Abstract
The Lumpy Skin Disease Virus (LSDV) Neethling vaccine strains have been used for decades for prophylactic immunization of domestic ruminants against the disease. Commercial products against Lumpy skin disease are supplied as live attenuated vaccines and often are associated with adverse reactions warranting studies towards development of safe and efficacious vaccine alternatives. The present study was designed to investigate the ability of Montanide™ Gel 01 PR adjuvanted inactivated Neethling vaccine strain of the lumpy skin disease to induce immune response in rabbits. Complete virus inactivation was achieved following treatment of live vaccine strain with binary ethyleneimine (BEI) at 2 mM final concentration. Inactivated virus antigen, formulated with Montanide™ Gel 01 was injected at 1,00E + 05 and 1,00E + 06 TCID50 per dose in rabbits. The second injection with same vaccine dosages was administered 21 days after the primary vaccination. Rabbits that received a 1,00E + 05 TCID50/dose of inactivated LSDV vaccine formulation induced maximum neutralizing antibody titres on day 13 post second vaccinations. Rabbits vaccinated and prime boosted with the 1,00E + 06 TCID50/dose of inactivated LSDV vaccine formulation, induced neutralizing antibody titres on day 14 after first vaccination. The maximum antibody titres for the 1,00E + 06 TCID50/dose of the inactivated LSDV vaccine formulation was obtained on day 35 post vaccination. The 1,00E + 06 TCID50 dose of the inactivated LSDV vaccine Montanide™ Gel-01 PR formulation induced higher neutralizing antibodies. The MontanideTM Gel-01 PR offers safer profile to oil adjuvants and can be developed further to protect target animals against LSDV in non-endemic areas.
Keywords: Lumpy skin disease, Virus Inactivation, Montanide™ Gel 01 adjuvant, Immunogenicity
1. Introduction
Lumpy skin disease (LSD) is an infectious disease of all breeds of cattle caused by a double-stranded DNA lumpy skin disease virus (LSDV). The virus belongs to the genus Capripoxvirus of family Poxviridae [1], [2]. LSD is defined by mild to severe symptoms, which include fever, nodules on the skin, enlarged lymph nodes, and oedema of the skin [3], [4]. The disease results in financial losses in affected cattle industries due to loss in milk production and infertility, abortion, damage to hides and sometimes death mainly due to secondary bacterial infections [5], [6]. LSD is endemic in many African countries, and first LSD outbreak in the Middle East region was reported since 2000. In 2015 the disease entered Europe via Turkey and affected North Eastern Greece and since then there have been major outbreak reports in Eastern Europe and surrounding Balkan countries, Russia [7], including reports in western China and eastern India [8]. The LSDV has spread into many regions of Asia, Mongolia, Vietnam, Combodia, Laos, Thailand, Malaysia, Indonesia, Sri Lanka, Bangladesh, Pakistan and others. There is an increased risk of LSD incursion in neighbouring countries with no previous reports of the occurrence of the disease due to movement of animals.
In comparison to other strategies used to control the spread of LSD, vaccination is reported to be most effective [9]. Several commercial live attenuated vaccines are available globally for prophylactic immunization of cattle against LSDV [9]. The live attenuated Neethling strain derived vaccines are effective and have played an important role in controlling the outbreaks in South East Europe in the past 6 years. In 2019 no LSD outbreaks were reported in South Europe following continued mass annual vaccination with live attenuated vaccines [8]. Two such vaccines used to provide immunity for cattle are: The Neethling strain LSD vaccine for cattle from Onderstepoort Biological Products (OBP) and the Lumpyvax supplied by (MSD Animal Health). Despite the success of the live LSDV vaccines, their use has been associated with the development of skin nodules in a considerable number of animals, following administration [10]. These reactions may be accompanied by a temporary drop in milk production [1]. Furthermore, these Capripoxvirus vaccines are capable of producing a large local reaction at the site of inoculation in Bos Taurus breeds, which farmers find unacceptable [11].
In an event of LSDV outbreaks, countries with no history of LSDV occurrence would hesitate to use live vaccines against the disease due to the safety concerns. Alternative LSDV vaccines must be considered as a safer option for the prophylactic immunization of cattle against LSDV. Recombinant LSDV vectored vaccines are still in developmental stages with improved safety in cattle [12], [13]. However, these live vectored vaccines are still associated with possibility of inducing mild form of disease. Currently no inactivated LSDV vaccines are commercially available and there is limited published research evaluating inactivated LSDV vaccines in cattle. Recently inactivated LSDV vaccine candidates were developed and immunity evaluated in cattle against challenge with virulent virus [14], [15]. The inactivated Neethling strain adjuvanted with oil was able to induce protection against virulent LSD challenge. The humoral response induced by inactivated LSD was comparable to the live attenuated vaccine and with improved safety [14]. The oil adjuvanted formulation induced swelling at site of injection associated with vaccine. In another research, inactivated field isolate of the lumpy skin virus was formulated with proprietary adjuvants [15]. The vaccine was safe for use and protected cattle against challenge infection. In this study an inactivated vaccine was prepared from the South African Neethling vaccine strain provided by the OBP. The vaccine was inactivated with lower concentration of BEI and formulated with MontanideTM Gel-01 adjuvant and immunogenicity evaluated in rabbits.
2. Materials and methods
2.1. Cells
The Mardin Darby Bovine Kidney (MDBK) cells were maintained at 37 °C with 5 % CO2 in Glasgow minimum essential media (GMEM), supplemented with 5 % (v/v) tryptose phosphate broth and 10 % (v/v) bovine serum. Cultures were grown at 37 °C with 5 % CO2 to reach 90 % confluency for propagation of the LSDV.
Bovine Dermis (BD) cells were used for determination of viable LSDV titre. Cells were cultured using completed minimum essential medium (MEM) containing 10 % bovine serum and 1 % glutamax. The cells were incubated at 37 °C with 5 % CO2 and confluent monolayer infected with LSDV.
2.2. Virus strains, propagation and quantification
The LSDV Neethling attenuated vaccine strain [1] vials were obtained from the OBP seed stock repository. The virus was amplified using confluent monolayer of MDBK cells at 37 °C until 90–100 % cytopathic effect (CPE) was observed. Harvested LSD virus was stored at 4 °C until required. The virus titre was measured using TCID50 assay in accordance with the method of Kaber [16].
2.3. Virus inactivation
The LSDV of known titre was inactivated with binary ethyleneimine (BEI) as per Bahnemann [17] and the final concentrations evaluated were 0.5, 1, 2, and 3 mM. The live virus control (without addition of BEI) was included and inactivation conducted at 37 °C with shaking at 130 rpm for all concentrations. The initial inactivation was carried out up to 36 h (with a 2 h sampling interval up to 24 hrs and again at 36 hrs). Immediately after sampling, sterile and cold 1 M sodium thiosulphate pentahydrate (H10Na2O8S2) was added to the samples at 10 % volume of BEI used for LSDV inactivation in order to neutralise BEI. Neutralized inactivated virus aliquots were stored at 4 °C until required. Virus inactivation was confirmed by titration using tissue culture infective methods and validated using the Real Time Cell Analysis system (RTCA) (xCELLigence™, DP ACEA Biosciences Inc, US) [18]. The RTCA system analyses and measures the electronic impedance in E-plates, which comprise micro-electrode arrays at the bottom surface of each well. The MDBK cell suspension (1–5 × 104 cells/mL) was used as the seeding rate for the RTCA assays. Cell growth status was monitored by recording the cell index (CI) hourly for up to 48 h to obtain exponential cell growth before infection with the LSDV-containing samples. Following infection, the E-Plates were further incubated at 37 °C with 5 % CO2 and monitored on the RTCA system up to 200 h.
2.4. Vaccine formulation
The LSDV antigens were formulated with Montanide™ Gel 01 PR adjuvant (SEPPIC, France) (10 % w/w) to 1,00E + 05 and 1,00E + 06 TCID50/dose at room temperature under sterile conditions using a high-speed homogenizer, D500 set at 13,000 rpms. Sterility tests of the vaccine formulations were conducted on blood tryptose agar with bovine blood, and inoculation on thioglycolate and soy media.
2.5. Ethical considerations
Experiments in animals were carried out in accordance with the standards as set out by Experimental Division at OBP. The animal experiment protocol was approved by the OBP Animal Ethics Committee (South African Veterinary Council Facility Registration Number: FR1514054). The research in animals was also approved by the national Department of Agriculture, Land Reform and Rural Development under Section 20 of the Animal Diseases Act (Act 35 of 1984) South Africa.
2.6. Animal housing and care
Immunogenicity of the adjuvanted inactivated LSDV vaccine formulations was assessed in rabbits using the New Zealand White strain. The research in animals was conducted according to the Animal Protection Act (Act 71 of 1962) and Animal Disease Act (Act 5 of 1985). Animals were handled in accordance with the standard operating procedures at the clinical department (OBP) in the small animal facility. Rabbits of mixed gender, 9–14 weeks old of age and weighing 2.5–3 Kg were commercially sourced from Hamariti Breeding Farm in Grootvlei (South Africa). The rabbits were placed individually in clean cages in a temperature-controlled environment at room temperature (±22 °C). Upon arrival animals were acclimatized for seven days and general health observations conducted daily prior to the start of the trial. The cages were labelled according to the animal groups and the unique animal identification allocated. Animals were fed Epol’s rabbit chow and clean water ad lib.
2.7. Immunogenicity in rabbits
Research in rabbits complied with the OBP animal ethics code and national guidelines for conducting research studies in animal. Twenty-four rabbits were randomly assigned into three groups (A to C) with each group containing 8 rabbits (Table 1). Rabbits were individually vaccinated subcutaneously (using 26 gauge needles) at the back of neck with 1 mL (1,00E + 05 and 1,00E + 06 TCID50/dose, for group A and B, respectively) of the adjuvanted inactivated LSDV vaccine on day 0. Group C vaccinated with placebo vaccine (OBP commercial sterile vaccine diluent) was kept as a negative control group. All animals were monitored daily for 21 days post each injection. The secondary injection was administered on day 22 and animals were monitored till end of the trial on day 42. Approximately 1–2 mL of blood samples were collected from each rabbit on days 0, 7, 14, 21, 28, 35 and 42. After the 3rd week of secondary vaccine inoculation, rabbits were sedated with ketamine fresenius at 100 mg/mL and xylavet 2 % 3 mg/Kg and bled through cardiac puncture followed euthanasia.
Table 1.
Vaccination of the adjuvanted inactivated LSDV vaccines in rabbits.
| Vaccine | Species | Injection route | Frequency of injections | Site of injection | Duration of the trial (days) | Number of animals |
|---|---|---|---|---|---|---|
| Adjuvanted inactivated LSDV (1,00E + 06 TCID50/dose) | Rabbits | S/C | 2 injections (D0, D22) | Back of neck | 42 | 8 |
| Adjuvanted inactivated LSDV (1,00E + 05 TCID50/dose) | Rabbits | S/C | 2 injections (D0, D22) | Back of neck | 42 | 8 |
| Placebo vaccine | Rabbits | S/C | 2 injections (D0, D22) | Back of neck | 42 | 8 |
| Total | 24 | |||||
2.8. Serum neutralising test (SNT) assay
The SNT test was used to detect specific neutralizing antibodies in serum samples of the vaccinated rabbits. The SNT method was performed as described by Frey and Liess [19]. Briefly, blood samples were centrifuged at 2500g at 4 °C for one hour. Serum samples were transferred to sterile cryovials and heat inactivated at 56 °C for 30 min. The heat inactivated serum samples were diluted fivefold using GMEM supplemented with 5 % (v/v) tryptose phosphate broth, amphotericin (5 µg/µL) and streptomycin (75 µL/mL). Positive and negative anti-LSDV serums, and live LSDV antigen were included as controls. For each dilution, 50 µL of 100 TCID50/mL LSDV antigen was added. After 1 h co-incubation of the sera with the virus at 37 °C, 100 µL of the BD cells were added in 96-well microtitre plates. The cell monolayer was examined for presence of CPE under light microscope. Antibody titres were expressed as the reciprocal of the serum dilution that inhibited 50 % of viral CPE [20].
2.9. Enzyme linked immunosorbent assay (ELISA)
A commercial ELISA kit, ID Screen® Capripox Double Antigen Multi-species (IDvet Innovative Diagnostics, France) was used as, a confirmatory test of obtained SNT data to detect antibodies in sera against LSDV induced by the vaccination in rabbits. This kit is recommended and validated to measure the concentration of antibodies in sera against LSDV, SPPV, and GTPV [21]. The ELISA assay was carried out according to the manufacturer’s instructions and the results were interpreted based on the calculated sample to positive (S/P) ratio.
3. Results
3.1. LSDV inactivation kinetics
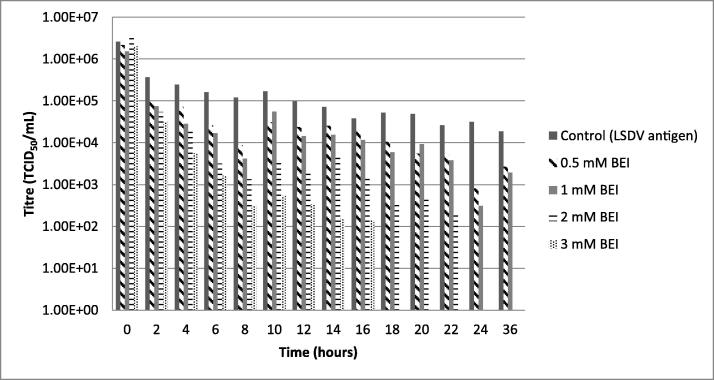
LSDV inactivation was evaluated at BEI concentrations of 0.5, 1, 2, and 3 mM for up to 36 h at 37 °C. Fig. 1 shows that the lowest concentrations of BEI (0.5 and 1 mM) did not completely inactivate LSDV within the 36 h. Decrease in virus titre from 1,00E + 06 TCID50/mL to 1,00E + 03.5 and 1,00E + 03 TCID50/mL were obtained, respectively. The LSDV inactivated with 2- and 3-mM BEI resulted in 00 TCID50/mL titres following 24- and 18-hours incubation, respectively. Results obtained with both these concentrations had trends similar to that obtained with cells only negative control which did not induce any CPE on infected cells. A slight decrease in titre from 1,00E + 06 TCID50/mL to 1,00E + 04.2 TCID50/mL was observed with the virus following 36 h of incubation at 37 °C under the same condition without inactivating agent.
Fig. 1.
Inactivation profile of LSDV using BEI as inactivating agent. LSDV inactivation was evaluated with BEI at 0.5, 1, 2 and 3 mM at 37 °C for 36 h. The data represents the average titers and standard deviation obtained from 3 independent experiments performed.
3.2. Vaccine formulation
The two LSDV antigens at 1,00E + 06 and 1,00E + 05 TCID50/dose were inactivated with 2 mM BEI for 24 h. Following BEI inactivation, the viral titre for these batches was zero TCID50/mL. In addition, no CPE was observed on BD cells infected with BEI inactivated LSDV antigen indicating complete inactivation of the virus. The RTCA assay was used to validate the TCID50 results obtained for the inactivated LSDV antigen. Growth profile of MDBK cells infected with inactivated LSDV for both vaccine dosages was similar to that of MDBK uninfected cell controls (Fig. 2). The CI index remained at 8 and above in the same range as the cells. The MDBK cells infected with active LSDV, displayed a profile of CPE with CI index decreasing from 8 to 0 in 7 days following infection which is typical of LSD virus. The inactivated LSDV antigen was formulated with Montanide™ Gel 01 at a dose of 1,00E + 05 and 1,00E + 06 TCID50/dose and subsequently tested for immunogenicity in a small animal model (rabbits). All formulated vaccines met quality control specifications for sterility, with no bacterial and fungal contaminants.
Fig. 2.
Validation of LSD inactivation with Real-Time Cell Analysis. The RTCA showing the growth profile of MDBK cells infected with BEI inactivated LSDV at 1,00E + 06 and 1,00E + 05 TCID50/mL and live LSDV. The MDBK cells only control was included.
3.3. Immunogenicity profile of adjuvanted inactivated LSDV vaccines.
Two groups of 8 rabbits were vaccinated with 2 injections of 1 mL adjuvanted inactivated LSDV vaccines 21 days apart, one group received 1,00E + 05 TCID50/dose and the other 1,00E + 06 TCID50/dose. The third group was injected with a placebo and used as a negative control. Rabbits were bled on day 0, 7, 14, 21, 28, 35 and 42. Serum was obtained and used to conduct SNTs and ELISA assays to determine antibody titres induced in rabbits following vaccinations. Antibody titres of ≥ 1:4 and ≥ 30 % measured by SNTs and ELISA, respectively are indicative of presence of neutralizing antibody titres. Serum collected on day 0 was free from LSDV antibodies as indicated by rabbits obtaining zero titres when tested by SNT and ELISA assays (Fig. 3A and B).
Fig. 3.
Determination of serological response induced by inactivated LSD Neethling vaccine. Data indicates mean antibody titres of rabbits vaccinated with the adjuvanted inactivated LSDV vaccine dosages. A) Serology as measured by SNT with P value of 0.0005 and B) Serology measured by ELISA with P value of < 0.0001.
3.3.1. Serum neutralization test (SNT)
Neutralizing antibody titres against LSDV were detected in group A and B rabbits vaccinated with the 1,00E + 06 and 1,00E + 05 TCID50 doses of inactivated LSDV vaccine, respectively. Rabbits vaccinated with lower vaccine dosage induced LSD neutralizing antibody titres by 21 days post primary vaccinations (Fig. 3A). No LSD antibody titres were detected on day 28 in animals vaccinated with a lower dose of the vaccine. However, a response was observed in the lower vaccine dose animals on day 35 (13 days post-secondary vaccination) with neutralizing antibody titres reaching a maximum of 18. The response obtained with inactivated LSDV vaccine at a higher dose (1,00E + 06 TCID50/dose) was superior. The response was significantly high with p-value of 0.0005. Specific LSD neutralizing antibodies were obtained from day 14 and had increased to 32 by day 35. Antibody titres in animals vaccinated with 1,00E + 06 TCID50 dose were consistently high when compared to the vaccine formulated with lower 1,00E + 05 TCID50dose. The antibody response trend in rabbits was comparable to serology in cattle [14] which had percentage reactors at 87 % by day 28. Rabbits vaccinated with placebo had zero antibody immune response throughout the trial (Fig. 3A).
3.3.2. Enzyme-linked immunosorbent assay (ELISA)
The SNT data obtained in the study was validated by an ELISA, and neutralizing antibody titres against LSDV detected prior to secondary vaccination were below the 30 % threshold. The high dose vaccine induced response at approximately 63 % 6 days post-secondary vaccination (Fig. 3B). The antibody response was significantly high (P < 0.0001) and increased to 134 % by day 35 and remained above 30 % threshold till the end of the trial on day 42. Rabbits vaccinated with the inactivated LSDV vaccine at low dose induced the mean neutralizing antibody titres of 68 % on day 35 (Fig. 3B). A slight decrease in antibody titre was observed on day 42 for both high (1,00E + 06 TCID50/dose) and low dose (1,00E + 05 TCID50/dose) of the vaccine, obtaining mean average antibody titres of 87 % and 57 %, respectively. The non-vaccinated control group did not develop any detectable antibody responses throughout the trial (Fig. 3B).
4. Discussion
Vaccination is the most effective control method against the spread of LSDV [9]. The live attenuated Neethling strain LSDV vaccines used in the latest cases of LSD outbreaks in the Eastern Europe has been shown to protect against the disease [8]. Approximately ten million LSDV vaccine doses were supplied by OBP alone during the 2015–2017 periods of LSD outbreaks across the affected countries in Eastern Europe. Since the last outbreaks reported in 2016, no new LSD outbreaks were reported in those affected countries, where full vaccination coverage has been achieved [8]. Although the live attenuated form of the vaccine is effective, it is associated with injection site reactions. The live attenuated vaccine causes local inflammation, mild form of the disease with the presence of skin lesions [10], [22], [23]. Inactivated vaccines are often considered safer, causing no clinical manifestations in animals as they consist of the dead pathogen, and thus can be recommended for immune-compromised animals [24], [25]. Furthermore, since the vaccine cannot replicate inside host, it reduces the risk of future LSD outbreaks as the strain cannot revert to its virulence state nor can it spread amongst animals [22].
In this study, an inactivated vaccine was prepared from the OBP Neethling LSDV vaccine strain. The LSDV was shown to be completely inactivated at a final concentration of 2 mM BEI and method of inactivation was validated using the RTCA system. The final concentration determined to fully inactivate Neethling LSDV was comparable to the concentrations required for inactivation of other pox viruses [26].
The inactivated LSDV vaccine in this study was evaluated at two doses: antigen concentrations; 1,00E + 06 TCID50/dose and a lower titre of 1,00E + 05 TCID50/dose. The inactivated LSDV vaccine formulated with Montanide™ Gel 01 was shown to be immunogenic in the rabbit model. Serological assays (SNT and ELISA) revealed that the 1,00E + 06 TCID50/dose of the Montanide™ Gel 01 adjuvanted inactivated LSDV vaccine induced high level of specific LSDV neutralizing antibody titres when compared to the 1,00E + 05 TCID50/dose of the vaccine. The antibody response increased after secondary vaccination and was comparable with both serological assays. The inactivated LSDV vaccine at 1,00E + 06 TCID50/dose induced earlier immunological response (6 days post booster), while, in contrast the lower dose of the vaccine (1,00E + 05 TCID50/dose) induced optimal antibody titres 13 days after second injection. A slight decrease in neutralising antibodies was observed at the end of the trial on day 42 for both vaccine dosages when using ELISA. The data in cattle [14] resulted similar serological trend by both ELISA and VNTs; on day 14 only 40 % reactors and on day 28 had increased to maximum of 87 %. In the field maximum of 80 % reactors was observed by day 28 and slight decrease to 68 % in 120 days. In the study by Wolff [15] protection was obtained from vaccination of cattle with inactivated and adjuvated LSDV-Serbia field strain. High antibody titres were obtained on day 42 when using both ELISA and SNT methods. Adverse reactions were detected following primary and second injections in all animals vaccinated with Adjuvant B at different vaccine dosages. The copolymer Adjuvant A did not cause adverse reactions in cattle.
In the current study Montanide™ Gel-01 formulation was used, and this adjuvant has been shown to induce protective antibody titres with other inactivated viruses. In a study done by Alsaid [27], a 20 % Montanide™ Gel-01 formulation with inactivated RVFV (1,00E + 07 TCID50/dose) induced protective antibody titres after 7 days of primary inoculation, which lasted for 13 months. Recently, Hamdi [14], [15] published data on the evaluation of an inactivated LSDV vaccine in cattle using Montanide oil adjuvant from SEPPIC as a vaccine delivery system. Limited inflammation at the injection site was reported and the side effect was attributed to the use of the oil adjuvant. The disadvantages associated with traditional oil adjuvants include the induction of mild to severe inflammation at site of inoculation and the viscosity of the oil adjuvant can cause side effects such as granulomas and cysts [28]. In addition, evaluation of inactivated LSDV-Serbia field strain with adjuvant A and B indicated the importance of adjuvant selection with Adjuvant-B formulated vaccine resulting in reaction in vaccinated animals. Animals vaccinated copolymer Adjuvant A was safe for use in cattle. In this study Montanide™ Gel-01 was used as an adjuvant for formulation of the inactivated LSDV. Montanide™ Gel adjuvants have been reported to have a good safety profile and can be used in sensitive animals when compared to oil adjuvants [29]. The use of a suitable adjuvant for inactivated vaccine formulation is a very critical factor as it can help elicit components of cell-mediated or humoral immune response, thereby improving the vaccine efficacy [28], [30]. The inactivated LSDV vaccine formulated with Montanide™ Gel-01 promises a safer combination that is worthy of evaluation in target species. Furthermore Montanide™ Gel-01 is successfully used in various viral vaccines. Future research from this study should focus on evaluating LSDV vaccine in target species (bovine) for safety and immunogenicity to provide an alternative and safer vaccine option for immunization against LSD. Protection against virulent challenge and duration of immunity are critical components that needs to be evaluated in future studies.
5. Conclusions
The pre-clinical study of the inactivated LSDV vaccine in rabbits indicated that the higher dosed formulation (1,00E + 06 TCID50/dose) elicits a greater antibody response when compared to the lower dosed formulation (1,00E + 05 TCID50/dose) as estimated by SNT and ELISA. As a proof-of-concept study, it was shown that Montanide™ Gel-01 adjuvanted inactivated LSD vaccine was able to induce immunological response in rabbits.
Author contribution
Nobalanda Mokoena contributed to the conception of the work. Matome Selina Matsiela performed experiments and analysed the results. Nobalanda Mokoena and Leeann Naicker contributed in planning of experiments, analysis and interpretation of the results. Vusi Saul Dibakwane contributed in the formulation of the vaccines. Matome Selina Matsiela and Nobalanda Mokoena took the lead in writing and revising the manuscript. Thandeka Khoza and Nomfundo Ntombela provided feedback to the shaping of the research.
Funding
The Research was financially supported by National Research Foundation (NRF), grant 115969 and internally funded from Onderstepoort Biological Products Research budget.
Availability of data and materials
The datasets and relevant reagents used during the current study are available from the corresponding author following the legal process of the Onderstepoort Biologial Products policies on material distribution.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr L Naicker reports financial support was provided by National Research Foundation South Africa.
Data availability
Data will be made available on request.
References
- 1.Weiss K. Lumpy skin disease virus, in Cytomegaloviruses. In: Rinderpest Virus. Lumpy Skin Disease Virus. Springer; 1968, 111–131. 10.1007/978-3-662-39771-8_3
- 2.Babiuk S., Bowden T.R., Boyle D.B., Wallace D.B., Kitching R.P. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound Emerg Dis. 2008;55:263–272. doi: 10.1111/j.1865-1682.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 3.Coetzer J, Tustin RC. Infectious diseases of livestock, England: Oxford University Press, 2nd ed. Tuppurainen, E. Lumpy skin disease; 2004, 1268-1276.
- 4.Adedeji A.J., Akanbi O.B., Adole J.A., Chima N.C., Baje M. Outbreak of lumpy skin disease in a dairy farm in Keffi, Nasarawa State, Nigeria. Sokoto J Vet Sci. 2018;16(3):80. doi: 10.4314/sokjvs.v16i3.13. [DOI] [Google Scholar]
- 5.Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med Vet Entomol. 2003;17(3):294–300. doi: 10.1046/j.1365-2915.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Irons P.C., Tuppurainen E.S.M., Venter E.H. Excretion of lumpy skin disease virus in bull semen. Theriogenology. 2005;63(5):1290–1297. doi: 10.1016/j.theriogenology.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Authority EFS, et al. Lumpy skin disease: III. Data collection and analysis. EFSA J 2019; 17: e05638. https://doi.org/10.2903/j.efsa.2019.5638. [DOI] [PMC free article] [PubMed]
- 8.Authority EFS, et al., Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J 2020; 18: e06010. https://doi.org/10.2903/j.efsa.2018.5176. [DOI] [PMC free article] [PubMed]
- 9.Tuppurainen E., Alexandrov T., Beltrán-Alcrudo D. Lumpy skin disease field manual–A manual for veterinarians. FAO Animal Prod Health Manual. 2017;20:1–60. [Google Scholar]
- 10.Ben-Gera J., Klement E., Khinich E., Stram Y., Shpigel N.Y. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease–The results of a randomized controlled field study. Vaccine. 2015;33(38):4837–4842. doi: 10.1016/j.vaccine.2015.07.071. [DOI] [PubMed] [Google Scholar]
- 11.Davies F.G. Lumpy skin disease, an African capripox virus disease of cattle in Africa. Br Vet J. 1991;147(6):489–503. doi: 10.1016/0007-1935(91)90019-J. [DOI] [PubMed] [Google Scholar]
- 12.Teffera M., Babiuk S. Potential of using capripoxvirus vectored vaccines against arboviruses in sheep, goats and cattle. Front Vet Sci. 2019;6:450. doi: 10.3389/fvets.2019.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace D.B., Mather A., Kara P.D., Naicker L., Mokoena N.B., Pretorius A., et al. Protection of cattle elicited using a bivalent lumpy skin disease virus-vectored recombinant Rift Valley fever vaccine. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdi J., Boumart Z., Daouam S., El Arkam A., Bamouh Z., Jazouli M., et al. Development and evaluation of an inactivated lumpy skin disease vaccine for cattle. Vet Microbiol. 2020;245:108689. doi: 10.1016/j.vetmic.2020.108689. [DOI] [PubMed] [Google Scholar]
- 15.Wolff J., Moritz T., Schlottau K., Hoffmann D., Beer M., Hoffmann B. Development of a safe and highly efficient inactivated vaccine candidate against Lumpy Skin Disease virus. Vaccines. 2021;9(1):4. doi: 10.3390/vaccines9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaber G. ’50% end point calculation’. Archiv fur Experimentelle Pathologies und Pharmakologie. 1931;162:480–483. [Google Scholar]
- 17.Bahnemann H.G. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch Virol. 1975;47(1):47–56. doi: 10.1007/BF01315592. [DOI] [PubMed] [Google Scholar]
- 18.Teng Z., Kuang X., Wang J., Zhang X.i. Real-time cell analysis – A new method for dynamic, quantitative measurement of infectious viruses and antiserum neutralizing activity. J Virol Methods. 2013;193(2):364–370. doi: 10.1016/j.jviromet.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Frey H.-R., Liess B. Vermehrungskinetik und Verwendbarkeit eines stark zytopathogenen VD-MD-Virusstammes für diagnostische Untersuchungen mit der Mikrotiter-Methode. Zentralblatt für Veterinärmedizin Reihe B. 1971;18(1):61–71. [PubMed] [Google Scholar]
- 20.Muench H.R. A simple method of estimating 50 per cent end points. Am J Hyg. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 21.Ochwo S., VanderWaal K., Munsey A., Nkamwesiga J., Ndekezi C., Auma E., et al. Seroprevalence and risk factors for lumpy skin disease virus seropositivity in cattle in Uganda. BMC Veterinary Res. 2019;15(1) doi: 10.1186/s12917-019-1983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedeković T., Šimić I., Krešić N., Lojkić I. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transboundary Emerg Dis. 2018;65(2):491–496. doi: 10.1111/tbed.12730. [DOI] [PubMed] [Google Scholar]
- 23.Lojkić I., Simic I., Kresic N., Bedekovic T. Complete genome sequence of a lumpy skin disease virus strain isolated from the skin of a vaccinated animal. Genome Announc. 2018;6:e00482–e518. doi: 10.1128/genomeA.00482-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meeusen E.N.T., Walker J., Peters A., Pastoret P.-P., Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20(3):489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gethmann J., Hüttner K., Heyne H., Probst C., Ziller M., Beer M., et al. Comparative safety study of three inactivated BTV-8 vaccines in sheep and cattle under field conditions. Vaccine. 2009;27(31):4118–4126. doi: 10.1016/j.vaccine.2009.04.072. [DOI] [PubMed] [Google Scholar]
- 26.Awad M., et al. Trials for preparation of inactivated sheep pox vaccine using binary ethyleneimine. Egyptian J Immunol. 2003;10:67–72. [PubMed] [Google Scholar]
- 27.Alsaid S., et al. Evaluation of the cellular and humoral immune response of sheep vaccinated with inactivated Rift Valley Fever Vaccine Adjuvanted with Montanide gel 01TM. J Appl Veterinary Sci. 2020;5 [Google Scholar]
- 28.Ibrahim E.-S., Gamal W.M., Hassan A.I., Mahdy S.-D., Hegazy A.Z., Abdel-Atty M.M. Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine. Veterinary World. 2015;8(10):1189–1198. doi: 10.14202/vetworld.2015.1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker R., Deville S., Dupuis L., Bertrand F., Aucouturier J. Adjuvant formulation for veterinary vaccines: Montanide™ Gel safety profile. Procedia Vaccinol. 2009;1(1):140–147. doi: 10.1016/j.provac.2009.07.026. [DOI] [Google Scholar]
- 30.Lombard M., Pastoret P.-P., Moulin A.M. A brief history of vaccines and vaccination. Revue Scientifique et Technique-Office International des Epizooties. 2007;26(1):29–48. doi: 10.20506/rst.26.1.1724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and relevant reagents used during the current study are available from the corresponding author following the legal process of the Onderstepoort Biologial Products policies on material distribution.
Data will be made available on request.