Abstract
Background
Breakthrough infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant (B.1.1.529) has occurred in populations with high vaccination rates.
Methods
In a longitudinal cohort study, pre-breakthrough infection sera for Omicron breakthroughs (n = 12) were analyzed. Assays utilized include a laboratory-developed solid phase binding assay to recombinant spike protein, a commercial assay to the S1 domain of the spike protein calibrated to the World Health Organization (WHO) standard, and a commercial solid-phase surrogate neutralizing activity (SNA) assay. All assays employed spike protein preparations based on sequences from the Wuhan-Hu-1 strain.
Results
Pre-breakthrough binding antibody titers ranged from 1:800 to 1:51,200 for the laboratory-developed binding assay, which correlated well and agreed quantitatively with the commercial spike S1 domain WHO calibrated assay. SNA was detected in 10/12 (83%) samples.
Conclusions
Neither high binding titers nor SNA were markers of protection from Omicron infection/re-infection.
Keywords: Binding antibodies, Neutralizing antibodies, SARS-CoV-2, Omicron, Breakthrough
Abbreviations: SNA, surrogate neutralizing activity; UI, No hx of natural infection prior to BT; NI, Hx of natural infection prior to BT; NII, Hx of natural infection 2x prior to BT; V1, Vaccinated once; V2, Vaccinated twice; V3, Vaccinated twice + booster vaccination; BT, Breakthrough infection
1. Introduction
The Omicron (B.1.1.529/BA.1) variant of SARS-CoV-2 progressed rapidly to become the predominant strain in the United States, comprising 99.5% of all new infections [1] by mid-December 2021. Omicron variants are notable for high transmissibility and significant antigenic differences in the spike protein compared to the original Wuhan-Hu-1 strain and earlier variants [2]. It is not surprising that prior natural or vaccine-induced immunity offer incomplete protection against Omicron breakthrough infection.
Antibody testing has been widely utilized in research, epidemiological and clinical settings during the coronavirus disease 2019 (COVID-19) pandemic, with over 85 tests commercially available under US FDA Emergency Use Authorization [3]. Most antibody detection methods employ solid phase binding assays such as enzyme-linked immunosorbent assays (ELISA), with some capable of detecting surrogate neutralizing antibody activity [4]. Antibody reactivity to recombinant spike proteins is widely used as a marker for humoral immunity [5], [6], [7], [8]; however a specific threshold for risk reduction or protection remains unclear [9] due to the lack of standardization across assays and the emergence of variants [10].
Here, we investigate two binding antibody assays and a receptor binding domain (RBD)-angiotensin converting enzyme 2 (ACE2) interaction inhibiting assay among previously vaccinated persons presenting with presumptive Omicron breakthrough infection. Our study compares three antibody tests: a two-step quantitative IgG binding assay to the full spike ectodomain (Icahn School of Medicine, Mount Sinai assay), a semi-quantitative assay for total serum immunoglobulins inhibiting RBD-ACE2 interactions (GenScript cPass) [11], and a quantitative binding titer assay for IgG to S1 domain (Ortho Clinical Diagnostics VITROS) [12], which is calibrated to the WHO standard. The primary aim of this work is to investigate the association between binding antibody titers, RBD-ACE2 interaction inhibition activity, and Omicron breakthrough infections. We also aim to assess the limitations of these three clinically available laboratory tests (Supplementary Table 1) in the context of Omicron variant infection, which is known to have significant properties of immune escape.
2. Methods
2.1. Study design and participants
We included participants who are enrolled in our IRB-approved (#20201026), ongoing, longitudinal SARS-CoV-2 immunity study (“CITY”) at the University of Miami Miller School of Medicine. Following written informed consent, participants answered a demographic and health history questionnaire. Nasal swabs (Ruhof, Mineola, NY) were collected at each visit to screen for active SARS-CoV-2 infection and whole blood samples (Becton Dickinson, Franklin Lakes, NJ) were processed for serum storage at −80 °C. All participants agreed to sample banking and consented to use in future research.
2.2. Omicron breakthroughs
Individuals with breakthrough infection (12/186 active participants [6.5%]) between December 15th, 2021, and January 7th, 2022, were included in this study. These dates corresponded with high (>98%) Omicron variant infection prevalence nationwide. Breakthrough infections were established with a positive molecular method, such as PCR. Banked serum samples obtained at the study visit prior to breakthrough infection were retrieved for the individuals described above.
2.3. Assays
-
–
Mount Sinai Laboratory binding (IgG) assay
The SARS-CoV-2 ELISAs were performed using a well-described recombinant spike protein binding assay developed by the Icahn School of Medicine at Mount Sinai [13], [14], [15]. Discrete titers were reported in values of 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, 1:12800, 1:25600, 1:51200, 1:102400, and 1:204800.
-
–
Genscript cPass™ combined IgG/IgM/IgA surrogate neutralizing assay (SNA)
This semi-quantitative SARS-CoV-2 assay for SNA measures the inhibition of RBD and ACE2 interactions, was performed in accordance with manufacturer’s instructions. Results were reported as percent neutralization with a threshold of 30% as the cutoff for SNA. Values from 30% to 100% SNA were considered positive and values <30% were considered negative for SNA. Threshold SNA was determined based on correlation studies with conventional live virus and pseudovirus neutralization activity [4]. All accuracy and precision control samples met criteria for within-run and between-run evaluations, and for the LOD/LOQ at 30% neutralization. There were no false positives in serum specimens pre-dating the SARS-CoV-2 outbreak or from donors vaccinated with the influenza vaccine.
-
–
Ortho Clinical VITROS binding (IgG) assay
This assay was performed following the manufacturer’s instructions on an Ortho-Clinical VITROS 7600 analyzer (Ortho Clinical Diagnostics, Raritan, NJ). Calibrated to the 1st WHO International Standard Anti-SARS-CoV-2 Immunoglobulin (Human), NIBSC [12], results were reported as IgG Binding Antibody Units/ml (BAU/ml) to recombinant spike S1 domain. The assay measurement range is 2.0–200 BAU/ml. Participant serum was diluted with manufacturer diluent to achieve a measurable result, followed by conversion of the result by the dilution factor to achieve the final BAU/ml concentration.
2.4. Statistical analysis
Following log-transformation, Pearson correlation coefficients and Bland-Altman plots were generated to examine the correlation and degree of agreement between the Mount Sinai Laboratory assay and Ortho-Clinical VITROS assay. All analyses and figures were generated in R Studio.
3. Results
3.1. Patient characteristics
As shown in Table 1 , the gender distribution was equal, and the median age was 50.5 years (range: 30–78). Six participants (50%) entered the study with a previous history of COVID-19, confirmed via NAAT testing. The participants largely identified as White (83.3%) and non-Hispanic (66.6%). None were known to be immunocompromised. All had received primary vaccination >90 days prior to breakthrough, to include Pfizer/BioNTech BNT162b2 (n = 8; 66.7%), Moderna mRNA-1273 (n = 3; 25%), and Johnson & Johnson Ad26.COV2.S (n = 1; 8.3%). Among those participants who had not yet received a booster (n = 5), two reported a history of previous COVID-19 at study entry. Seven received a booster vaccine (58.3%), with 2 receiving Pfizer/BioNTech BNT162b2 (28.6%) and 5 receiving Moderna mRNA-1273 (71.4%) (Table 2 ). Three of the participants who opted for a Moderna mRNA-1273 booster received a half dose (i.e., 50 mcg vs. 100 mcg).
Table 1.
Participant characteristics for Omicron breakthrough infections.
| n | 12 |
|---|---|
| Gender (M/F) | 6 (50%), 6 (50%) |
| Age (Range) | 50.5 (30–78) |
| Race White Black/African American Other |
10 (83.3%) 1 (8.3%) 1 (8.3%) |
| Ethnicity Hispanic Not Hispanic |
4 (33.3%) 8 (66.6%) |
| COVID-19 Status at Study Entry Positive Negative |
6 (50%) 6 (50%) |
| Vaccine Manufacturer (Dose 1 + 2) n Pfizer/BioNTech BNT162b2 Moderna mRNA-1273 Johnson & Johnson Ad26.COV2.S |
12 (100%) 8 (66.7%) 3 (25%) 1 (8.3%) |
| Median Days Since 2nd Dose to Breakthrough (Range)1 | 172 (90–357) |
| Vaccine Manufacturer (Dose 3) n Pfizer/BioNTech BNT162b2/ Moderna mRNA-12732 |
7 (58.3%) 2 (28.6%) 5 (71.4%) |
| Median Days from 3rd Dose to Breakthrough (Range)3 | 61 (47–148) |
| Median Days from Pre-Breakthrough Sample Collection to Breakthrough (Range) | 31.5 (11–86) |
| Symptomatic Breakthrough | 11 (91.7%) |
Individuals with breakthrough infection following Dose 3 were not included.
Three individuals received half dose boosters (i.e., 50 mcg booster dose vs. 100 mcg for primary doses).
Individuals with breakthrough infection following Dose 2 were not included.
Table 2.
Mt. Sinai Laboratory, Ortho-Clinical VITROS, and Genscript cPASS Assay Results.
| Participant |
# of SARS-CoV-2 Challenges (Infection(s) and Vaccines) Prior to BT |
Days Between Pre-Breakthrough Sample and Last Positive NAAT Test |
Icahn SOM at Mt. Sinai Assaya |
cPass Assayb |
Ortho-Clinical VITROSc |
|
|---|---|---|---|---|---|---|
| 1 | 3 | NI, V2 | 75 | 51,200 | 98, POS | 6600 |
| 2 | 2 | UI, V2 | 33 | 800 | <30, NEG | 57.4 |
| 3 | 2 | UI, V2 | 16 | 1600 | 58, POS | 103 |
| 4f | 3 | NII, V1 | 11 | 1600 | 90, POS | 116 |
| 5d | 3 | UI, V3 | 27 | 51,200 | 98, POS | 6160 |
| 6d | 3 | UI, V3 | 70 | 51,200 | 98, POS | 7490 |
| 7d, e | 4 | NI, V3 | 23 | 12,800 | 98, POS | 2250 |
| 8d, e | 4 | NI, V3 | 36 | 25,600 | 98, POS | 2710 |
| 9d | 3 | UI, V3 | 86 | 51,200 | 98, POS | 13,500 |
| 10 | 3 | NI, V2 | 33 | 6400 | <30, NEG | 635 |
| 11d, e | 4 | NI, V3 | 30 | 25,600 | 98, POS | 4890 |
| 12d | 3 | UI, V3 | 19 | 6400 | 98, POS | 1210 |
UI = No hx of natural infection prior to BT; NI = Hx of natural infection prior to BT; NII: Hx of natural infection 2x prior to BT; V1 = Vaccinated once; V2 = Vaccinated twice; V3 = Vaccinated twice + booster vaccination; BT = Breakthrough infection.
Discrete antibody titers may be reported from 1:100 to 204,800, though in this study discrete titers were only observed up to 51,200.
Surrogate neutralizing activity was reported from <30% to 100% (<30% = Neg, 30–100% = Pos).
Undiluted linear range 2–200 BAU/ml, samples diluted as needed to achieve result in linear range of 2–200 BAU/ml.
Received a booster vaccination.
Received a Moderna mRNA-1273 half dose.
Received Johnson & Johnson Ad26.COV2.S as primary vaccination.
3.2. Breakthrough infection characteristics
All breakthroughs were classified as mild with limited symptoms and lack of medical intervention or hospitalization. The median days from vaccination to breakthrough infection in twice-vaccinated individuals was 172 days (range: 90–357), and 61 days (range: 48–140) in those who had been boosted (Table 2). As seen in Supplementary Table 2, the most frequently reported symptoms were cough (66.6%) and congestion/rhinorrhea (66.6%), sore throat (41.6%) and headache (41.6%). Only one participant reported no symptoms. No participant reported nausea, vomiting, or dyspnea.
3.3. Assay results
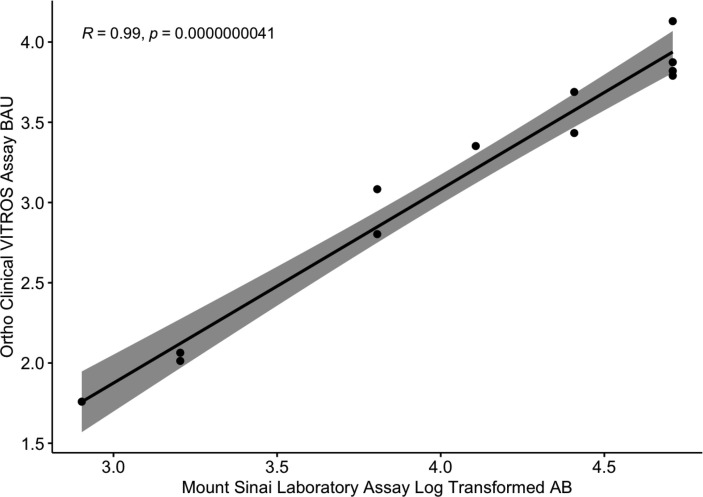
All participants had detectable antibodies with the Mount Sinai assay (Table 2), and all had detectable antibodies (BAU/ml) with the VITROS assay (Fig. 1 ). Titers ranged from 1:800 to 1:51,200 for the Mount Sinai assay (median: 1:19200; range: 1:800–51200) and from 57.4 to 13,500 BAU/ml for the VITROS assay (median: 2710; range: 103–13500). The cPass assay showed 10/12 (83.3%) participants with positive SNA and 2/12 (16.7%) lacking SNA (<30%) as determined by manufacturer specifications. Among those participants with positive surrogate neutralizing activity, 8/10 (80%) had 98% SNA, and two with 90% SNA and 58% SNA respectively. Notably, the two individuals found negative for SNA were also found to have lower values for the binding assays (i.e., Mount Sinai and VITROS).
Fig. 1.
The Mount Sinai Laboratory Assay and the Ortho Clinical VITROS Assay are strongly correlated. Discrete antibody titers from the Mount Sinai Laboratory assay and binding antibody units (BAU/ml) from the Ortho Clinical VITROS assay were log-transformed prior to analysis. The assays were strongly correlated (t = 19, Pearson’s r = 0.99, 95% CI = 0.949–0.996; p = <0.001). The grey shaded area indicates the standard error margins.
3.4. The Icahn school of medicine at mount sinai and the ortho-clinical VITROS assay correlate well
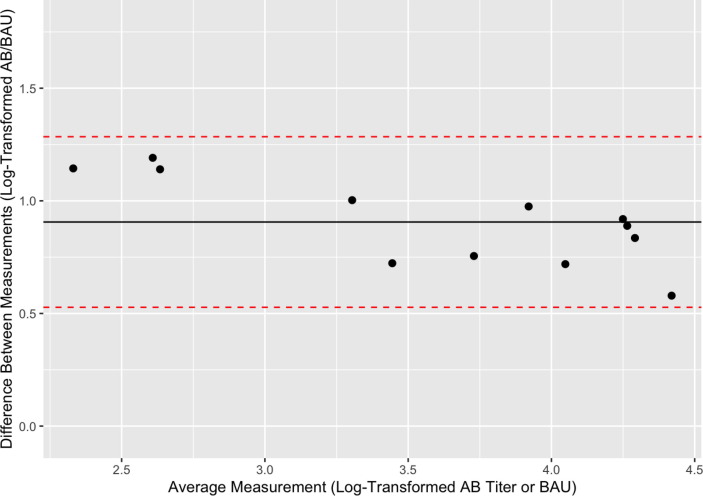
Log-transformed pre-breakthrough antibody titers ranged from 2.90 to 4.71. Bland-Altman analysis demonstrated good agreement between assays (Fig. 2 ). The mean difference was 0.91, with a 95% confidence interval of 0.527 to 1.285. Additionally, the Mount Sinai Laboratory assay endpoints were strongly correlated with the Ortho-Clinical VITROS assay endpoints (Pearson correlation of r(10) = 0.99, p = <0.00001). We also examined the correlation between the Mount Sinai Laboratory assay and the cPass assay and found that the two assays were positively associated (Pearson correlation of r(10) = 0.69, p = 0.013), though to a lesser degree than the VITROS assay.
Fig. 2.
The Mount Sinai Laboratory Assay and the Ortho Clinical VITROS Assay quantitatively agree. A Bland-Altman plot was generated in order to describe agreement between the Mount Sinai Laboratory assay and the Ortho Clinical VITROS assays. The y-axis demonstrates the difference between paired, log-transformed measurements (i.e., discrete AB values from the Mount Sinai vs. BAU from the Ortho Clinical VITROS assay) for each participant. The x-axis represents the average of the log-transformed measurement from each assay. The mean difference (0.91) between values is indicated by the black line, while the red dotted line represents the 95% confidence interval limits (0.527–1.285) for the average difference between assays. All data points fell within the limits of agreement, indicating good agreement between the assays.
Additionally, we stratified participants based on the number of SARS-CoV-2 “antigenic challenges” experienced (i.e., combinations of vaccination and/or natural infection). Individuals with only two SARS-CoV-2 “antigenic challenges” appeared to have lower antibody titers (or less reactivity) across all three assays, although there was no statistically significant relationship between the quantity of “antigenic challenges” and increased antibody titers. Those who had received booster vaccinations had higher antibody titers than those who did not except for one participant who had received their 2nd dose <90 days before breakthrough infection.
4. Discussion
The goal of this study was to investigate the association between binding antibody titers and RBD-ACE2 interaction inhibition activity and Omicron breakthrough infections across three readily available clinical assays. The binding antibody assays (Mount Sinai Laboratory and Ortho-Clinical VITROS) correlated and quantitatively agreed with each other. Given that the Ortho-Clinical VITROS assay is calibrated to the WHO standard, this finding adds additional validity to this and future work, particularly when conducting comparisons across assays. Further, when examined alongside previous work [16] incorporating BAU/ml to quantify antibody titer robustness, 80% of our double vaccinated group had “low” (<2190 BAU/ml) antibodies while the triple vaccinated group consisted of mostly “medium” (2190–3800 BAU/ml) to “high” (>3800 BAU/ml) antibodies. Studies [17], [18] have cited a threshold value of <250 BAU/ml with ∼90% vaccine efficacy against wild type virus. Three (25%) of our participants fell below this threshold at the time of breakthrough, but the majority had extensive antibody activity against ancestral spike.
We also found that higher binding titers were generally suggestive of higher SNA, though magnitude (percent neutralization) alone was an insufficient marker of protection in preventing breakthrough infection in our cohort. In spite of a recent study [19] suggesting adequate viral neutralizing activity following booster vaccination, we provide additional evidence in a longitudinal cohort with three distinct assays that robust antibody levels against the ancestral-strain fail to establish proof of sufficient protection against antigenically distant variants. Overall, these results indicate that the observation of high binding titers or SNA to ancestral spike/RBD alone may not adequately confer protection from breakthrough infection with the Omicron variant [20]. In order to achieve more robust immune protection, the next generation of SARS-CoV-2 vaccine preparations will be directed towards Omicron spike determinants [21]. Parallel development of solid phase serological assays (binding and SNA) should also employ Omicron antigen targets to serve as markers of humoral immune response to circulating variants.
This work has several limitations, principally the small sample size and variable immune experience of the cohort. Further, as is the case for the majority of the available antibody assays, the Mount Sinai Laboratory assay and the cPass assay have not yet been calibrated to the WHO standard. Our study shows a good correlation between the Mount Sinai Laboratory and the Ortho-Clinical VITROS assay. Further standardization of clinical serological binding assays to an international standard will allow better correlations of immunity between independent clinical trials.
Finally, this study examined only samples immediately prior to breakthrough infection so factors regarding temporal relationship of infection, clinical presentation, and sample collection may have affected our observations. Additional studies including individuals who appear to be susceptible to re-infection or who are poor immunologic responders to SARS-CoV-2 infection and/or vaccination, are needed to better understand differential immune kinetics in those populations. Of particular interest will be the characterization of T-cell immune responses in individuals who developed strong antibody responses yet experienced breakthrough infection. Importantly, since we did not compare binding antibody titers of breakthrough cases with non-breakthrough cases, we cannot draw conclusions regarding where non-breakthrough binding antibody titers would fall or if they would trend differently than those of individuals who may have been exposed but were not infected. Our data suggest additional studies are required to understand the role of SNA assays in assessing immune protection to infection or re-infection.
Although mounting evidence suggests that both primary vaccination and boosters lessen the likelihood of symptomatic infection, hospitalization and death following infection with Omicron, there remains an urgent need for updated variant-specific and ultimately “variant-proof” vaccines and early treatment modalities. Though the severity of the COVID-19 disease course is related to antigen-specific adaptive immune responses, particularly in breakthrough cases [22], [23], [24], [25], the precise mechanisms for individual susceptibility to breakthrough infections remain unclear. Greater clarification on the specifics of the humoral response to infection and vaccination is needed, especially the roles of mucosal antibodies and non-neutralizing antibodies. Future work should also incorporate cellular immunity profiling to better define the immune landscape amongst diverse populations affected by SARS-CoV-2.
Funding
This work was partly funded by the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051 as part of the PARIS/SPARTA studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
As ever, we are grateful to our research participants for their samples, time, and interest in this work. At the University of Miami Miller School of Medicine, we thank Aria Nawab, Daniel Muniz, Felipe Echeverri Tribin, Margaret Roach, Elizabeth Varghese, and the entire phlebotomy team at the Clinical Translational Research Site. We would also like to thank the SARS-CoV-2 serology team at the Icahn School of Medicine at Mount Sinai, including Dominika Bielak and Gagandeep Singh.
All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.08.058.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.CDC. Variant Proportions. 2022 1/15/2022. <https://covid.cdc.gov/covid-data-tracker/#variant-proportions>.
- 2.Shiehzadegan S., et al. Analysis of the delta variant B.1.617.2 COVID-19. Clin Practice. 2021;11(4):778–784. doi: 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. In Vitro Diagnostic EUAs - Serology and Other Adaptive Immune Response Tests for SARS-CoV-2. 2022 1/24/2022. <https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2>.
- 4.Tan C.W., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 5.Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 6.Earle K.A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steensels D., et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry J., et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE. 2022;17(4):e0266852. doi: 10.1371/journal.pone.0266852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromer D., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GenScript. cPass SARS-CoV-2 Neutralization Antibody Detection Kit. Instructions for Use; Version 6.0. <https://www.fda.gov/media/143583/download>.
- 12.VITROS. VITROS Anti-SARS-CoV-2 IgG Quantitative. Instructions for Use; Version 1.0; <https://www.fda.gov/media/150675/download>.
- 13.Amanat F., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadlbauer D., et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57(1):e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wajnberg A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert PB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 0(0), eab3435. [DOI] [PMC free article] [PubMed]
- 17.Maillard A., et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139(1):134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblatt D., et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40(2):306–315. doi: 10.1016/j.vaccine.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wratil PR, SM, Priller A, et al., Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med; 2022. [DOI] [PubMed]
- 20.Stærke N.B., et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat Commun. 2022;13(1):4466. doi: 10.1038/s41467-022-32254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X. Omicron: call for updated vaccines. J Med Virol. 2022;94(4):1261–1263. doi: 10.1002/jmv.27530. [DOI] [PubMed] [Google Scholar]
- 22.Juthani P.V., et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21(11):1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque A., Pant A.B. Mitigating Covid-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J Autoimmun. 2022;127:102792. doi: 10.1016/j.jaut.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brosh-Nissimov T., et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen P.A., et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.