Abstract
Oral squamous cell carcinoma (OSCC) commonly affects older patients; however, several studies have documented an increase in its incidence among younger patients. Therefore, it is important to investigate if this trend is also found in different geographic regions. The pathology files of diagnostic and therapeutic institutions from different parts of the globe were searched for OSCC cases diagnosed from 1998 to 2018. Data regarding the sex, age, and tumor location of all cases, as well as the histologic grade and history of exposure to risk habits of cases diagnosed as OSCC in young patients (≤ 40 years of age) were obtained. The Chi-square test was used to determine any increasing trend. A total of 10,727 OSCC cases were identified, of which 626 cases affected young patients (5.8%). Manipal institution (India) showed the highest number of young patients (13.2%). Males were the most affected in both age groups, with the tongue and floor of the mouth being the most affected subsites. OSCC in young individuals were usually graded as well or moderately differentiated. Only 0.9% of the cases occurred in young patients without a reported risk habit. There was no increasing trend in the institutions and the period investigated (p > 0.05), but a decreasing trend was observed in Hong Kong and the sample as a whole (p < 0.001). In conclusion there was no increase of OSCC in young patients in the institutions investigated and young white females not exposed to any known risk factor represented a rare group of patients affected by OSCC.
Keywords: Oral squamous cell carcinoma, Oral cancer, Young patients, Incidence, Prevalence, Epidemiology
Introduction
Oral cancer represents the 18th most common malignancy worldwide with 354,864 new cases estimated for 2018, representing approximately 2% of all human cancers in this period. In addition to this high frequency, oral cancer is also associated with a high mortality rate, with 177,384 new deaths being estimated as a consequence of this disease (1.9% of all cancer-related deaths) [1].
Although oral cancer represents an important health problem worldwide, its importance is even more relevant in specific geographic regions where its incidence ranks top among human cancers. This for the most part is due to local cultural habits associated with the use of oral carcinogenic agents such as tobacco. In South America, Brazil has the highest incidence of oral cancer [2], while this malignancy is one of the more common human cancers in India, Pakistan, Taiwan, and Sri-Lanka.
Oral squamous cell carcinoma (OSCC) is the most common malignant neoplasm of the oral cavity, accounting for over 90% of all malignancies [3–5]. It usually affects the tongue and the floor of the mouth, being most prevalent in male patients older than 50 years of age [2]. However, some studies from the United States of America and Europe have demonstrated a rising incidence of cases affecting younger patients [6, 7]. Although some of these individuals have been exposed to tobacco earlier in life, simulating the biological context of the more common older population, a specific group of young individuals with OSCC, usually white women, have no reported exposure to known risk habits [8].
During the last decade, our group has investigated the clinicopathological and molecular aspects of oral cancer in young patients to better understand its pathogenesis in this important clinical scenario [9–13]. In the current study, we aim to determine if the increased incidence of OSCC in young patients previously reported in the USA and European countries is also occurring in other parts of the world.
Material and Methods
All cases diagnosed as OSCC during a 20-year period (1998 to 2018) were retrospectively retrieved from the files of the oral pathology services of the Universidade Federal de Minas Gerais (Belo Horizonte/Brazil), Manipal Academy of Higher Education (Manipal/India), University of Pretoria (Pretoria/South Africa), University of Sheffield (Sheffield/UK), Universidad Nacional de Córdoba (Córdoba/Argentina), Universidad Autónoma Metropolitana - Xochimilco (Mexico City/Mexico), University of Basque Country (Bilbao/Spain) and the University of Hong Kong (Hong Kong/China). Demographic data regarding age, sex, and tumor location were collected for all OSCC cases, while data on the histologic tumor grade according to the WHO criteria and the history of exposure to known risk factors for OSCC development including tobacco use, alcohol intake, and betel quid chewing, were collected for the young patient group. Tumor tissue were available from either diagnostic biopsies or surgical specimens depending on the institution of origin that comprised both hospitals and oral pathology services specialized in the treatment and diagnosis of oral cancer.
Patients were categorized as younger individuals when aged 40-years or less. All cases affecting the oropharynx and lips were excluded given their unique etiological association with HPV infection and ultraviolet radiation, respectively. Special histologic subtypes of OSCC including carcinoma cuniculatum, papillary, verrucous, spindle, acantholytic, adenosquamous, and basaloid squamous cell carcinomas, and those with unavailable information regarding the affected site and the patients’ age were also removed from the analysis. Finally, OSCC cases affecting patients with known syndromes or predisposing systemic conditions were also excluded.
Comparisons were made between younger patients and those older than 40 years of age to determine differences between these two age cohorts regarding sex and affected site (tongue + floor of the mouth vs buccal mucosa + alveolar ridge + gingiva + retromolar trigone vs palate vs oral cavity, NOS). In addition, comparisons were made between data collected according to the period in which cases were diagnosed using the following sequence: 1998–2003, 2004–2009, 2010–2014, and 2015–2018, to detect possible variations and trends across the years. The Pearson Chi-square and Fisher’s exact tests were used to analyze categorical variables. All analyses were performed using version 22.0 of the SPSS statistical package (IBM Corp., Armonk, NY, USA), whereby a significant result was obtained when the p value was ≤ 0.05.
This study followed the principles established by the Helsinki Declaration on human research and was performed following approval by the Ethical Committee of the Universidade Federal de Minas Gerais (CAAE# 91158918.0.0000.5149).
Results
The distribution and clinicopathological features of all OSCC cases and those affecting young patients from each institution are described in Table 1. Taken together, the institutions diagnosed 10,727 cases of OSCC, with 626 cases affecting patients ≤ 40 years of age, representing 5.8% of this sample. There were 6,567 cases affecting males (61.2%) and 4,160 cases affecting females (38.8%). The mean age of all OSCC cases was 62-years (range 5 to 100 years). The most commonly affected subsites were the tongue and the floor of the mouth (60.6%), although the buccal mucosa/alveolar ridge/gingiva/retromolar trigone regions were the most affected subsites in cases from Manipal institution, India (63.7%).
Table 1.
Distribution and clinicopathological features of oral squamous cell carcinoma affecting patients ≤ 40 years old and > 40 years old in referral oral diagnosis centers from different regions of world (1998–2018)
| Belo Horizonte (Brazil) |
Cordoba (Argentina) |
Mexico City (Mexico) |
Sheffield (UK) |
Bilbao (Spain) |
Pretoria (South Africa) |
Hong Kong (China) |
Manipal (India) |
Total | |
|---|---|---|---|---|---|---|---|---|---|
| No. of OSCC cases | 934 | 170 | 383 | 2051 | 174 | 1186 | 5192 | 637 | 10,727 |
| Sex distribution | |||||||||
| Male | 685 (73.3%) | 97 (57.1%) | 167 (43.6%) | 1256 (61.2%) | 95 (54.6%) | 797 (67.2%) | 3033 (58.4%) | 437 (68.6%) | 6567 (61.2%) |
| Female | 249 (26.7%) | 73 (42.9%) | 216 (56.4%) | 795 (38.8%) | 79 (45.4%) | 389 (32.8%) | 2159 (41.6%) | 200 (31.4%) | 4160 (38.8%) |
| Mean age (range) (years) | 59.5 (18–98) | 63.1 (18–90) | 64.3 (26–96) | 65.0 (6–95) | 64.4 (25–95) | 59.7 (5–99) | 64.5 (5–100) | 55.7 (18–86) | 62.0 (5–100) |
| Affected site | |||||||||
| Buccal mucosa/Alveolar ridge/Gingiva/Retromolar trigone | 255 (27.3%) | 52 (30.6%) | 151 (39.4%) | 622 (30.3%) | 82 (47.1%) | 211 (17.8%) | 1215 (23.4%) | 406 (63.7%) | 2994 (27.9%) |
| Tongue/Floor of Mouth | 614 (65.7%) | 98 (57.6%) | 202 (52.7%) | 1246 (60.8%) | 78 (44.8%) | 690 (58.2%) | 3395 (65.4%) | 180 (28.3%) | 6503 (60.6%) |
| Palate | 65 (7.0%) | 15 (8.8%) | 24 (6.2%) | 134 (6.5%) | 14 (8.1%) | 49 (4.1%) | 367 (7.1%) | 24 (3.8%) | 692 (6.5%) |
| Oral mucosa, NOS | 0 (0.0%) | 5 (2.9%) | 6 (1.5%) | 49 (2.4%) | 0 (0.0%) | 236 (19.9%) | 215 (4.1%) | 27 (4.2%) | 538 (5.0%) |
| No. of OSCC in patients ≤ 40 years (%) | 43 (4.6%) | 9 (5.3%) | 25 (6.5%) | 76 (3.7%) | 12 (6.8%) | 65 (5.5%) | 312 (6.0%) | 84 (13.2%) | 626 (5.8%) |
| Sex distribution | |||||||||
| Male | 28 (65.1%) | 6 (66.6%) | 14 (56%) | 45 (59.2%) | 4 (33.3%) | 34 (52.3%) | 163 (52.2%) | 64 (76.2%) | 358 (57.2%) |
| Female | 15 (34.9%) | 3 (33.4%) | 11 (44%) | 31 (40.8%) | 8 (66.7%) | 31 (47.7%) | 149 (47.8%) | 20 (23.8%) | 268 (42.8%) |
| Mean age (range) | 34 (18–40) | 30.4 (18–40) | 34.9 (26–40) | 33.5 (6–40) | 33.2 (25–40) | 34.0 (5–40) | 32.7 (5–40) | 34.6 (18–40) | 33.4 (5–40) |
| Microscopic differentiation | |||||||||
| Well differentiated | 14 (32.6%) | 6 (66.7%) | 17 (68%) | 28 (36.8%) | 11 (91.7%) | 12 (18.5%) | 12 (14.3%) | 100 (31.8%) | |
| Moderately differentiated | 6 (14.0%) | 3 (33.3%) | 6 (24%) | 38 (50.0%) | 1 (8.3%) | 15 (23.1%) | NA | 57 (67.9%) | 126 (40.1%) |
| Poorly differentiated | 4 (9.3%) | 0 (0.0%) | 2 (8%) | 10 (13.2%) | 0 (0.0%) | 33 (50.8%) | 8 (9.5%) | 57 (18.2%) | |
| Not specified | 19 (44.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (7.7%) | 7 (8.3%) | 31 (9.9%) | |
| Affected site | |||||||||
| Buccal mucosa/Alveolar ridge/Gingiva/Retromolar trigone | 11 (25.6%) | 4 (44.4%) | 4 (16%) | 12 (15.8%) | 1 (8.3%) | 11 (16.9%) | 23 (7.4%) | 48 (57.1%) | 114 (18.2%) |
| Tongue/Floor of mouth | 28 (65.1%) | 5 (55.6%) | 20 (80%) | 63 (82.9%) | 11 (91.7%) | 37 (56.9%) | 262 (84.0%) | 32 (38.1%) | 458 (73.2%) |
| Palate | 4 (9.3%) | 0 (0.0%) | 1 (4%) | 1 (1.3%) | 0 (0.0%) | 4 (6.2%) | 18 (5.8%) | 1 (1.2%) | 29 (4.6%) |
| Oral mucosa, NOS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 13 (20.0%) | 9 (2.8%) | 3 (3.6%) | 25 (4.0%) |
| Tobacco use | |||||||||
| Yes | 22 (51.2%) | 1 (11.1%) | 7 (28%) | 11 (14.5%) | 7 (58.3%) | 13 (20.0%) | NA | 41 (48.8%) | 102 (32.5%) |
| No | 11 (25.6%) | 0 (0.0%) | 5 (20%) | 16 (21.1%) | 3 (25.0%) | 7 (10.8%) | 43 (51.2%) | 85 (27.1%) | |
| Not specified | 10 (23.2%) | 8 (88.9%) | 13 (52%) | 49 (64.4%) | 2 (16.7%) | 45 (69.2%) | 0 (0.0%) | 127 (40.4%) | |
| Alcohol use | |||||||||
| Yes | 13 (30.2%) | 0 (0.0%) | 1 (4%) | 13 (17.1%) | 7 (58.3%) | 3 (4.6%) | NA | 7 (8.3%) | 44 (14.0%) |
| No | 18 (41.9%) | 1 (11.1%) | 11 (44%) | 10 (13.2%) | 2 (16.7%) | 2 (3.1%) | 77 (91.7%) | 121 (38.5%) | |
| Not specified | 12 (27.9%) | 8 (88.9%) | 13 (52%) | 53 (69.7%) | 3 (25.0%) | 60 (92.3%) | 0 (0.0%) | 149 (47.5%) | |
| Exposure to other risk factors | |||||||||
| Yes | 23 (53.6%) | 1 (11.1%) | 7 (28%) | 18 (23.7%) | 9 (75.0%) | 13 (20.0%) | NA | 64 (76.2%) | 135 (43.0%) |
| No | 10 (23.2%) | 0 (0.0%) | 5 (20%) | 8 (10.5%) | 1 (8.3%) | 7 (10.8%) | 20 (23.8%) | 51 (16.2%) | |
| Not specified | 10 (23.2%) | 8 (88.9%) | 13 (52%) | 50 (65.8%) | 2 (16.7%) | 45 (69.2%) | 0 (0.0%) | 128 (40.8%) |
NOS Not otherwise specified
Regarding only the young patients, males also outnumbered females (M:F ratio of 1.3:1.0), with a mean age at diagnosis of 33.4-years (range 5 to 40 years). The tongue and the floor of the mouth were the most affected subsites (73.2%), although buccal mucosa/alveolar ridge/gingiva/retromolar trigone regions were again the most affected subsites in Manipal, India (57.1%). Most of the cases diagnosed in young patients were histologically graded as well or moderately differentiated tumors (31.8% and 40.1%, respectively), whereas only 18.2% of the cases were diagnosed as poorly differentiated. Data concerning the exposure of young patients to known risk habits showed that 32.5% of patients were exposed to tobacco, 14% reported alcohol consumption, and 43% to other risk habits, including pan masala and betel quid, especially in Manipal, India. In contrast, 16.2% of the young patients with OSCC reported no history of exposure to known risk habits. Moreover, we found 17 OSCC affecting patients with 18 years-old or less, and only 4 cases affecting patients with 10 years-old or less.
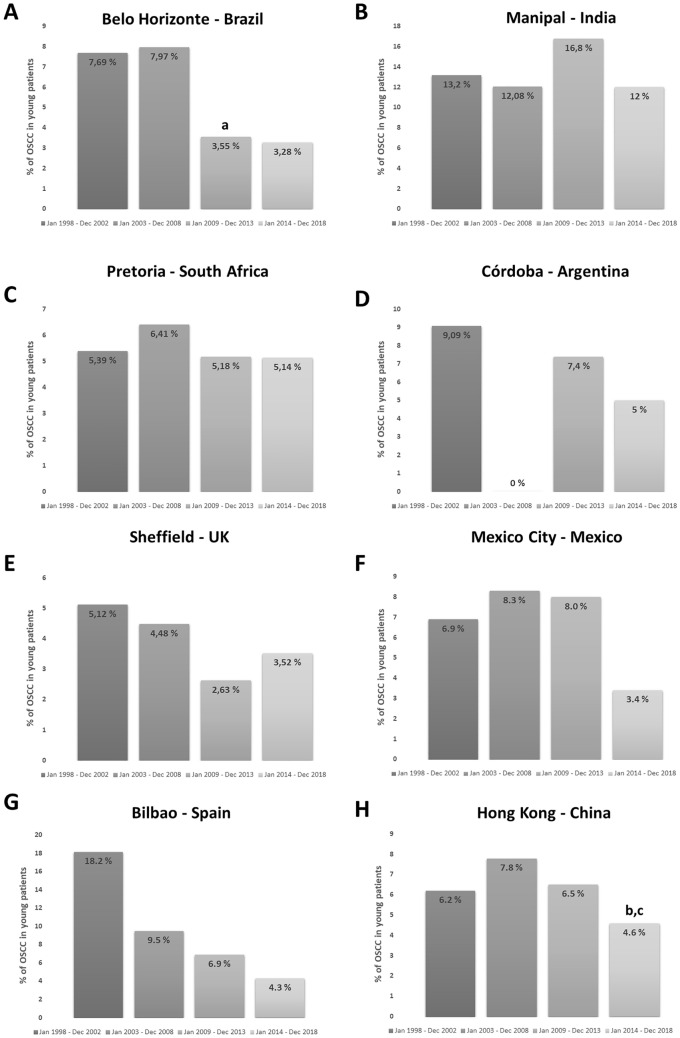
The results of the variation in the frequency and trends of OSCC affecting young patients through the 20-year period investigated are illustrated for each service and the whole population in Fig. 1. Arbitrarily aggregating data in five-year increments, we could not demonstrate any statistically significant increase in the number of young patients affected by OSCC in any of the institutions investigated or in the whole sample (p > 0.05). On the contrary, there was a significant decrease in the number of cases when we compared 2003–2008 to 2009–2013 periods in Belo Horizonte/Brazil (p < 0.05), from 2003–2008 to 2014–2018 (p < 0.001) and from 2009–2013 to 2014–2018 (p < 0.001) in Hong Kong/China, and from 2003–2008 to 2014–2018 in the whole sample evaluated (p < 0.01).
Fig. 1.
Distribution of OSCC in young patients from 1998 to 2018 in different parts of the world for every five years. A Belo Horizonte (Brazil). B Cordoba (Argentina). C Mexico City (Mexico). D Pretoria (South Africa). E Sheffield (UK). F Bilbao (Spain). G Hong Kong (China) and H Manipal (India). a, b, c Statistically significant difference. a between 2003/2008 and 2009/2013. b between 2003/2008 and 2014/2018. c 2009/2013 and 2014/2018
Investigating only the group of patients comprised of young female patients never exposed to any known risk habits, we found 9 cases in India, 8 in Brazil, 7 in South Africa (6 white females and 1 black female), 5 in the UK, 1 in Spain and 0 in Mexico and Argentina. Unfortunately, this data was not available for Hong Kong patients. Moreover, Eastern countries (China and India) presented significantly more cases (7,3%) affecting young patients than in Western countries (4.9%) (p < 0.001).
Discussion
Oral cancer is a significant health problem worldwide, with its development strongly associated with sociocultural habits, leading to significant variability in its incidence among different parts of the world [2]. Epidemiological studies have shown a decrease in its overall incidence, followed by a rise in the number of HPV-positive oropharyngeal cancer during the last decades [14, 15]. However, oral cancer still remains the most common human malignancy among males in some Asiatic countries [16]. OSCC usually affects patients over 50 years of age; however, a series of studies has revealed an increase in the number of cases affecting younger individuals, especially white females without exposure to known risk habits [15]. Although these studies used large-scale populational databases, they were restricted to very few countries. Therefore, by accessing the files of referral centers worldwide we attempted to determine if this increasing trend might also occur in other regions. Our findings did not indicate a significant rise in the number of young patients diagnosed with OSCC during the last two decades, as recently described in Republic of Korea [17].
Many literature references indicating an increase in the incidence of OSCC in young patients were developed in the USA using the SEER database. Davies et al. [18] showed a significant increase in the number of cases affecting young patients. This finding was corroborated by Shiboski et al. [19], demonstrating increasing trends among white females younger than 40 years of age. These results were further supported by Patel et al. [6] and Tota et al. [20] using updated versions of the SEER database. Simultaneously, a population-based study published by Annertz et al. [21], which was later updated by the same group [7], also documented an increase in the incidence among young patients in European Nordic countries. These results were also found in institution-based studies [22–24].
Although these studies are well designed to investigate the incidence of OSCC in young patients and represent the basis of our current knowledge on this subject, they are limited to specific geographic regions and contain important methodological limitations associated with the characteristics of the databases used by these authors. The SEER-based studies have time overlaps and do not cover the whole American territory. In all studies, lip cancer, known to be associated with exposure to ultraviolet radiation, was also included in the samples and might account for some of the cases in this young age group. Histologic subtyping was not performed in these population studies, resulting in some OSCC subtypes that have different clinical features and biological behaviors being included in the overall analyses. Finally, few studies investigated the association of OSCC with known risk factors.
Given the results reported by these studies, there is an important need to validate these observations in other geographic regions. In this study, we used institutional databases to investigate whether the changing trend in the last twenty years reported by Muller et al. [24] occurred in other regions. Although institutional data is less representative than population-based studies, the centers included in this series are important references for the diagnosis and treatment of oral cancer in these regions. Moreover, using institutional data allowed us to overcome some of the above-described limitations of the population-based studies, including histologic confirmation, the exclusion of lip/oropharyngeal cancers, the exclusion of cases with known predisposing syndromes, and the possibility of analyzing the exposure to known risk factors. However, our findings must also be validated in population-based studies in each of these countries, since unknown local environmental factors may predispose the onset of OSCC in young people.
In the present survey, we only observed 30 cases of OSCC in young females not exposed to known risk factors. Although we failed to demonstrate an increase in the frequency of oral cancer in young patients in the institutions investigated, it is evident the presence of a unique group of patients that develop OSCC earlier in life, without associated risk factors. In contrast, we also observed a group of young patients diagnosed with OSCC who were exposed to some type and degree of risk factors. These patients therefore represent conventional oral cancer patients, as the period of exposure may be similar to older patients [25] and earlier age of smoking onset increases head and neck cancer incidence [26]. A study by Choi et al. [27] did not find a significant difference between young and old groups according to tobacco and alcohol exposure. On the other hand, Farquhar et al. [15] demonstrated that younger patients (< 45 years) were more likely to abstain from tobacco usage (51% vs 39%) compared with older individuals. Conversely, Patel et al. [6] claimed that the decrease in tobacco smoking in the USA demonstrated by other population-based studies, would suggest a lack of association between tobacco use and the increase of OSCC among young patients.
The highest number of young patients observed in our study originated from Manipal, India (84 cases). Most of these patients reported usage of at least one known risk factor for oral cancer. These findings stem from the well-known local sociocultural habits of chewing and/or smoking different forms of tobacco [16]. Amongst the Indian population, only a small minority of patients with OSCC reported no associated form of tobacco exposure. In India, the use of other products, different from cigarette smoking, also seems to impact clinical presentation, since the buccal mucosa/alveolar ridge/gingiva/retromolar trigone regions were the most affected subsites [16]. These sites seem most exposed to the hazardous effects of tobacco in this population, whereas the floor of the mouth and tongue represents the most affected subsites in all other geographic regions [28]. The tongue and the floor of the mouth are often simultaneously affected by advanced stage OSCC, are typically associated with similar etiological factors, and show similar biological behavior. Therefore, for statistical purposes, we evaluated these two locations together and the use of the term oral cancer may be more appropriate than tongue cancer.
Distinguishing oral cancer from lip cancer and oropharyngeal cancer is crucial, as these cancers are associated with different etiological factors, exhibit different clinical behaviors, and may be treated with different therapeutic approaches. However, many studies do not separate these patients, resulting in inconsistent conclusions. After observing an increase in the incidence of oropharyngeal cancer in developed countries, HPV infection was determined as a new etiological agent [14]. Therefore, it has been speculated whether this virus would also play an important role in the development of OSCC in young patients. Although Kaminagakura et al. [29] observed evidence to support this theory, these results could not be reproduced by other studies [11, 30–32].
Regarding the histologic differentiation of oral cancer in young patients, we observed that the majority of the cases were classified as well to moderately differentiated tumors, as previously described [24]. Unfortunately, since this information was not available for all OSCC affecting older patients, we could not determine if well-differentiated tumors were more common in younger individuals. Interestingly, our sample had several cases showing microinvasion of the connective tissue, suggesting that these cases presented in early clinical stages. In this study, less common histologic subtypes of OSCC were excluded, as some subtypes may be associated with specific clinicopathological features, such as adenosquamous carcinoma whose origin is still debatable [33], and verrucous carcinoma that presents with a distinct clinical presentation and biological behavior [34].
In addition to the high variability present in the literature concerning the most appropriate cut-off value to categorize patients as either young or older individuals, the molecular basis and the prognostic significance of OSCC affecting young patients are also debatable. We performed a comprehensive literature review [11] to integrate the available data regarding the molecular alterations described in OSCC of young patients and observed minor molecular differences compared to their older counterparts. An important limitation of most of the studies attempting to investigate the molecular basis of oral cancer in young patients is the lack of uniformity in the design of both control and study groups. Therefore, future contributions require the appropriate separation of clinicopathological groups, especially the white female group of patients never exposed to risk factors [8].
Although predisposing syndromes and medical conditions associated with a significantly higher risk of developing oral cancer are uncommon, they must be considered in studies dealing with young patients. OSCC cases affecting children and adolescents might be explained by the presence of pre-existing diseases such as Fanconi’s anemia, xeroderma pigmentosum, among others. In this study, we attempted to exclude all patients with known syndromes or predisposing systemic conditions, which could, however, at least partially explain the development of OSCC in 5 and 6 years-old patients in our sample. Therefore, a detailed genetic investigation of these very young patients is highly desirable. Another relevant scenario is the possible association of oral cancer and HIV/AIDS, which has been more commonly described in recent years [35–38]. In our study, there were 5 young patients diagnosed with oral cancer and HIV/AIDS in the sample retrieved from Pretoria, South Africa. However, the etiological association of HIV/AIDS and OSCC development are incompletely elucidated.
In conclusion, using data retrieved from eight referral centers from around the world covering a 20-year period, we did not observe an increasing trend in the frequency of OSCC in patients younger than 40-years-old, nor specifically in young white females never exposed to known risk factors. This result must, however, be validated using population databases in these countries and further collaborative studies are encouraged.
Funding
This study was supported by the Minas Gerais State Research Foundation (FAPEMIG), the Coordination for the Improvement of Higher Education Personnel (CAPES) (Financial code 001) and the National Council for Scientific and Technological Development (CNPq) (Brazil).
Declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the Universidade Federal de Minas Gerais (91158918.0.0000.5149).
Consent to Participate
Data used in this study were retrospectively obtained from institutional files and patients’ identification was not accessed.
Consent to Publish
Not applied.
Footnotes
The original online version of this article was revised: The original version of this article unfortunately contained a mistake. The spelling of the co-author name Raghu Radhakrishnan was incorrect. The original article has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/14/2022
A Correction to this paper has been published: 10.1007/s12105-022-01503-z
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Curado MP, Johnson NW, Kerr AR, Silva DRM, Lanfranchi H, Pereira DL, et al. Oral and oropharynx cancer in South America: incidence, mortality trends and gaps in public databases as presented to the Global Oral Cancer Forum. Transl Res Oral Oncol. 2016;1:1–7. [Google Scholar]
- 3.Scully C, Bagan JV. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–399. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues PC, Miguel MCC, Bagordakis E, Fonseca FP, Aquino SN, Santos-Silva AR, et al. Clinicopathological prognostic factors of oral tongue squamous cell carcinoma: a retrospective study of 202 cases. Int J Oral Maxillofac Surg. 2014;43:795–801. doi: 10.1016/j.ijom.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 5.De Morais EF, Mafra RP, Gonzaga AKG, Souza DLB, Pinto LP, Silveira EJD. Prognostic factors of oral squamous cell carcinoma in young patients: a systematic review. J Oral Maxillofac Surg. 2017;75:1555–1566. doi: 10.1016/j.joms.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 7.Annertz K, Anderson H, Palmér K, Wennerberg J. The increase in incidence of cancer of the tongue in the Nordic countries continues into the twenty-first century. Acta Oto-Laryngol. 2012;132:552–557. doi: 10.3109/00016489.2011.649146. [DOI] [PubMed] [Google Scholar]
- 8.de Castro JG, Santos-Silva AR, Folgueira AZK, Toporcov T. Tongue cancer in the young. Curr Opin Oncol. 2016;28:193–194. doi: 10.1097/CCO.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca FP, Coletta RD, Azevedo MB, Prado Ribeiro AC, Pires Soubhia AM, Miyahara GI, et al. Stromal myofibroblasts in squamous cell carcinoma of the tongue in young patients—a multicenter collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:483–489. doi: 10.1016/j.oooo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Miranda GM, Santos-Silva AR, Jardim JF, Fonseca FP, Lopes MA, Almeida OP, et al. Different patterns of expression of cell cycle control and local invasion-related proteins in oral squamous cell carcinoma affecting young patients. J Oral Pathol Med. 2018;47:32–39. doi: 10.1111/jop.12601. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos Costa SF, Brennan PA, Gomez RS, Fregnani ER, Santos-Silva AR, Martins MD, et al. Molecular basis of oral squamous cell carcinoma in young patients: Is it any different from older patients? J Oral Pathol Med. 2018;47:541–546. doi: 10.1111/jop.12642. [DOI] [PubMed] [Google Scholar]
- 12.Ambele MA, Pepper MS, van Heerden M, van Heerden WFP. Molecular profile of tongue cancer in an 18-year-old female patient with no recognizable risk factor. Laryngoscope Investig Otolaryngol. 2009;4:310–313. doi: 10.1002/lio2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambele M, van Zyl AW, Pepper M, van Heerden MB, van Heerden WFP. Amplification of 3q26.2, 5q14.3, 8q24.3, 8q22.3, and 14q32.33 are possible common genetic alterations in oral cancer patients. Front Oncol. 2020;10:683. doi: 10.3389/fonc.2020.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123:2219–2229. doi: 10.1002/cncr.30588. [DOI] [PubMed] [Google Scholar]
- 15.Farquhar DR, Tanner AM, Masood MM, Patel SR, Hackman TG, Olshan AF, et al. Oral tongue carcinoma among young patients: an analysis of risk factors and survival. Oral Oncol. 2018;84:7–11. doi: 10.1016/j.oraloncology.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Abdulla R, Adyanthaya S, Kini P, Mohanty V, D'Souza N, Subbannayya Y. Clinicopathological analysis of oral squamous cell carcinoma among the younger age group in coastal Karnataka, India: a retrospective study. J Oral Maxillofac Pathol. 2018;22:180–187. doi: 10.4103/jomfp.JOMFP_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon M, Lee DK, Choi SH, Nam SY, Kim SY, Lee YS. Clinicopathological characteristics of young never smoker females with oral cavity squamous cell carcinoma: a STROBE compliant retrospective observational study. Medicine. 2021;100:e23871. doi: 10.1097/MD.0000000000023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies S, Severson R. Increasing incidence of cancer of the tongue in the United States among young adults. Lancet. 1987;17:910–911. doi: 10.1016/S0140-6736(87)91393-6. [DOI] [PubMed] [Google Scholar]
- 19.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma. Increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 20.Tota JE, Anderson WF, Coffey C, Califano J, Cozen W, Ferris RL, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017;67:146–152. doi: 10.1016/j.oraloncology.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Annertz K, Anderson H, Biorklund A, Möller T, Kantola S, Mork J, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer. 2002;101:95–99. doi: 10.1002/ijc.10577. [DOI] [PubMed] [Google Scholar]
- 22.Shemen LJ, Klotz J, Schottenfeld D, Strong EW. Increase in tongue cancer in young men. J Am Med Assoc. 1984;252:1857. doi: 10.1001/jama.1984.03350140017016. [DOI] [PubMed] [Google Scholar]
- 23.Schantz SP, Byers RM, Goepfert H. Tobacco and cancer of the tongue in young adults. J Am Med Assoc. 1988;259:1943–1944. doi: 10.1001/jama.1988.03720130021012. [DOI] [PubMed] [Google Scholar]
- 24.Müller S, Pan Y, Li R, Chi AC. Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971–2006. The Emory University experience. Head Neck Pathol. 2008;2:60–66. doi: 10.1007/s12105-008-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q, Wang C, Li B, Kim K, Li J, Mao M, et al. The impact of age on oral squamous cell carcinoma: a longitudinal cohort study of 2,782 patients. Oral Dis. 2019;25:730–741. doi: 10.1111/odi.13015. [DOI] [PubMed] [Google Scholar]
- 26.Chang CP, Chang SC, Chuang SC, Berthiller J, Ferro G, Matsuo K, et al. Age at start of using tobacco on the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium (INHANCE) Cancer Epidemiol. 2019;63:101615. doi: 10.1016/j.canep.2019.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi G, Song JS, Choi SH, Nam SY, Kim SY, Roh JL, et al. Comparison of squamous cell carcinoma of the tongue between young and old patients. J Pathol Transl Med. 2019;53:369–377. doi: 10.4132/jptm.2019.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pontes FS, Carneiro JT, Jr, Fonseca FP, da Silva TS, Pontes HA, Pinto Ddos Jr. S. Squamous cell carcinoma of the tongue and floor of the mouth: analysis of survival rate and independent prognostic factors in the Amazon region. J Craniofac Surg. 2011;22:925–930. doi: 10.1097/SCS.0b013e31820fe1cb. [DOI] [PubMed] [Google Scholar]
- 29.Kaminagakura E, Villa LL, Andreoli MA, Sobrinho JS, Vartanian JG, Soares FA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer. 2012;130:1726–1732. doi: 10.1002/ijc.26185. [DOI] [PubMed] [Google Scholar]
- 30.Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010;32:499–503. doi: 10.1002/hed.21220. [DOI] [PubMed] [Google Scholar]
- 31.Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 32.Campbell BR, Netterville JL, Sinard RJ, Mannion K, Rohde SL, Langerman A, et al. Early onset oral tongue cancer in the United States: a literature review. Oral Oncol. 2018;87:1–7. doi: 10.1016/j.oraloncology.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonseca FP, Ramos LM, Vargas PA, de Almeida OP, Lopes MA, Santos-Silva AR. Oral adenosquamous carcinoma: evidence that it arises from the surface mucosal epithelium. Histopathology. 2012;61:321–323. doi: 10.1111/j.1365-2559.2012.04257.x. [DOI] [PubMed] [Google Scholar]
- 34.Peng Q, Wang Y, Quan H, Li Y, Tang Z. Oral verrucous carcinoma: From multifactorial etiology to diverse treatment regimens (Review) Int J Oncol. 2016;49:59–73. doi: 10.3892/ijo.2016.3501. [DOI] [PubMed] [Google Scholar]
- 35.Lucas S, Nelson AN. HIV and the spectrum of human disease. J Pathol. 2015;235:229–241. doi: 10.1002/path.4449. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura K. Current status of HIV/AIDS in the ART era. J Infect Chemother. 2017;23:12–16. doi: 10.1016/j.jiac.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Thrift AP, Chiao EY. Are non-HIV malignancies increased in the HIV-infected population? Curr Infec Dis Rep. 2018;20:22. doi: 10.1007/s11908-018-0626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Méndez-Martínez R, Maldonado-Frías S, Vázquez-Vega S, Caro-Vega Y, Rendón-Maldonado JG, Guido-Jiménez M, et al. High prevalent human papillomavirus infections of the oral cavity of asymptomatic HIV-positive men. BMC Infec Dis. 2020;20:27. doi: 10.1186/s12879-019-4677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]