely post-radiation sarcomas [9, 20, 21, 35]. Among 71 cases with gender information, no gender predilection was identified, as 39 cases occurred in males and 32 cases occurred in females. Location was reported in all the cases; some exclusively involved the nasal cavity or the paranasal sinuses, though a combination of both locations was often seen. Forty-five patients (60%) had tumor involvement of the nasal cavity [2–5, 7, 9, 12–14, 17, 19, 24, 25, 28, 29, 31, 33, 38–40, 42–45]. Tumors extending into multiple sinuses were seen in 23 cases (30.7%) [2, 3, 7, 9, 10, 12, 13, 24, 27, 29–31, 33, 38, 39, 42, 44, 45]. Several cases had single sinus involvement, with or without nasal cavity extension. The maxillary sinus was the most common solitary sinus involved with 23 cases (30.6%) identified [3, 4, 6, 8, 9, 11, 14, 21–23, 26, 28, 31–33, 35, 36, 42]. Exclusive involvement of the ethmoid sinus was identified in 9 cases (12%), and involvement of the sphenoid sinus was observed in 5 cases (6.7%) [3, 4, 6, 15, 16, 18–21, 25, 29, 37, 41]. Tumors primarily originating from the nasopharynx were the rarest; only two cases were identified, though a few other cases extended into this region [6, 7, 9, 34].
Table 1.
Clinicopathologic characteristics of sinonasal leiomyosarcomas
| Case | Author | Age/Gender | Site | Previous irradiation or chemotherapy | Follow-up |
|---|---|---|---|---|---|
| 1 | Dobben | 69/F | Nasopharynx | NS | Recurrence treated with surgery; NR at 1 year |
| 2 | Pimpinella and Marquit | 56/M | Nasal cavity, sinuses and nasopharynx | NS | Recurrence treated with surgery; NR 4 months later |
| 3 | Kawabe et al | 60/M | L maxillary sinus | NS | Recurrence treated with radio-chemotherapy; death at 7 months |
| 4 | Kawabe et al | 52/M | L maxillary sinus | NS | Recurrence treated with curettage and radiotherapy; lung metastasis and death at 1 year |
| 5 | Fu and Perzin | 18/F | L nasal cavity + sinuses | NS | Multiple recurrences treated with excision and radiotherapy; NR 1 year later |
| 6 | Fu and Perzin | 37/F | L nasal cavity + sinuses | NS | Multiple recurrences and metastasis to chest wall treated with chemotherapy at 3.5 years |
| 7 | Fu and Perzin | 36/M | R nasal cavity, sinuses and nasopharynx | NS | NR; death from metastatic liver adenocarcinoma 1.5 years later |
| 8 | Fu and Perzin | 56/M | L nasal cavity, sinuses and nasopharynx | NS | Multiple recurrences treated with radiotherapy; Death from persistent tumor over 3.5 years later |
| 9 | Fu and Perzin | 40/F | L nasal cavity + sinuses | "Radium treatments" to both nasal cavities for sinus symptoms | Multiple recurrences treated with surgery and radiotherapy; death from persistent tumor 1 year later |
| 10 | Fu and Perzin | 45/M | L nasal cavity and maxillary sinus | NS | Persistent tumor; death 1 year later |
| 11 | Mindell et al | 70/M | L nasal cavity | NS | NR at 2 years |
| 12 | Dropkin et al | 75/M | Nasal cavity + sinuses | None | NR at 26 months |
| 13 | Jakobiec et al | 39/M | Paranasal sinuses | None | Exenteration of L ethmoid, maxillary sinus and orbit. Alive with disease 9 months later |
| 14 | Kakar et al | 31/M | R maxillary sinus | NS | Death after 1 year |
| 15 | Kakar et al | 55/F | R ethmoid sinus + nasal cavity | NS | Extensive recurrence at 9 months; lost to follow-up afterward |
| 16 | Ohashi et al | 53/F | Maxillary sinus | NS | NS |
| 17 | Josephson et al | 87/F | Nasal cavity + sinuses | Radiotherapy for BCC | NR at 3 years; Death from cardiovascular disease at 3 years |
| 18 | Kuruvilla et al | 22/M | L nasal cavity | None | NR at 15 months |
| 19 | Kuruvilla et al | 68/F | R nasal cavity | None | NR at 9 years |
| 20 | Kuruvilla et al | 86/M | R nasal cavity | None | NR at 9 years |
| 21 | Kuruvilla et al | 72/F | R nasal cavity | None | NR at 20 months |
| 22 | Kuruvilla et al | 66/M | L nasal cavity + sinuses | None | NR at 58 months; Multiple recurrences later treated with radical surgery and chemotherapy; death at 6 years |
| 23 | Kuruvilla et al | 51/F | R nasal cavity + maxillary sinus | None | Persistent tumor at 3 months |
| 24 | Kuruvilla et al | 28/F | R nasal cavity + ethmoid sinus | None | NR at 9 months |
| 25 | Kuruvilla et al | 43/F | L nasal cavity + sinuses | None | NR at 19 months |
| 26 | Kuruvilla et al | 60/F | R nasal cavity + ethmoid sinus | None | NR at 19 months |
| 27 | Lalwani and Kaplan | 66/M | Nasal cavity + sinuses | Radio-chemotherapy | Persistent tumor; palliative radio-chemotherapy; alive with disease several months after diagnosis |
| 28 | Martin-Hirsch et al | 45/M | R maxillary sinus + nasal cavity | Radiotherapy | Maxillectomy and orbital exenteration after recurrent tumor |
| 29 | Sankary et al | 32/F | Sphenoid sinus | NS | Persistent tumor with sphenoid sinus resection; death after 1 year |
| 30 | Reich et al | 74/F | R ethmoid sinus | Radio-chemotherapy for Hodgkin lymphoma, chemotherapy for breast carcinoma | NR at 13 months |
| 31 | Lippert et al | 44/M | R nasal cavity | NS | Recurrence treated with surgery; NR at 48 months |
| 32 | Ikeda et al | 43/M | L sphenoid sinus | NS | Partial remission after radiotherapy |
| 33 | Kawaura et al | 64/M | Ethmoid + nasal cavity | NS | NR at 2 years |
| 34 | Rubin et al., Dunkel et al. ** | 12/NS | Ethmoid sinus | Radiotherapy | NR at 24 months |
| 35 | Rubin et al., Dunkel et al. ** | 23/NS | Sphenoid sinus | Radiotherapy | Alive with disease 148 months later |
| 36 | Rubin et al., Dunkel et al. ** | 32/NS | Ethmoid sinus | Radiotherapy | NR at 6 months |
| 37 | Dunkel et al | 10/F | R maxillary sinus | Radiotherapy | Persistent tumor treated with radiotherapy; Death at 16 months |
| 38 | Dunkel et al | 18/NS | L maxillary sinus | Radiotherapy | NR at 29 months |
| 39 | Tanaka et al | 69/M | R maxillary sinus | NS | Recurrence at 4 months |
| 40 | Klippenstein et al | 29/M | R maxillary sinus | Radio-chemotherapy | Recurrence after 4 years treated with orbital and maxillary exenteration and radiotherapy; NR after |
| Stammberger et al | F | Lateral nasal wall | NS | Death at 21 months from metastatic breast cancer | |
| 41 | Batra et al | 41/F | L nasal cavity + sinuses | NS | NR at 4 months |
| 42 | Keck et al | 68/F | R ethmoid and nasal cavity | NS | NR at 29 months |
| 43 | Sumida et al | 77/M | L maxillary sinus | NS | Metastasis to lungs; death at 3 months |
| 44 | Fusconi et al | 57/F | R ethmoid and maxillary sinus | NS | Recurrence treated with radio-chemotherapy; NR at 48 months |
| 45 | Montgomery et al | 55/F | Nasal cavity | NS | NS |
| 46 | Montgomery et al | 34/F | Maxillary sinus | NS | Alive with metastatic disease 24 months later |
| 47 | Huang et al | 41/M | L nasal cavity | NS | NR at 28 months |
| 48 | Huang et al | 57/F | L nasal cavity + sinuses | NS | NR at 175 months |
| 49 | Huang et al | 37/M | L ethmoid sinus + nasal cavity | NS | NR at 24 months |
| 50 | Newman et al | 40/M | Ethmoid and maxillary sinus | Radiotherapy | NS |
| 51 | Prasad et al | 27/F | R paranasal sinuses + nasal cavity | NS | Death after 3 days |
| 52 | Prasad et al | 42/F | L maxillary sinus + nasal cavity | NS | NR at 18 months |
| 53 | Sedghizadeh et al | 30/M | L maxillary sinus | Radiotherapy | NR after 3 years |
| 54 | Ulrich et al | 24/M | Nasal cavity + sinuses | Radiotherapy | Alive with disease at 29 months |
| 55 | Kuo et al | 23/M | Nasopharynx | NS | NS |
| Podoj and Šmid | 66/F | Ethmoid + lateral nasal wall | NS | NR at 2 years | |
| 56 | Qureshi et al | 15/M | Maxillary sinus | Radiotherapy | Persistent tumor treated with radio-chemotherapy; death at 3 months |
| 57 | Chew et al | 36/M | L maxillary sinus | NS | NR at 3 years |
| 58 | Ramakrishnan et al | 77/M | R sphenoid sinus | None | Lost to follow-up |
| 59 | Fitzpatrick et al | 35/M | Nasal cavity + sinuses | Radiotherapy | Lost to follow-up |
| 60 | d'Adesky et al | 72/F | L nasal cavity + sinuses | NS | Radiotherapy; NR at 1 year |
| 61 | Papoian et al | 83/F | R nasal cavity | NS | Recurrence after 4 years treated with surgery and radiotherapy; NR 16 months after |
| 62 | Sadashiva et al | 53/M | Sphenoid sinus | NS | Lost to follow-up |
| 63 | Agaimy et al | 77/M | R nasal cavity + maxillary sinus | None | Multiple lung metastasis with resection; alive with disease at 88 months |
| 64 | Agaimy et al | 48/F | L nasal cavity + sinuses | No | After recurrence and lung metastases, NR at 17 years |
| 65 | Agaimy et al | 43/F | R nasal cavity | Radiotherapy | Lung metastasis and possible recurrence at 16 months; lost to follow-up |
| 66 | Agaimy et al | 61/M | Nasal cavity | NS | NS |
| 67 | Agaimy et al | 28/M | Maxillary sinus | NS | Multiple recurrences over 16 months; lost to follow-up |
| 68 | Agaimy et al | 26/F | Maxillary sinus | Chemotherapy and stem cell transplant | Lung metastasis at 20 months treated with surgery; NR at 8.2 years |
| 69 | Agaimy et al | 27/M | Maxillary sinus | Radiotherapy | Local recurrence at 2 years and death shortly after |
| 70 | Agaimy et al | 66/F | Nasal cavity | No | NR at 6.6 years |
| 71 | Agaimy et al | 66/M | Maxillary sinus | Radiotherapy for fibrous dysplasia at a young age | Brain extension of tumor; death at 0.5 years |
| 72 | Kwok et al | 81/M | Nasal cavity | No | NR at 9 months |
| 73 | Saadoun et al | 84/F | Nasal cavity + sinuses | No | NR at 9 months |
| 74 | Ohta et al | 49/M | L nasal cavity and sinuses | No | NR at 36 months |
| 75 | Current Case | 64/F | L nasal cavity + maxillary sinus | No | NR at 1 month |
F female, M male, R right, L left, NS not specified, NR no recurrence, GVHD graft versus host disease
**Patients are reported by both authors in separate articles
Clinically, an infiltrative polypoid or pedunculated mass is characteristic of sinonasal LMS [3, 5, 9, 39]. The most common symptom reported was nasal obstruction, followed by epistaxis [3, 11, 25]. Facial pain and ocular signs such as exophthalmos and strabismus have been described as well [3, 9, 11]. CT findings show bulky masses that often demonstrate cystic changes and areas of central necrosis without calcification [31, 37, 40]. The tumor frequently invades surrounding structures, resulting in bony destruction [31]. In the present case, extension into the maxillary sinus and thinning the orbital floor was apparent without invasion into the orbit. While these findings are nonspecific and don’t allow for a definitive diagnosis of LMS on radiology alone, imaging studies are essential for surgical planning and preservation of vital structures such as the orbit [22].
Grossly, the tumor appears as a polypoid or pedunculated mass that exhibit a whorled appearance on the cut surface [3]. Apart from the overlying respiratory epithelium, sinonasal LMS is largely analogous to conventional LMS histologically. The tumor is unencapsulated and infiltrative, with interlacing fascicles of spindle-shaped cells [24]. The nuclei are characteristically “cigar shaped'' with blunt ends and are associated with a fibrillary eosinophilic cytoplasm [24, 37]. Hypercellularity, nuclear pleomorphism, and mitoses are frequently identified and correlate with tumor grade. Grading of extrauterine leiomyosarcoma typically follows the FNCLCC guidelines which consist of three grades based on the degree of mitotic activity, necrosis, and cellular differentiation. This grading system highly correlates with prognosis and can accurately predict tumor-specific distant metastases and mortality [46–48]. The present case fulfills the criteria of a high-grade LMS, showing hypercellularity, pleomorphic and hyperchromatic nuclei, and areas of numerous atypical mitoses within a sparse stroma [11, 37].
In review of the literature, earlier reports (prior to 1990) often used stains in conjunction with histology or ultrastructural studies. Masson’s trichrome stain was most notably used in the identification of these tumors; red cytoplasmic staining of longitudinal fibrils supports compatibility with smooth muscle cells as opposed to blue for collagen fibers [7, 9, 10]. In addition to the latter stain, electron microscopy provides valuable insight into the ultrastructure of LMS. Smooth muscle differentiation is characterized by elongated tumor cells with longitudinal myofibrils and dense bodies, invested by a clear basement membrane material [8, 11, 12]. These methods are not currently used as routine diagnostic tools. In the past couple decades, immunohistochemical stains have emerged as a mainstay for diagnosis. Among prior reports with inclusion of muscle markers, the antibodies for SMA were the most common and were expressed in nearly all cases [3, 6, 14, 16, 18, 22, 25, 26, 28, 29, 32, 33, 35–39, 41, 42, 45]. However, while immunoreactivity for SMA is sensitive for smooth muscle, it lacks specificity. Expression is observed in other cell types, such as myofibroblasts and pericytes, due to the presence of myofilaments [49]. Tumors such as synovial sarcoma, malignant fibrous histiocytoma, fibrosarcoma, fibromatosis, and nodular fasciitis may show reactivity for SMA [32, 38]. As observed in the present case, additional muscle markers, such as MSA and desmin, further support smooth muscle differentiation [3, 6, 14–16, 22, 23, 25, 28, 29, 32, 33, 37, 39, 40, 42, 45]. Moreover, in some studies, h-caldesmon has shown superior smooth muscle distinction in comparison to other muscle markers [50, 51]. In one report, diffuse staining of h-caldesmon was exclusively seen in LMS compared to myofibroblastic tumors [51]. In our review of sinonasal LMS, 6 of 9 cases showed unequivocal expression of h-caldesmon [42]. Inclusion of h-caldesmon in an immunohistochemical panel may seem prudent in conjunction with other muscle markers. Several prior studies also report immunostaining of the cellular proliferation marker MIB-1(Ki-67). Most cases had a proliferation index above 15%, with the highest at 40%, suggesting a role when histologic features are confounding [25, 29, 41, 43]. Few cases in our review showed unusual immunohistochemical findings. In addition to SMA and desmin reactivity, one case of sinonasal LMS showed rare expression of cytokeratin CAM 5.2, EMA, and Bcl-2 [45]. Another two cases showed focal S100 positivity, but the diagnosis of LMS was supported by ultrastructural and immunohistochemical findings [3]. Although we have included these cases in our review, unusual IHC findings should warrant further investigation for cases suspected as LMS, as it is possible that these cases may be reclassified as synovial sarcoma and biphenotypic sinonasal sarcoma (BSNS), respectively. In the present case, we believe sinonasal LMS is the most appropriate diagnosis, as supported by the characteristic histologic features and strong expression of all muscle markers.
Low-grade LMS may be difficult to distinguish from sinonasal leiomyomas, as mild cellular atypia and variable mitotic activity are seen in the former [29]. Apart from lack of atypia and mitotic activity, sinonasal leiomyomas are usually circumscribed and smaller in size [42]. One report describes MIB-1 as a valuable discriminator between LMS and leiomyoma. The proliferation index was considerably higher (≥ 15%) in low-grade LMS compared to leiomyoma (≤ 5%) [29]. Aside from leiomyoma, the differential diagnosis of sinonasal LMS includes several malignant spindle cell tumors such as malignant peripheral nerve sheath tumor (MPNST), undifferentiated spindle cell, synovial sarcoma, and BSNS [3, 38, 42]. MPNST consists of highly cellular fascicles of spindle cells with wavy nuclei and may be distinguished from LMS with variable S100 and SOX10 staining and lack of reactivity to muscle markers [1]. In addition, loss of nuclear expression of H3K27me3 is highly sensitive and specific for MPNST in an appropriate setting [52]. Undifferentiated spindle cell carcinoma lacks features of smooth muscle differentiation such as blunt-ended nuclei and a fascicular growth pattern and may show variable cytokeratin, p60, and p40 reactivity [42, 53]. An in-situ component and negative staining for desmin support its diagnosis as well [42]. Synovial sarcoma may exhibit a monophasic spindle cell pattern that can be confused with LMS, but often expresses cytokeratin positivity, CD99, and Bcl-2, which is rarely identified in LMS [38, 45]. Most importantly, LMS does not harbor rearrangements of the SS18 gene, which are frequently identified in synovial sarcoma [38]. Lastly, BSNS is a recently recognized sarcoma that shows neural and myogenic differentiation. BSNS is characterized by consistent PAX3 rearrangements detected by fluorescence in situ hybridization (FISH) [1, 54]. BSNS also reliably expresses nuclear β-catenin, which is consistently negative in LMS [54].
A previous history of RB is commonly associated with sinonasal LMS. RB is the most common primary ocular malignancy in children and infants [55]. Most patients with the hereditary form of RB have bilateral disease, while unilateral disease is frequently associated with the sporadic type [20]. Genetic studies have established the deletion of the RB-1 gene on chromosome 13 as the responsible event for hereditary RB [20]. In our review, a previous history of retinoblastoma was documented in 14 cases (18.7%) of sinonasal LMS. Of the 14 cases, 4 showed unilateral orbital involvement, while the remainder were bilateral. Although a family history of retinoblastoma was only reported in three cases, it is reasonable to conclude that nearly all bilateral cases are associated with genetic inheritance [21]. Remarkably, two of the three cases with a documented family history occurred in patients with unilateral retinoblastoma. This is unusual, as patients with the germline defect frequently develop mutations in retinal cells of both eyes, resulting in bilateral RB. Individuals with hereditary unilateral RB have been described with an inheritance pattern referred to as low-penetrance retinoblastoma [32].
The occurrence of a secondary malignancy in patients with a history of hereditary retinoblastoma is not exceptional. In one cohort study, 14.3% of patients with hereditary RB developed a secondary tumor, compared to 1.9% patients with non-hereditary RB [56]. In this study, among hereditary RB patients, LMS was the most common secondary tumor identified, followed by osteosarcoma and melanoma [56]. A secondary sarcoma usually occurs between 1 to 4 decades after initial RB treatment, and two-thirds of tumors occur within the field of radiation [20]. While it is evident that radiation is a significant risk and may be involved in the induction of a sarcoma, a significant subset of tumors occurs independently of the head and neck region. Furthermore, as secondary tumors are more common in hereditary RB compared to the sporadic form, a synergistic role between inheritance and radiation therapy is supported [42]. In reported cases, all patients with a previous history of RB were treated with radiotherapy [6, 14, 20, 21, 23, 30, 32, 33, 35, 38, 42]. Prior radiotherapy was identified in 19 cases [6, 9, 12–14, 16, 20, 21, 23, 30, 32, 33, 35, 38, 42]. Aside from RB, other conditions treated with radiation therapy included: unspecified sinus disease, basal cell carcinoma, granulomatosis with polyangiitis, Hodgkin lymphoma, and fibrous dysplasia [9, 12, 13, 16, 42]. Locations for Hodgkin lymphoma and fibrous dysplasia were not specified, so the precise field of radiation is undetermined for these cases [42]. Accordingly, patients with previous head and neck irradiation, irrespective of tumor type, may have an increased risk of developing sinonasal LMS.
Fewer reports have discussed a prior history of chemotherapy and the occurrence of a secondary sarcoma. In reported cases, prior chemotherapy was identified in 4 (5.3%) patients. Only one patient with bilateral RB was treated with chemoradiotherapy. Exposure to chemotherapy, particularly cyclophosphamide, has been associated with development of sinonasal LMS. [16, 24, 40]. A case report describes long-term cyclophosphamide therapy for breast carcinoma in a patient who developed sinonasal LMS 15 years later [16]. The patient, however, also had previous irradiation for Hodgkin lymphoma 40 years prior to development of LMS [16]. Cyclophosphamide has also been implicated in the development of urinary LMS [57]. One report describes development of urinary LMS in two patients 5–21 years after extensive treatment with cyclophosphamide [57]. It is difficult to draw conclusions, however, as few cases with an extensive history of chemotherapy have been described in sinonasal LMS. Further research is needed to clarify the risk of chemotherapeutic agents in the induction of sinonasal LMS.
Radical surgical therapy is the primary treatment modality for sinonasal LMS. Patients were treated with surgical excision and resection in all prior reports whenever feasible, as the tumor has been described as relatively radioresistant [11, 33]. In many cases, clear margins are challenging to attain owing to tumor extension into several paranasal sinuses and the orbit [40]. Few reports describe surgical debulking or partial excision of the tumor when complete resection was impractical, due to tumor involvement of the cranial base or structures such as the cribriform plate [26, 33]. Several of these patients were treated with palliative radiotherapy or chemotherapy which resulted in residual disease or death at follow-up [15, 21, 26, 33, 37]. Predominantly, radiotherapy was used as an adjunct to surgical excision or resection. Of the 74 cases where the treatment regimen was provided, radiotherapy was initially used in 26 (35.1%) cases, and several cases included follow-up with radiotherapy due to persistent or recurrent tumor. Chemotherapy was often used in conjunction with radiotherapy but was largely considered palliative [3, 27]. Nevertheless, it is notable that one report discusses a 50% tumor reduction after neoadjuvant chemotherapy, implying it may have a role in large unresectable tumors [33] (Table 1).
Of 67 cases with follow-up data, recurrence was seen in 20 cases (29.9%) [3, 4, 6–9, 14, 17, 22, 23, 27, 40, 42]. Tumor persistence, without a disease-free interval, was seen in 6 cases (8.8%) [3, 9, 13, 15, 21, 35]. Among 12 patients with a previous history of RB and follow-up data, recurrence of sinonasal LMS was seen in 8 patients (66.7%) [14, 20, 21, 23, 33, 35, 42]. Three of these patients received radiation therapy for progressive and recurrent tumors, and two patients died during treatment [21, 23, 35]. Compared to RB, secondary tumors are more aggressive, and less responsive to radiation therapy [30]. Death from sinonasal LMS was seen in 13 cases (19.4%), ranging from within 1 month after initial treatment to 72 months post-treatment, with a mean of 17 months [3, 4, 6, 8, 9, 15, 21, 26, 31, 35, 42]. Metastasis of sinonasal LMS was seen in 8 cases (11.8%), with a range of 3 months to 156 months, a mean of 41 months, and a median of 22 months [6, 8, 9, 26, 28, 42]. Of 7 cases with the location of metastatic disease specified, 6 were in the lung [8, 26, 42]. One report discusses a patient with no evidence of recurrence at nearly 5 years after the initial surgery, who later died at 6 years due to subsequent recurrence [3]. Despite radical surgeries and adjuvant chemoradiotherapy, extension into crucial anatomical structures facilitates the insidious nature of this tumor.
Sinonasal LMS is an aggressive head and neck tumor with significant potential for recurrence and metastasis. The diagnosis of sinonasal LMS is contingent on histology and immunohistochemistry, as there are several microscopic mimics. Patients with a history of RB and radiotherapy are of particular concern, as the tumor often occurs at an earlier age, and recurrence was observed in most of these individuals. In patients with metastatic disease, a predilection for the lung is seen. Since recurrence is high and the potential for late metastasis exists, life-long follow-up in these patients would be beneficial, especially among those with previous history of RB.
Acknowledgements
We would like to acknowledge Dr. Jeb Justice, MD from the Department of Otolaryngology at the University of Florida, who helped us collect the appropriate documents in preparation of this manuscript.
Author Contributions
All authors SSS, BS, EN, MEL and EMD confirm they have meaningfully contributed to the research and read and approved the final manuscript.
Funding
The authors did/did not receive support from any organization for the submitted work nor was there any funding to conduct the study or to assist with manuscript preparation.
Data Availability
It is possible upon reasonable request, deidentified for maintenance of annonymity and compliance with IRB request.
Code Availability
Not applicable.
Declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was waived by the Institutional Review Board of the University of Florida.
Consent to Participate
It was received from the patient directly.
Consent for Publication
It was obtained from the patient for whom identifying information is uniquely included in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hornick J. Practical soft tissue pathology: a diagnostic approach. 2. Amsterdam: Elsevier; 2018. [DOI] [PubMed] [Google Scholar]
- 2.Dropkin LR, Tang CK, Williams JR. Leiomyosarcoma of the nasal cavity and paranasal sinuses. Ann Otol Rhinol Laryngol. 1976;85(3):399–403. doi: 10.1177/000348947608500313. [DOI] [PubMed] [Google Scholar]
- 3.Kuruvilla A, Wenig BM, Humphrey DM, Heffner DK. Leiomyosarcoma of the sinonasal tract. A clinicopathologic study of nine cases. Arch Otolaryngol Head Neck Surg. 1990;116(11):1278–1286. doi: 10.1001/archotol.1990.01870110050005. [DOI] [PubMed] [Google Scholar]
- 4.Kakar PK, Singh B, Puri ND, Lahiri AK. Leiomyosarcoma of paranasal sinuses. J Laryngol Otol. 1978;92(4):333–336. doi: 10.1017/S0022215100085418. [DOI] [PubMed] [Google Scholar]
- 5.Mindell RS, Calcaterra TC, Ward PH. Leiomyosarcoma of the head and neck: a review of the literature and report of two cases. Laryngoscope. 1975;85(5):904–910. doi: 10.1288/00005537-197505000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Dobben Gd. Leiomyosarcoma of the nasopharynx. AMA Arch Otolaryngol. 1958;68(2):211–213. doi: 10.1001/archotol.1958.00730020219013. [DOI] [PubMed] [Google Scholar]
- 7.Pimpinella RJ, Marquit B. Leiomyosarcoma of nose, nasopharynx and paranasal sinuses. Ann Otol Rhinol Laryngol. 1965;74(3):623–630. doi: 10.1177/000348946507400304. [DOI] [PubMed] [Google Scholar]
- 8.Kawabe Y, Kondo T, Hosoda S. Two cases of leiomyosarcoma of the maxillary sinuses. Arch Otolaryngol. 1969;90(4):492–495. doi: 10.1001/archotol.1969.00770030494016. [DOI] [PubMed] [Google Scholar]
- 9.Fu YS, Perzin KH. Nonepithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: a clinicopathologic study. IV. Smooth muscle tumors (leiomyoma, leiomyosarcoma) Cancer. 1975;35(5):1300–1308. doi: 10.1002/1097-0142(197505)35:5<1300::AID-CNCR2820350508>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Jakobiec FA, Mitchell JP, Chauhan PM, Iwamoto T. Mesectodermal leiomyosarcoma of the antrum and orbit. Am J Ophthalmol. 1978;85(1):51–57. doi: 10.1016/S0002-9394(14)76664-4. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi Y, Nakai Y, Muraoka M, Takano H. Asymptomatic leiomyosarcoma of maxillary sinus accompanied by primary mucocele. Arch Otorhinolaryngol. 1984;240(1):73–78. doi: 10.1007/BF00464348. [DOI] [PubMed] [Google Scholar]
- 12.Josephson RL, Blair RL, Bedard YC. Leiomyosarcoma of the nose and paranasal sinuses. Otolaryngol Head Neck Surg. 1985;93(2):270–274. doi: 10.1177/019459988509300228. [DOI] [PubMed] [Google Scholar]
- 13.Lalwani AK, Kaplan MJ. Paranasal sinus leiomyosarcoma after cyclophosphamide and irradiation. Otolaryngol Head Neck Surg. 1990;103(6):1039–1042. doi: 10.1177/019459989010300627. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Hirsch DP, Habashi S, Benbow EW, Farrington WT. Post-irradiation leiomyosarcoma of the maxilla. J Laryngol Otol. 1991;105(12):1068–1071. doi: 10.1017/S0022215100118213. [DOI] [PubMed] [Google Scholar]
- 15.Sankary S, Sherwin RN, Malone PS, et al. Clonal chromosomal aberrations in a leiomyosarcoma of the sinonasal tract. Cancer Genet Cytogenet. 1993;65(1):21–26. doi: 10.1016/0165-4608(93)90053-O. [DOI] [PubMed] [Google Scholar]
- 16.Reich DS, Palmer CA, Peters GE. Ethmoid sinus leiomyosarcoma after cyclophosphamide treatment. Otolaryngol Head Neck Surg. 1995;113(4):495–498. doi: 10.1016/S0194-5998(95)70094-3. [DOI] [PubMed] [Google Scholar]
- 17.Lippert BM, Godbersen GS, Lüttges J, Werner JA. Leiomyosarcoma of the nasal cavity. Case report and literature review. ORL J Otorhinolaryngol Relat Spec. 1996;58(2):115–120. doi: 10.1159/000276810. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Kudo T, Shimomura A, et al. A case of leiomyosarcoma of the sphenoid sinus. Tohoku J Exp Med. 1997;182(3):265–270. doi: 10.1620/tjem.182.265. [DOI] [PubMed] [Google Scholar]
- 19.Kawaura M, Nameki H, Fujii M, Kanzaki J. Use of vertical median forehead flap in the reconstruction of the anterior skull base: report of two cases. Auris Nasus Larynx. 1997;24(4):379–383. doi: 10.1016/S0385-8146(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 20.Rubin CZ, Rosenfield NS, Abramson SJ, Abramson DH, Dunkel IJ. The location and appearance of second malignancies in patients with bilateral retinoblastoma. Sarcoma. 1997;1(2):89–93. doi: 10.1080/13577149778353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkel IJ, Gerald WL, Rosenfield NS, Strong EW, Abramson DH, Ghavimi F. Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy: the Memorial Sloan-Kettering experience. Med Pediatr Oncol. 1998;30(1):59–62. doi: 10.1002/(SICI)1096-911X(199801)30:1<59::AID-MPO14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Westesson PL, Wilbur DC. Leiomyosarcoma of the maxillary sinus: CT and MRI findings. Br J Radiol. 1998;71(842):221–224. doi: 10.1259/bjr.71.842.9579188. [DOI] [PubMed] [Google Scholar]
- 23.Klippenstein KA, Wesley RE, Glick AD. Orbital leiomyosarcoma after retinoblastoma. Ophthalmic Surg Lasers. 1999;30(7):579–583. doi: 10.3928/1542-8877-19990701-17. [DOI] [PubMed] [Google Scholar]
- 24.Batra PS, Kern RC, Pelzer HJ, Haines GK. Leiomyosarcoma of the sinonasal tract: report of a case. Otolaryngol Head Neck Surg. 2001;125(6):663–664. doi: 10.1067/mhn.2001.120394. [DOI] [PubMed] [Google Scholar]
- 25.Keck T, Mattfeldt T, Kühnemann S. Leiomyosarcoma of the ethmoidal cells. Rhinology. 2001;39(2):115–117. [PubMed] [Google Scholar]
- 26.Sumida T, Hamakawa H, Otsuka K, Tanioka H. Leiomyosarcoma of the maxillary sinus with cervical lymph node metastasis. J Oral Maxillofac Surg. 2001;59(5):568–571. doi: 10.1053/joms.2001.22691. [DOI] [PubMed] [Google Scholar]
- 27.Fusconi M, Magliulo G, Della Rocca C, Marcotullio D, Suriano M, de Vincentiis M. Leiomyosarcoma of the sinonasal tract: a case report and literature review. Am J Otolaryngol. 2002;23(2):108–111. doi: 10.1053/ajot.2002.30628. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery E, Goldblum JR, Fisher C. Leiomyosarcoma of the head and neck: a clinicopathological study. Histopathology. 2002;40(6):518–525. doi: 10.1046/j.1365-2559.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang HY, Antonescu CR. Sinonasal smooth muscle cell tumors: a clinicopathologic and immunohistochemical analysis of 12 cases with emphasis on the low-grade end of the spectrum. Arch Pathol Lab Med. 2003;127(3):297–304. doi: 10.5858/2003-127-0297-SSMCT. [DOI] [PubMed] [Google Scholar]
- 30.Newman SA, Yoo J, Jones N, Beasley N, Gullane P. Radiation-Induced Leiomyosarcoma of the Ethmoid Sinus. Skull Base. 2003;13(3):179–182. doi: 10.1055/s-2003-43329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad KC, Alva TB, Khadilkar U, Madhu D. Leiomyosarcoma of the maxillary sinuses: report of two cases. Ear Nose Throat J. 2004;83(2):122. doi: 10.1177/014556130408300213. [DOI] [PubMed] [Google Scholar]
- 32.Sedghizadeh PP, Angiero F, Allen CM, Kalmar JR, Rawal Y, Albright EA. Post-irradiation leiomyosarcoma of the maxilla: report of a case in a patient with prior radiation treatment for retinoblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(6):726–731. doi: 10.1016/j.tripleo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich CT, Feiz-Erfan I, Spetzler RF, et al. Sinonasal leiomyosarcoma: review of literature and case report. Laryngoscope. 2005;115(12):2242–2248. doi: 10.1097/01.mlg.0000183767.97518.09. [DOI] [PubMed] [Google Scholar]
- 34.Kuo R, Huang JK, Lee KS, Chen BF, Yang FS. Leiomyosarcoma in the nasopharynx: MR imaging findings. AJNR Am J Neuroradiol. 2007;28(7):1373–1374. doi: 10.3174/ajnr.A0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi S, Mistry R, Natrajan G, Gujral S, Laskar S, Banavali S. Leiomyosarcoma of the maxilla as second malignancy in retinoblastoma. Indian J Cancer. 2008;45(3):123–125. doi: 10.4103/0019-509X.44069. [DOI] [PubMed] [Google Scholar]
- 36.Chew YK, Noorizan Y, Khir A, Brito-Mutunayagam S. Leiomyosarcoma of the maxillary sinus. Med J Malays. 2009;64(2):174–175. [PubMed] [Google Scholar]
- 37.Ramakrishnan VR, Said S, Kingdom TT. Primary leiomyosarcoma of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2009;135(9):949–952. doi: 10.1001/archoto.2009.114. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick SG, Woodworth BA, Monteiro C, Makary R. Nasal sinus leiomyosarcoma in a patient with history of non-hereditary unilateral treated retinoblastoma. Head Neck Pathol. 2011;5(1):57–62. doi: 10.1007/s12105-010-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.d'Adesky C, Duterme JP, Lejeune D, et al. Leiomyosarcoma of the inferior nasal concha: a case report and literature review. B-ENT. 2012;8(3):213–217. [PubMed] [Google Scholar]
- 40.Papoian V, Yarlagadda BB, Devaiah AK. Multifocal, recurrent sinonasal leiomyosarcoma: case report and review of literature. Am J Otolaryngol. 2014;35(2):254–256. doi: 10.1016/j.amjoto.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Sadashiva N, Nandeesh BN, Shukla D, Bhat D, Somanna S, Devi BI. Isolated sphenoid sinus lesions: experience with a few rare pathologies. J Neurosci Rural Pract. 2017;8(1):107–113. doi: 10.4103/0976-3147.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agaimy A, Semrau S, Koch M, Thompson LDR. Sinonasal leiomyosarcoma: clinicopathological analysis of nine cases with emphasis on common association with other malignancies and late distant metastasis. Head Neck Pathol. 2018;12(4):463–470. doi: 10.1007/s12105-017-0876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwok MMK, Lee S, Hosking P. Leiomyosarcoma: a rare sinonasal malignancy. BMJ Case Rep. 2018;2018:bcr-2017. doi: 10.1136/bcr-2017-224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saadoun R, Obermueller T, Franke M, Schell A, Mückner K, Riemann R. Leiomyosarcoma of the nasal cavity. Ear Nose Throat J. 2020;2020:145561320961204. doi: 10.1177/0145561320961204. [DOI] [PubMed] [Google Scholar]
- 45.Ohta N, Noguchi N, Shinohara S, et al. Endoscopic treatment of sinonasal leiomyosarcoma: a case report in light of the literature. Yonago Acta Med. 2021;64(2):217–221. doi: 10.33160/yam.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 47.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53(3):530–541. doi: 10.1002/1097-0142(19840201)53:3<530::AID-CNCR2820530327>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, Jacquemier J, Duplay H, Sastre-Garau X, Costa J. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–362. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 49.Eyden B. Smooth-muscle-type myofilaments and actin in reactive and neoplastic nonmuscle cells. Ultrastruct Pathol. 2000;24(5):347–351. doi: 10.1080/019131200750035085. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe K, Tajino T, Sekiguchi M, Suzuki T. H-Caldesmon as a specific marker for smooth muscle tumors. Comparison with other smooth muscle markers in bone tumors. Am J Clin Pathol. 2000;113(5):663–668. doi: 10.1309/JNQX-F4KM-Q0Q0-7XK8. [DOI] [PubMed] [Google Scholar]
- 51.Ceballos KM, Nielsen GP, Selig MK, O'Connell JX. Is anti-h-caldesmon useful for distinguishing smooth muscle and myofibroblastic tumors? An immunohistochemical study. Am J Clin Pathol. 2000;114(5):746–753. doi: 10.1309/K5JP-A9EN-UWN7-B5GG. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer IM, Fletcher C, Hornick J. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4–13. doi: 10.1038/modpathol.2015.134. [DOI] [PubMed] [Google Scholar]
- 53.Bishop JA, Montgomery EA, Westra WH. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. Am J Surg Pathol. 2014;38(2):257–264. doi: 10.1097/PAS.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rooper LM, Huang SC, Antonescu CR, Westra WH, Bishop JA. Biphenotypic sinonasal sarcoma: an expanded immunoprofile including consistent nuclear β-catenin positivity and absence of SOX10 expression. Hum Pathol. 2016;55:44–50. doi: 10.1016/j.humpath.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aerts I, Lumbroso-Le Rouic L, Gauthier-Villars M, Brisse H, Doz F, Desjardins L. Retinoblastoma. Orphanet J Rare Dis. 2006;1:31. doi: 10.1186/1750-1172-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacCarthy A, Bayne AM, Brownbill PA, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951–2004. Br J Cancer. 2013;108(12):2455–2463. doi: 10.1038/bjc.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanguay C, Harvey I, Houde M, Srigley JR, Têtu B. Leiomyosarcoma of urinary bladder following cyclophosphamide therapy: report of two cases. Mod Pathol. 2003;16(5):512–514. doi: 10.1097/01.MP.0000068237.38715.D9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It is possible upon reasonable request, deidentified for maintenance of annonymity and compliance with IRB request.
Not applicable.