Abstract
Objectives
Venous thromboembolism (VTE) represents an important cause of maternal morbidity and mortality. Estimates of bleeding associated with therapeutic‐dose anticoagulation are variable. We describe the frequency of bleeding in pregnant women receiving therapeutic anticoagulation for VTE by means of a systematic review of the literature.
Data Sources
Medical Literature Analysis and Retrieval System, Embase, Scopus, Web of Science, and ClinicalTrials.gov were searched. Databases were searched from inception to February 27, 2022. There was no language or geographic location restriction.
Methods of Study Selection
The search yielded 2773 articles with 2212 unique citations. Studies were included if they described pregnant women treated for an acute VTE with therapeutic‐dose anticoagulation and a defined bleeding outcome was reported.
Tabulation, Integration, and Results
Five studies met inclusion criteria. Included studies were judged to have a serious to critical risk of bias using the Risk of Bias in Nonrandomized Studies of Intervention tool. The rate of bleeding, as defined by respective studies, ranged between 2.9% and 30.0%. Two studies included control groups, one of which found no significant difference in the risk of bleeding between groups, while the other found a significantly increased bleeding risk associated with therapeutic anticoagulation.
Conclusion
Among pregnant women anticoagulated for VTE, the reported bleeding risk is variable. The ability to draw definite conclusions is limited by the scarcity and low quality of the studies, the small number of included patients, and the heterogeneity of bleeding definitions used. Large‐scale studies with standardized bleeding definitions are required to provide acute bleeding estimates and optimize the care of these patients.
Systematic Review Registration
PROSPERO, CRD42021276771.
Keywords: anticoagulation, hemorrhage, pregnancy, venous thromboembolism
Essentials.
In pregnancy, blood clots, or venous thromboembolism (VTE), are treated with blood thinners.
The risk of bleeding with the use of blood thinner in pregnancy is not well described.
There was a wide range of bleeding complications in this review, between 2.9% and 30.0%.
Larger studies looking at this question are needed to better inform patients and doctors.
1. INTRODUCTION
Women are at an increased risk of venous thromboembolism (VTE) during pregnancy and the postpartum period. VTE, which includes pulmonary embolism (PE) and deep vein thrombosis, complicates 1–2 of 1000 pregnancies and is an important cause of maternal morbidity and mortality. 1 The risk of VTE is 10‐ to 20‐fold higher in pregnancy than in matched nonpregnant women. 2 The increased risk of thrombosis reflects physiological changes during pregnancy resulting in hormonally induced decreased venous capacitance, decreased venous outflow, increased concentrations of coagulation factors and peripartum vascular injury. 3
Weight‐adjusted subcutaneous low‐molecular‐weight heparin (LMWH) is the standard anticoagulant therapy for both the prevention and the treatment of VTE during pregnancy. 4 , 5 Therapeutic‐dose anticoagulant therapy reduces mortality and the risk of VTE recurrence in the pregnant population. 6 , 7 While LMWH is considered safe in pregnancy, variable estimates are reported in the literature pertaining to the risk of bleeding with the use of therapeutic anticoagulation for VTE in pregnancy. 8 , 9 , 10 Postpartum hemorrhage (PPH) is the leading direct cause of maternal death worldwide. 11 As such, understanding the risk of bleeding for women on therapeutic anticoagulation is paramount to inform clinical care and anticipate the health care needs of this complex population.
Previous studies have evaluated the risk of bleeding associated with the use of LMWH in pregnancy. Reviews in the area either combined data from patients receiving different doses and indications for LMWH 12 , 13 or focused on the therapeutic efficacy rather than the bleeding complications associated with the use of LMWH for VTE. 14 The aim of the current systematic review was to evaluate the risk of antepartum and postpartum bleeding in women receiving therapeutic anticoagulation for VTE during pregnancy.
2. MATERIAL AND METHODS
This systematic review was conducted according to a prespecified protocol following the reporting guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement. 15 The protocol was registered on PROSPERO (CRD42021276771). 16 Review objectives, criteria for study selection, and bias assessment method were defined a priori.
2.1. Sources
A search strategy was developed in conjunction with a medical librarian. An electronic search was conducted from database inception to February 27, 2022, using the following databases: Medical Literature Analysis and Retrieval System, Excerpta Medical Database (Embase), Scopus, Web of Science, and ClinicalTrials.gov. The search strategy was based on two key concepts: (1) anticoagulation using key words including “heparin,” “low‐molecular‐weight heparin” and (2) bleeding using key words such as “antepartum” or “postpartum hemorrhage” (Appendix A). The search strategy was adapted for each database based on its specific nomenclature. There was no language or geographic location restriction applied. A manual search of the reference lists of all included studies and relevant review articles was additionally performed. All results were imported into an EndNote X9 library to remove duplicates and then transferred into Distiller SR (version 2.35, Evidence Partners; 2021) to ensure rigorous methodology and reporting.
2.2. Study selection
Included studies (i) described women treated for an acute VTE during pregnancy (ii) receiving weight‐adjusted therapeutic‐dose anticoagulation with LMWH, and (iii) reported a defined bleeding outcome. Bleeding events during the antepartum and postpartum periods, as defined by the study, were evaluated. Studies in which the main indication for therapeutic anticoagulation was not VTE (e.g., anticoagulation for mechanical heart valve) or the dose of anticoagulation used was not therapeutic (i.e., prophylactic or intermediate dose) were excluded. Studies including populations with mixed indications for therapeutic anticoagulation (e.g., VTE and antiphospholipid syndrome) were included so long as the majority of included patients (i.e., >50.0%) were treated for VTE. Case reports, editorials, commentaries, conference abstracts, and review articles were excluded. The articles identified in the literature search were screened by title and abstract by two independent reviewers (CS, LG). Articles deemed potentially relevant were retrieved for full‐text review. Discrepancies during full‐text review were resolved through discussion and by the opinion of a third reviewer (IM) when necessary.
2.3. Data extraction and quality assessment
An electronic data extraction form was developed and used by two independent reviewers (CS, LG). Disagreements were resolved by consensus or by a third reviewer (IM). Study and patient characteristics, intervention, and outcome definitions were collected. Clinical bleeding outcomes, as defined by investigators in each study, were extracted.
The Risk of Bias in Nonrandomized Studies of Intervention (ROBINS‐I) tool was used for quality assessment by two independent reviewers (CS, TC). 17 Disagreements were resolved by consensus. The ROBINS‐I tool was selected as it provides a comprehensive assessment of traditional epidemiological biases including confounding, selection, and information biases, as well as bias relating to how authors handle missing data and the choice of outcome. 17 All eligible publications were included in the qualitative synthesis regardless of their assessed risk of bias. This was decided beforehand because of preexisting knowledge of the literature and the known critical risk of confounding in retrospective cohort studies without control groups.
2.4. Statistical analysis
We used a descriptive analytical approach to synthesize the incidence of bleeding associated with the use of therapeutic anticoagulation for VTE during pregnancy. Due to the known substantial heterogeneity between included publications in terms of bleeding definitions, it was decided that it would not be meaningful to meta‐analyze the extracted data.
3. RESULTS
3.1. Search results
A total of 2773 articles were identified with our search, 2212 of which remained after duplicates were removed; 2155 publications were excluded following title and abstract screening, and 57 articles underwent full‐text review. Articles were excluded if they described patients treated with an anticoagulant at a dose other than therapeutic (n = 17), if the main indication for anticoagulation was other than VTE (n = 25), or if the article had no specified bleeding outcome (n = 3). Review articles (n = 4), case reports (n = 2), and a conference abstract (n = 1) were also excluded following full‐text review. Five articles meeting all inclusion criteria were included in our systematic review (Figure 1).
FIGURE 1.
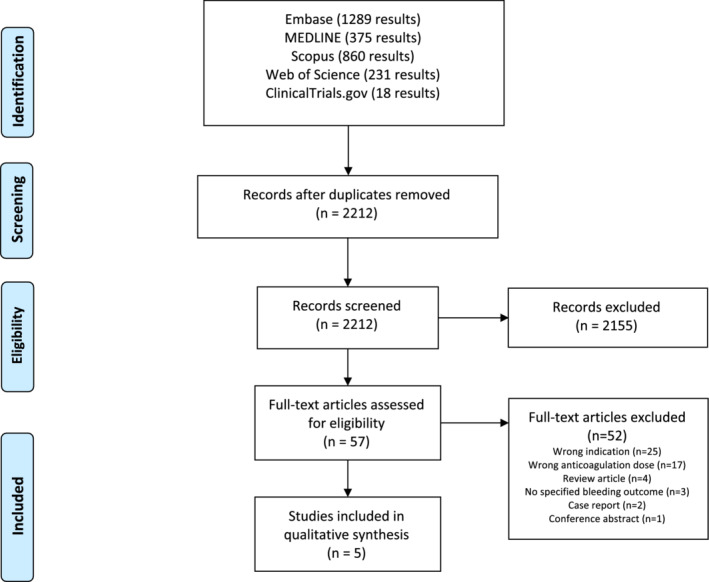
Flow diagram of the systematic search used to identify studies performed on February 27, 2022
3.2. Study characteristics
The characteristics of included studies can be found in Table 1. Four retrospective cohort 8 , 9 , 10 , 18 and one prospective cohort studies 19 were included, representing a total of 1487 participants (611 receiving therapeutic‐dose anticoagulation and 876 controls). Therapeutic‐dose anticoagulation was administered with LMWH or unfractionated heparin. Two studies included control groups of pregnant women without acute VTE who were not receiving anticoagulation, 8 , 9 while the remaining studies had no control group. 10 , 18 , 19 The bleeding definitions used in individual studies were variable. Two studies used the ISTH major bleeding definition, which includes fatal bleeding and/or bleeding in a critical area or organ, and/or bleeding causing a fall in hemoglobin level of 20 g/L or leading to an intravenous transfusion of two or more units of whole blood or red cells. 18 , 19 One study used a composite end point of major hemorrhagic complication comprising bleeding requiring surgery, hospital readmission, admission to the intensive care unit, red blood cell transfusion, or fluid resuscitation of 1 L or more of crystalloids for bleeding concerns. 10 The two remaining studies used PPH as a bleeding end point, with one study using different bleeding thresholds according to the mode of delivery (i.e., PPH of 500 ml or more and severe PPH of 1000 ml or more for vaginal delivery, and a blood loss of 1000 ml or more for cesarean section [CS]), 8 while the other study defined PPH and severe PPH without differentiation by mode of delivery. 9
TABLE 1.
Studies evaluating bleeding in pregnant women treated for acute VTE
| Study | Year of publication | Study design | Population size (n) | Control group size (n) | Intervention | Bleeding definition | Bleeding estimate |
|---|---|---|---|---|---|---|---|
| Blanco‐Molina et al. 19 | 2007 | Prospective cohort study | 136 | NA | LMWH | ISTH definition of major bleeding 20 | Major bleeding: 4/136 (2.9%) |
| Chan et al. 18 | 2012 | Retrospective cohort study | 60 | NA | Once or twice daily LMWH | ISTH definition of major bleeding 20 | Major bleeding: 3/60 (5.0%; 95% CI, 1.0–14.0%); 6 total bleeding events (6/60, 10.0%) |
| Côté‐Poirier et al. 10 | 2020 | Retrospective cohort study | 232 | NA | Therapeutic‐dose LWMH or IVH | Major hemorrhagic complication a | Major hemorrhagic complication: 9/149 (6.0%; 95% CI, 2.8–11.1) for VD and 7/83 (8.4%; 95% CI, 3.5–16.6) for CS |
| Knol et al. 8 | 2012 | Retrospective cohort study | 88 | 352 | Therapeutic‐dose nadroparin (175 units/kg/day) |
VD: PPH ≥500 ml, severe PPH ≥1000 ml CS: PPH ≥1000 ml |
PPH: 30.0% vs. 18.0% in treatment vs. control group (OR, 1.9; 95% CI, 1.1–3.5) for VD and 12.0% vs. 4.0% in treatment vs. control group (OR, 2.9; 95% CI, 0.5–19.4) for CS Severe PPH:5.6% vs. 5.0% (OR, 1.1; 95% CI, 0.4–3.6) for VD |
| Roshani et al. 9 | 2011 | Retrospective cohort study | 95 | 524 | Weight‐based therapeutic‐dose LMWH as defined by manufacturer | PPH > 500 ml, severe PPH > 1000 ml |
PPH: 18.0% in treatment group vs. 22.0% in control group (RR, 0.8; 95% CI, 0.5–1.4) Severe PPH: 6.0% in both groups (RR, 1.2; 95% CI, 0.5–2.9) |
Abbreviation: CI, confidence interval; CS, cesarean section; ICU, intensive care unit; IVH, intravenous heparin; LMWH, low‐molecular‐weight heparin; NA, not available; OR, odds ratio; PPH, postpartum hemorrhage; RR, risk ratio; VD, vaginal delivery.
Major hemorrhagic complication defined as significant bleeding that occurred after the resumption of therapeutic anticoagulation requiring surgery, hospital readmission, admission to the intensive care unit, red blood cell transfusion, or fluid resuscitation of 1 L or more of crystalloid (prescribed specifically for a bleeding concern after therapeutic anticoagulation resumption, so not related to the immediate intrapartum and postpartum period).
3.3. Quality assessment
Based on ROBINS‐I, the risk of bias of most included studies ranged from serious to critical (Appendix B). Retrospective cohort studies without control groups were evaluated as having critical risk of confounding due to the absence of controls. 10 , 18 , 19 All studies were judged to have a serious risk of bias in the measurement of the outcome due to the subjective nature of the bleeding outcome measurement in the absence of a standardized assessment method, and the vulnerability to influence from knowledge of the intervention in the absence of blinding of outcome assessors.
3.4. Bleeding complications
Reported major bleeding events according to the ISTH definition of major bleeding ranged between 2.9% and 5.0% in women receiving therapeutic anticoagulation for an acute VTE during pregnancy. 18 , 19 When evaluating PPH, one study showed a higher risk of PPH in women receiving therapeutic anticoagulation when compared to controls undergoing vaginal delivery (30.0% vs. 18.0%, odds ratio [OR], 1.9; 95% confidence interval [CI], 1.1–3.5; p = 0.03), 8 while another did not find a significant difference between the intervention and control groups (18.0% vs. 22.0%; relative risk [RR] for PPH, 0.8; 95% CI, 0.5–1.4). 9 In the study using the composite end point for major hemorrhagic complication, the outcome occurred in 6.0% (95% CI, 2.8–11.1) of vaginal deliveries and 8.4% (95% CI, 3.5–16.6) of CS deliveries. 10
4. DISCUSSION
Our systematic review describes published rates of bleeding with therapeutic‐dose anticoagulation for VTE during pregnancy. Five observational cohort studies reported bleeding outcomes in this patient population and were evaluated as having serious to critical risk of bias, mainly due to the absence of a control group and subjective measurement of the bleeding outcome. We observed estimates of major bleeding as defined by the ISTH ranging between 2.9% and 5.0% and estimates of PPH between 12.0% and 30.0% associated with the use of therapeutic anticoagulation for VTE during pregnancy.
Whether the use of therapeutic‐dose anticoagulation for the treatment of VTE in pregnancy increases the incidence of bleeding remains unclear. While some studies in our review included a comparator group and showed inconsistent results, 8 , 9 others only reported incidence estimates in the total study population exposed to anticoagulants. 10 , 18 , 19 The reported incidence of postpartum bleeding is 5% to 15% in the general population according to the World Health Organization estimates, although the global incidence and severity of bleeding events in pregnancy remains unknown. 21 While some studies included in our review reported bleeding rates that were overall within a similar range as the general population, 10 , 18 , 19 others reported bleeding rates beyond what would be expected. 8 , 9
Several reasons may explain the reported variability in bleeding rates. First, bleeding definitions are not standard across studies. This may indirectly be reflective of the lack of agreement across national guidelines regarding the definition of PPH. The Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists (ACOG) take into consideration the mode of delivery to define PPH as blood loss greater than 500 ml for vaginal deliveries and greater than 1000 ml for CS. 22 , 23 , 24 The French College of Gynecologists and Obstetricians and the Royal Australian and New Zealand College of Obstetricians and Gynecologists define PPH as any blood loss greater than 500 ml and severe PPH as any blood loss greater than 1000 ml, irrespective of mode of delivery. 25 , 26 The Royal College of Obstetricians and Gynecologists (RCOG) in the United Kingdom further divides PPH into three categories: minor (500 ml to 1 L), moderate (greater than 1–2 L) and major (greater than 2 L) bleeding events. 27 In addition to varying bleeding thresholds and discrepancies in considering delivery mode, visual estimation of the amount of blood loss has been recognized as an unreliable measure of hemorrhagic events. 21 Moreover, the measurement of the bleeding outcome is vulnerable to ascertainment bias associated with the knowledge of the intervention, therapeutic‐dose anticoagulation, received by study participants.
Second, variability in bleeding rates may be due to differences in study populations. For example, aspirin use during pregnancy, which was reported in only two of the five included studies, 8 , 10 has been shown to be associated with an increased risk of postpartum bleeding. 28 Maternal comorbidities and pregnancy complications including gestational diabetes, placenta previa or abruption, preeclampsia, and eclampsia have been shown to increase the risk of postpartum hemorrhage. 29 , 30 These were not reported in included studies including control groups and may have differentially influenced bleeding rates. 8 , 9
Third, variability in peripartum clinical practices may have influenced rates of bleeding. Obstetric interventions, including the management of the third stage of labor, differ by center, and this variation may further explain the variability in reported bleeding outcomes. The highest rates of PPH were reported in two studies performed in the Netherlands. This may be partly explained by the fact that an active management of the third stage of delivery with prophylactic administration of oxytocics and early cord clamping is not routinely performed in these centers, although these interventions have been shown to reduce the amount of blood loss. 8 , 9 Anticoagulation may also not be routinely held antepartum, which may further increase peripartum blood loss. 6 In addition, the time interval between delivery and resumption of anticoagulation in the postpartum period influences the risk of bleeding complications in patients treated with therapeutic anticoagulation for VTE, with shorter intervals leading to a higher risk of major hemorrhagic complication. 10 Time interval for postpartum anticoagulation was not standardized across studies and was reported in only one included study. 10
The lack of standard bleeding definitions has affected the reliability of previously published bleeding estimates associated with the use of anticoagulation during pregnancy. A previous systematic review of anticoagulation for VTE during pregnancy including treatment with therapeutic and nontherapeutic doses of heparins reported an incidence of major PPH (defined by the RCOG as blood loss greater than 2 L) of 1.90% (95% CI, 0.8%–3.6%), with insufficient information provided on bleeding events in individual studies to apply a standardized bleeding classification. Given the limited information in some individual studies with regards to bleeding events, this rate should be interpreted cautiously. 31 Another systematic review of pregnant patients with VTE treated with various doses of anticoagulation compared to controls receiving either thromboprophylaxis or no anticoagulation reported no significant difference in the rate of antepartum bleeding events between the two groups (OR, 1.08; 95% CI, 0.84–1.40). 14 Our review contrasts the existing literature by reporting a wide range of bleeding events in patients receiving therapeutic‐dose anticoagulation for VTE. It also complements previous reports by highlighting the urgent need for standardized bleeding definitions.
Our systematic review has limitations. First, the observational nature of the included studies with small numbers of patients carries an inherent risk of bias with regards to population selection and measure of outcome. Second, the lack of standardization in bleeding definitions precluded a meta‐analysis of reported bleeding outcomes. The ACOG's reVITALize program has put forward a definition of PPH that includes cumulative blood loss regardless of route of delivery and signs or symptoms of hypovolemia, which should alert clinicians to the consequences of blood loss. 32 Recently, the ISTH Scientific and Standardization Committee on Control of Anticoagulation has proposed a standardized classification of antepartum and postpartum bleeding events that classifies bleeding severity according to therapeutic interventions or consequence of blood loss rather than the bleeding event itself. 21 Variable definitions of bleeding outcomes in studies evaluating anticoagulation use in pregnancy, whether prophylactic or other doses, is recognized by the committee. They propose uniform definitions of antepartum and postpartum bleeding events during pregnancy. 21 These tools will undoubtedly help the standardization of bleeding severity in pregnant women receiving anticoagulation and facilitate future research. Moreover, the ongoing PREP & GO study will prospectively evaluate intrapartum and postpartum bleeding using standardized bleeding definitions for women on prophylactic and therapeutic doses of anticoagulation for VTE‐related indications. This will help inform decisions on optimal anticoagulation management and identify future research priorities for this patient population. 33 Third, the lack of information and adjustment with regards to confounders including maternal comorbidities, comedication, and management of the third stage of labor may have further influenced the risk of bleeding associated with the use of therapeutic anticoagulation for VTE in pregnancy. Finally, other patient‐important outcomes that may impact life such as minor bleeding, wound complications, and access to epidural anesthesia were not systematically evaluated. 34
5. CONCLUSION
Among women who received therapeutic anticoagulation for VTE during pregnancy, reported bleeding risks are variable. The available observational studies do not provide reliable bleeding estimates or inform clinicians as to whether the risk of clinically significant blood loss is increased with the use of anticoagulation compared to nonanticoagulated patients. The ability to make definite inference is limited by the observational nature of studies, the small number of patients, and the heterogeneity of bleeding definitions. Larger‐scale studies with standardized bleeding outcomes are required to evaluate the bleeding risk associated with therapeutic anticoagulation to optimize the care of this patient population.
AUTHOR CONTRIBUTIONS
Conceptualization: Camille Simard, Isabelle Malhamé, Vicky Tagalakis. Methodology: Camille Simard, Isabelle Malhamé, Antonios Douros, Kristian Filion, Vicky Tagalakis. Formal analysis and investigation: Camille Simard, Lindsey Gerstein, Teresa Cafaro, Isabelle Malhamé. Original draft preparation: Camille Simard, Isabelle Malhamé, Vicky Tagalakis. Review and editing: Camille Simard, Lindsey Gerstein, Teresa Cafaro, Kristian Filion, Antonios Douros, Isabelle Malhamé, Vicky Tagalakis.
FUNDING INFORMATION
Camille Simard was supported by a CanVECTOR studentship award for this publication; the CanVECTOR Network receives grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT‐142654).
ETHICS STATEMENT
Ethics was not obtained as this is a systematic review of the litterature.
RELATIONSHIP DISCLOSURE
Dr Camille Simard is supported by a studentship award from the Canadian Venous Thromboembolism Research Network (CanVECTOR) for this project. The CanVECTOR Network received grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT‐142654). Dr Camille Simard has no other conflict of interest to declare. Dr Lindsey Gertein, Dr Teresa Cafaro, Dr Kristian Filion, Dr Antonios Douros, Dr Isabelle Malhamé, And Dr. Vicky Tagalakis have no conflict of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors would like to thank Genevieve Gore, liaison librarian at the Schulich Library of Physical Sciences, Life Sciences, and Engineering at McGill University for her assistance with the search strategy.
Simard C, Gerstein L, Cafaro T, et al. Bleeding in women with venous thromboembolism during pregnancy: A systematic review of the literature. Res Pract Thromb Haemost. 2022;6:e12801. doi: 10.1002/rth2.12801
Handling Editor: Dr Vania Morelli
Contributor Information
Camille Simard, Email: camille.simard@mail.mcgill.ca, @camillemsimard.
Teresa Cafaro, @Tcafaro_MD.
Antonios Douros, @AntoniosDouros.
Isabelle Malhamé, @IsabelleMalhame.
Vicky Tagalakis, @VTagalakis.
REFERENCES
- 1. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025‐2033. [DOI] [PubMed] [Google Scholar]
- 2. Bailly J, Jacobson BF, Louw S. Safety and efficacy of adjusted‐dose enoxaparin in pregnant patients with increased risk for venous thromboembolic disease. Int J Gynaecol Obstet. 2019;145(1):70‐75. [DOI] [PubMed] [Google Scholar]
- 3. James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29(3):326‐331. [DOI] [PubMed] [Google Scholar]
- 4. Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317‐3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527‐553. [DOI] [PubMed] [Google Scholar]
- 6. Middeldorp S, Ganzevoort W. Hematologic complications in pregnancy how i treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133‐2142. [DOI] [PubMed] [Google Scholar]
- 7. Rath W, von Tempelhoff GF, Tsikouras P. How to reduce maternal mortality from venous thromboembolism. Clin Appl Thromb Hemost. 2018;24(9_suppl):6s‐7s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knol HM, Schultinge L, Veeger NJGM, Kluin‐Nelemans HC, Erwich JJHM, Meijer K. The risk of postpartum hemorrhage in women using high dose of low‐molecular‐weight heparins during pregnancy. Thromb Res. 2012;130(3):334‐338. [DOI] [PubMed] [Google Scholar]
- 9. Roshani S, Cohn DM, Stehouwer AC, et al. Incidence of postpartum haemorrhage in women receiving therapeutic doses of low‐molecular‐weight heparin: results of a retrospective cohort study. BMJ Open. 2011;1(2):e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cote‐Poirier G, Bettache N, Cote AM, et al. Evaluation of complications in postpartum women receiving therapeutic anticoagulation. Obstet Gynecol. 2020;136(2):394‐401. [DOI] [PubMed] [Google Scholar]
- 11. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
- 12. Greer IA, Nelson‐Piercy C. Low‐molecular‐weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106(2):401‐407. [DOI] [PubMed] [Google Scholar]
- 13. Sirico A, Saccone G, Maruotti GM, et al. Low molecular weight heparin use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta‐analysis. J Maternal‐Fetal Neonatal Med. 2019;32(11):1893‐1900. [DOI] [PubMed] [Google Scholar]
- 14. Chen GC, Gao H, Zhang L, Tong T. Evaluation of therapeutic efficacy of anticoagulant drugs for patients with venous thromboembolism during pregnancy: a systematic review and meta‐analysis. Eur J Obstetr Gynecol Reproduct Biol. 2019;238:7‐11. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simard C, Gerstein L, Cafaro T, Malhamé I, Douros A, Filion KB, Tagalakis V. Bleeding Complications in Women with Venous Thromboembolism during Pregnancy: A Systematic Review of the Literature. PROSPERO: International prospective register of systematic reviews; 2021. Available from: http://www.crd.york.ac.uk/prospero/display_record.php?RecordID=276771 [DOI] [PMC free article] [PubMed]
- 17. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan N, Merriman E, Hyder S, Woulfe T, Tran H, Chunilal S. How do we manage venous thromboembolism in pregnancy? A retrospective review of the practice of diagnosing and managing pregnancy‐related venous thromboembolism at two major hospitals in Australia and New Zealand. Intern Med J. 2012;42(10):1104‐1112. [DOI] [PubMed] [Google Scholar]
- 19. Blanco‐Molina Á, Rota L, Di Micco P, et al. Venous thromboembolism during pregnancy, postpartum or during contraceptive use: findings from the RIETE registry. Thromb Haemost. 2010;103(2):306‐311. [DOI] [PubMed] [Google Scholar]
- 20. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 21. Tardy B, Chalayer E, Kamphuisen PW, et al. Definition of bleeding events in studies evaluating prophylactic antithrombotic therapy in pregnant women: a systematic review and a proposal from the ISTH SSC. J Thromb Haemost. 2019;17(11):1979‐1988. [DOI] [PubMed] [Google Scholar]
- 22. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130(4):e168‐e186. [DOI] [PubMed] [Google Scholar]
- 23. Lalonde A. Prevention and treatment of postpartum hemorrhage in low‐resource settings. Int J Gynaecol Obstet. 2012;117(2):108‐118. [DOI] [PubMed] [Google Scholar]
- 24. Leduc D, Senikas V, Lalonde AB. No. 235‐active Management of the Third Stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2018;40(12):e841‐e855. [DOI] [PubMed] [Google Scholar]
- 25. Sentilhes L, Vayssière C, Deneux‐Tharaux C, et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR). Eur J Obstet Gynecol Reprod Biol. 2016;198:12‐21. [DOI] [PubMed] [Google Scholar]
- 26. Royal Australian and New Zealand College of Obstetricians and Gynaecologists . Management of postpartum hemorrhage. March 2011. Available from: http://www.ranzcog.edu.au/collegestatements‐guidelines.html
- 27. Royal College of Obstetrician and Gynaecologists . Postpartum hemorrhage: prevention and management. April 2011. Available from: https://www.rcog.org.uk/en/guidelines‐research‐services/guidelines/gtg52/
- 28. Hastie R, Tong S, Wikström AK, Sandström A, Hesselman S, Bergman L. Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population‐based cohort study. Am J Obstet Gynecol. 2021;224(1):95.e1‐e12. [DOI] [PubMed] [Google Scholar]
- 29. Muche AA, Olayemi OO, Gete YK. Effects of gestational diabetes mellitus on risk of adverse maternal outcomes: a prospective cohort study in Northwest Ethiopia. BMC Pregnancy Childbirth. 2020;20(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1‐449.e7. [DOI] [PubMed] [Google Scholar]
- 31. Romualdi E, Dentali F, Rancan E, et al. Anticoagulant therapy for venous thromboembolism during pregnancy: a systematic review and a meta‐analysis of the literature. J Thromb Haemost. 2013;11(2):270‐281. [DOI] [PubMed] [Google Scholar]
- 32. Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124(1):150‐153. [DOI] [PubMed] [Google Scholar]
- 33. International Network of VENous Thromboembolism Clinical Research Networks (INVENT) . A prospective cohort study evaluating peripartum anticoagulation management among pregnant women with VTE and its impact on patient outcomes (PREP & GO). Available from: https://www.invent‐vte.com/studies/study/~850‐prep‐go
- 34. Kealy MA, Small RE, Liamputtong P. Recovery after caesarean birth: a qualitative study of women's accounts in Victoria, Australia. BMC Pregnancy Childbirth. 2010;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


