Abstract
Background & Aims
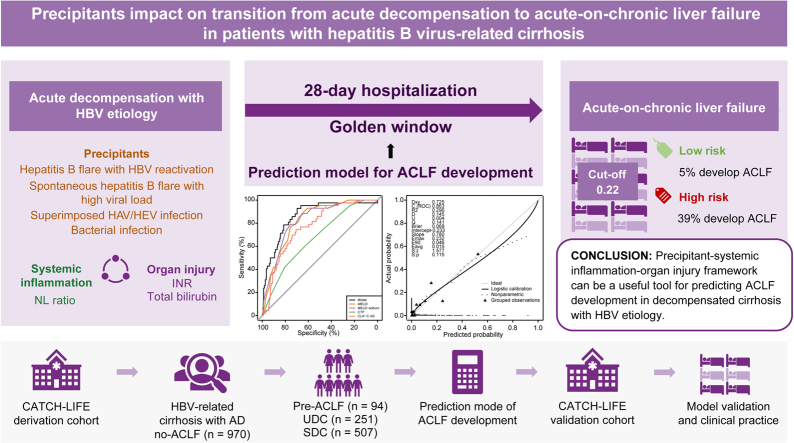
Pre-acute-on-chronic liver failure (ACLF) is a distinct intermediate stage between acute decompensation (AD) and ACLF. However, identifying patients with pre-ACLF and predicting progression from AD to ACLF is difficult. This study aimed to identify pre-ACLF within 28 days, and to develop and validate a prediction model for ACLF in patients with HBV-related decompensated cirrhosis.
Methods
In total, 1,736 patients with HBV-related cirrhosis and AD were enrolled from 2 large-scale, multicenter, prospective cohorts. ACLF occurrence within 28 days, readmission, and 3-month and 1-year outcomes were collected.
Results
Among 970 patients with AD without ACLF in the derivation cohort, the 94 (9.6%) patients with pre-ACLF had the highest 3-month and 1-year LT-free mortality (61.6% and 70.9%, respectively), which was comparable to those with ACLF at enrollment (57.1% and 67.1%); the 251 (25.9%) patients with unstable decompensated cirrhosis had mortality rates of 22.4% and 32.1%, respectively; while the 507 (57.9%) patients with stable decompensated cirrhosis had the best outcomes (1-year mortality rate of 2.6%). Through Cox proportional hazard regression, specific precipitants, including hepatitis B flare with HBV reactivation, spontaneous hepatitis B flare with high viral load, superimposed infection on HBV, and bacterial infection, were identified to be significantly associated with ACLF occurrence in the derivation cohort. A model that incorporated precipitants, indicators of systemic inflammation and organ injuries reached a high C-index of 0.90 and 0.86 in derivation and validation cohorts, respectively. The optimal cut-off value (0.22) differentiated high-risk and low-risk patients, with a negative predictive value of 0.95.
Conclusions
Three distinct clinical courses of patients with AD are validated in the HBV-etiology population. The precipitants significantly impact on AD-ACLF transition. A model developed by the precipitant–systemic inflammation–organ injury framework could be a useful tool for predicting ACLF occurrence.
Clinical trial number
NCT02457637 and NCT03641872.
Lay summary
It was previously shown that patients with decompensated cirrhosis could be stratified into 3 groups based on their short-term clinical prognoses. Herein, we showed that this stratification applies to patients who develop cirrhosis as a result of hepatitis B virus infection. We also developed a precipitant-based model (i.e. a model that incorporated information about the exact cause of decompensation) that could predict the likelihood of these patients developing a very severe liver disease called acute-on-chronic liver failure (or ACLF).
Keywords: acutely decompensated cirrhosis, acute-on-chronic liver failure, hepatitis B virus, precipitants, prediction model
Abbreviations: ACLF, acute-on-chronic liver failure; AD, acute decompensation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CATCH-LIFE study, Chinese AcuTe-On-Chronic LIver FailurE Study; CLIF, Chronic Liver Failure; CLIF-C AD, CLIF consortium acute decompensation score; CRP, C-reactive protein; EASL, European Association for the Study of the Liver; HR, hazard ratio; iMELD, integrated MELD; INR, international normalized ratio; LT, liver transplantation; MELD, model for end-stage liver disease; OFs, organ failures; NL, neutrophil-lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value; PVT, portal vein thrombosis; SDC, stable decompensated cirrhosis; UDC, unstable decompensated cirrhosis; WBC, white blood cell
Graphical abstract
Highlights
-
•
Three distinct clinical trajectories were validated in patients with HBV-related cirrhosis.
-
•
Specific precipitants were associated with an increased risk of developing ACLF.
-
•
Models incorporating precipitants performed better for the prediction of ACLF development.
Introduction
Over the past decades, cirrhosis has become a progressively pressing concern because of the constantly increasing death and disability it causes, as well as its increasing global prevalence.1,2 Decompensation, which is defined as the presence of variceal bleeding, ascites, encephalopathy or jaundice, marks a prognostic watershed in the natural history of cirrhosis, leading to a dramatic decline in median survival from more than 12 years in compensated cirrhosis to approximately 2–4 years in decompensated cirrhosis.3
One of the major clinical challenges in the management of patients with decompensated cirrhosis is that patients with acute decompensation (AD) (usually at first decompensation) are susceptible to acute-on-chronic liver failure (ACLF) which is defined by organ failures (OFs) and accounts for approximately 1 million deaths per year worldwide.4 The high short-term mortality of patients with ACLF and the lack of specific drugs or therapies, apart from liver transplantation (LT), has led to the concept of a ‘golden window’ for early intervention at the AD-ACLF transition. Thus, the first essential step is identifying patients who are more likely to develop ACLF and predicting the progression from AD to ACLF.
Recently, the PREDICT study delineated the clinical courses of patients with AD without ACLF on admission through the follow-up of patients for OF and readmission events within 3 months.5 A distinct pre-ACLF state with a high grade of systemic inflammation, in contrast to unstable decompensated cirrhosis (UDC) and stable decompensated cirrhosis (SDC), was clearly identified but failed to be accurately predicted.
It has been well recognized that HBV-related ACLF (HBV-ACLF) displayed specific phenotypes and prognoses that are different from those of patients with ACLF of another etiology.[6], [7], [8] For instance, HBV exacerbation is the most frequent potential precipitant during the evolving disease course from HBV-related chronic liver disease to ACLF,9,10 whereas bacterial infection and severe acute alcoholic hepatitis are frequent in precipitating Western ACLF.11 It seems that HBV-ACLF is more homogenous than ACLF described in the CANONIC study and may therefore be more predictable. Actually, the COSSH-II score could predict the short-term outcomes of patients with HBV-ACLF with a concordance index (C-index) of >0.80, which was not achieved by prognostic scores developed in Western ACLF.9 Therefore, it is likely that HBV etiology is a feasible clinical setting to predict the AD-ACLF transition.
Due to scant information on the current topic, we identified 1,736 patients with HBV-related cirrhosis and AD from 2 large-scale prospective observational cohorts. Our aims were to verify the differential clinical trajectories in HBV-related cirrhosis and AD, identify risk factors associated with ACLF development, and finally, to develop and validate an AD-ACLF prediction model to differentiate high-risk and low-risk patients on admission in clinical practice.
Patients and methods
Patients
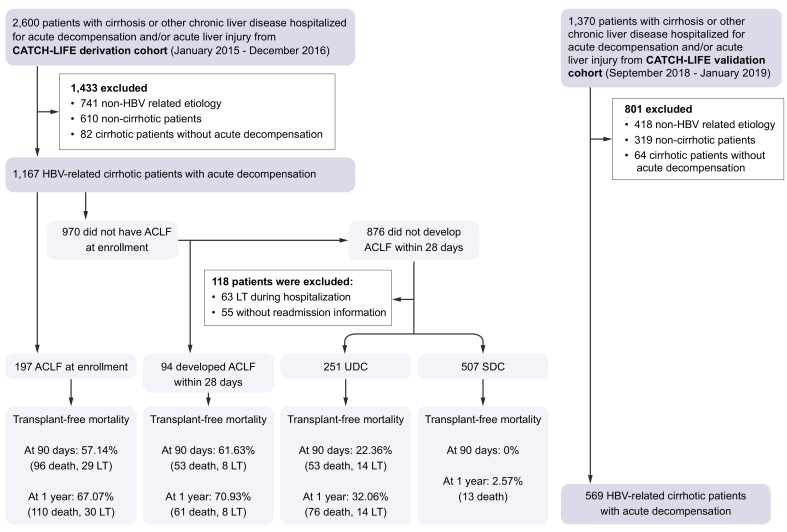
Data were collected from 2 large, multicenter, prospective, observational cohorts from the Chinese Acute on Chronic Liver Failure (CATCH-LIFE) study, which was performed in areas where HBV is endemic; the derivation cohort was based on data from January 2015 to December 2016 (NCT02457637) and the validation cohort from September 2018 to March 2019 (NCT03641872). The design and baseline characteristics of these cohorts have been reported elsewhere.12,13 Briefly, 2,600 and 1,370 patients with cirrhosis or other chronic liver diseases hospitalized for AD and/or acute liver injury were consecutively included, respectively. The study protocol and informed consent form were approved by the Ethics Committee of Renji Hospital (the lead center of the CATCH-LIFE study), Shanghai Jiaotong University School of Medicine [Approval No. (2014) 148 k and (2016) 142 k]. Appropriate approvals were obtained from all participants or their legal surrogates before recruitment. All patients hospitalized for AD of cirrhosis were eligible for the analysis.
Patients with HBV-related etiology and cirrhosis with AD were selected. HBV-related etiology was defined as seropositive status for the HBV surface antigen at 6 months or beyond.14 Cirrhosis was diagnosed based on a composite of clinical signs and findings provided by imaging examination or signs of portal hypertension on endoscopy.15 AD upon hospitalization was defined as the onset of overt ascites, hepatic encephalopathy, variceal bleeding, bacterial infection or jaundice (total bilirubin [TB] >5 mg/dl) or their combination within 1 month before enrolment.16,17 ACLF was diagnosed based on the EASL-CLIF criteria (supplementary information).18 Detailed management of patients was provided in the supplementary information.
Pre-ACLF was defined as patients who developed ACLF within 28 days after enrolment. Patients who did not develop ACLF 28 days after enrolment were followed up for 3 months for readmission; those who died or underwent LT during hospitalization were censored. UDC was defined as at least one readmission during the 3-month follow up after discharge, and SDC was defined as patients who experienced no readmission during the 3-month follow up. Hepatitis B flare was defined as an upsurge of alanine aminotransferase (ALT) by more than 5 times the upper limit of the normal value, which is a modification of the definition by AASLD.14 HBV reactivation in the setting of the study was caused by viral resistance and withdrawal of nucleoside/nucleotide analogues (NUCs), and it was defined as an increase in HBV-DNA by >2log IU/ml in patients who had ever achieved a serum HBV-DNA of <20 IU/ml through NUC therapy. For patients without a history of NUC therapy, hepatitis B flare with HBV-DNA >106 was considered high load, and hepatitis B flare with HBV-DNA <106 was considered a low load.
Superimposed HAV/HEV infection on HBV was defined by the presence of anti-HAV/HEV serum IgM and IgG. Hepatotoxic drug exposure was defined as exposure within 3 months before admission. Active alcohol intake was defined as more than 2 standard drinks per day for women (approximately 40 g of alcohol) and 3 standard drinks per day for men (approximately 50-60 g of alcohol).19 Surgery was defined as surgery within the past 3 months. Portal vein thrombosis (PVT) was diagnosed based on imaging examination at present or previous admission.16
Data collection
We collected data on demographics, decompensation and precipitation events on the first day of hospitalization. Findings of clinical manifestations, physical examinations, and laboratory measurements were collected at enrolment and 4, 7, 14, 21, and 28 days, respectively, including the day before discharge and 24 hours prior to death or LT. Data regarding cirrhosis severity were collected, with particular attention given to liver function scores and OFs. Prognostic information, including the 28-day, 90-day, and 1-year outcomes (death and LT), were recorded. Patients who were lost to follow-up were censored at last follow-up date. We also recorded the occurrence of ACLF and readmission of patients with AD without ACLF at enrolment during the 28-day and 3-month follow-ups, respectively. All-cause readmission details were obtained through scheduled post-discharge telephone calls and/or acquired from the outpatient and inpatients records of hospitals. Patients discharged to a hospice or transferred to other care facilities were not considered a readmission.
Development and validation of a model for predicting ACLF development
To predict AD-ACLF transition during the 28-day hospitalization, a multivariable prediction model was fitted using derivation cohort. No data were missing in categorical variables. The distributions of the missing data for each variable in the derivation cohort are shown in Fig. S1. We supposed that these missing values could be considered at random and performed a multiple imputation based on a mixed model including all potential predictors significantly associated with pre-ACLF in the univariate analysis. Independent predictors were screened by a univariate Cox proportional hazard regression, and only variables with p <0.1 were introduced into the multivariable regression analysis. The collinearity for continuous variables was assessed through a correlation analysis. One of the two variables was removed based on clinical relevance if the magnitude of the correlation coefficient was >0.6. Competing risk regression was performed to isolate the effect of risk factors on ACLF development from that of LT. A multivariate logistic regression model was utilized based on Akaike information criterion to obtain the probability of each observation. The model performance was assessed by testing discrimination and calibration. Model discrimination was assessed by the C-index, and calibration was evaluated by plotting predicted and observed events. The model was internally validated within the derivation cohort, followed by external testing in the validation cohort by calculating the prediction score of the formula.
Statistical analysis
Continuous variables were represented as mean ± SD or median (IQR) in congruence with their distribution, while categorical variables were expressed as counts and percentages. The Student’s t test or Mann–Whitney U test was used to compare continuous variables depending on their distribution, and the chi-square test or Fisher’s exact test was used for categorical parameters. All non-normal data were transformed to natural logarithms for the regression analysis. Survival probabilities were estimated using the Kaplan–Meier estimate, and the overall or pairwise comparisons of groups were conducted using the log-rank test. The C-index of the new model was compared with generic prognostic scores, including the integrated model for end-stage liver disease (iMELD) score, model for end-stage liver disease-sodium (MELD-sodium) score, Chronic Liver Failure Consortium acute decompensation score (CLIF-C AD), and the Child-Pugh score, by using the Delong’s test. A 2-tailed p <0.05 was considered statistically significant in all statistical analyses. All statistical analyses were conducted with R version 4.0.2 (http://www.r-project.org).
Results
Differential clinical courses of hospitalized patients with HBV-related cirrhosis and AD
In total, 1,167 eligible patients with HBV-related cirrhosis and AD were finally analyzed from 2,600 patients screened in the derivation cohort (Fig. 1). Patients were more often male (853, 81.1%), with a median age of 49 years and a median MELD score of 19 (IQR 13–26). All patients had 90-day outcomes while 4 patients were lost to follow-up at 1 year. Comparison between ACLF, pre-ACLF, UDC, and SDC with regards to demographic, clinical characteristics and outcomes is presented in Table 1. In total, 197 patients were diagnosed with ACLF at enrollment according to EASL-CLIF criteria. Among no-ACLF patients, 94 pre-ACLF patients had high LT-free mortality rates at 3-month and 1-year follow-ups (61.63% and 70.93%, respectively), 251 patients with UDC showed lower mortality rates (22.36% and 32.06%, respectively), whereas 507 patients with SDC showed very low mortality rates (0.00% and 2.57%, respectively).
Fig. 1.
Patient screening flow chart according to diagnosis of ACLF and 3-month readmission in the derivation and validation cohort.
ACLF, acute-on-chronic liver failure; LT, liver transplantation.
Table 1.
Baseline characteristics and outcomes in patients with ACLF, pre-ACLF, UDC and SDC.
| Characteristics | ACLF (n = 197) |
Pre-ACLF (n = 94) |
UDC (n = 251) |
SDC (n = 507) |
p∗ | p# | p§ |
|---|---|---|---|---|---|---|---|
| Male, n (%) | 176 (89.3) | 75 (79.8) | 211 (84.1) | 391 (77.1) | 0.042 | 0.436 | 0.033 |
| Age (years), (median (IQR)) | 47.6 [41.4, 54.3] | 49.6 [41.9, 57.59] | 50.87 [44.4, 57.6] | 47.8 [42.2, 56.5] | 0.252 | 0.374 | 0.011 |
| Previous decompensation, n (%) |
71 (23.1) |
39 (28.5) |
196 (51.6) |
251 (34.5) |
0.003 |
0.005 |
<0.001 |
| Decompensation, n (%) | |||||||
| HE | <0.001 | 0.129 | 0.255 | ||||
| Grade 0 | 141 (71.6) | 92 (97.9) | 235 (93.6) | 485 (95.7) | |||
| Grade 1 | 13 (6.6) | 0 (0.0) | 9 (3.6) | 7 (1.4) | |||
| Grade 2 | 30 (15.2) | 1 (1.1) | 6 (2.4) | 12 (2.4) | |||
| Grade 3 | 11 (5.6) | 0 (0.0) | 1 (0.4) | 3 (0.6) | |||
| Grade 4 | 2 (1.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | |||
| Bacterial infection | 96 (48.7) | 54 (57.4) | 69 (27.5) | 121 (23.9) | 0.206 | <0.001 | 0.320 |
| Variceal bleeding | 10 (5.1) | 8 (8.5) | 53 (21.1) | 86 (17.0) | 0.380 | 0.010 | 0.197 |
| Ascites |
154 (78.2) |
77 (81.9) |
172 (68.5) |
351 (69.2) |
0.560 |
0.020 |
0.909 |
| Laboratory data, median (IQRs) | |||||||
| TB (mg/dl) | 24.5 [17.3, 31.0] | 18.2 [8.6, 26.9] | 3.4 [1.4, 11.2] | 3.4 [1.5, 10.6] | 0.001 | <0.001 | 0.748 |
| INR | 2.8 [2.3, 3.4] | 2.06 [1.8, 2.3] | 1.5 [1.3, 1.8] | 1.5 [1.3, 1.7] | <0.001 | <0.001 | 0.013 |
| Cr (mg/dl) | 0.9 [0.7, 1.6] | 0.8 [07, 1.0] | 0.8 [0.7, 1.0] | 0.8 [0.7, 0.9] | <0.001 | 0.601 | 0.138 |
| BUN (mEq/L) | 5.5 [3.6, 11.1] | 5.1 [3.7, 6.9] | 5.0 [3.7, 7.0] | 4.6 [3.6, 6.3] | 0.062 | 0.576 | 0.051 |
| ALT (U/L) | 172.5 [57.0, 595.6] | 200.5 [60.2, 429.9] | 42.7 [23.6, 117.0] | 61.90 [29.0, 194.5] | 0.769 | <0.001 | <0.001 |
| AST (U/L) | 191.0 [89.0, 470.7] | 197.5 [97.5, 470.0] | 63.1 [34.6, 151.9] | 70.0 [40.0, 165.6] | 0.737 | <0.001 | 0.105 |
| Hemoglobin (g/L) | 117.0 [104.0, 131.0] | 118.0 [100.0, 134.8] | 109.0 [91.5, 126.5] | 113.5 [93.0, 129.0] | 0.988 | 0.006 | 0.112 |
| WBC count (109/L) | 7.2 [5.0, 10.6] | 6.1 [4.4, 8.1] | 4.2 [3.0, 6.4] | 4.4 [3.0, 6.2] | 0.003 | <0.001 | 0.970 |
| PLT (109/L) | 81.0 [50.0, 112.0] | 73.0 [47.3, 103.5] | 62.0 [42.0, 95.5] | 72.0 [51.0, 108.0] | 0.544 | 0.056 | 0.001 |
| NL ratio | 4.84 [3.01, 7.80] | 3.60 [2.35, 5.89] | 2.58 [1.68, 4.48] | 2.3 [1.5, 3.5] | 0.004 | <0.001 | 0.009 |
| Sodium (mEq/L) | 135.0 [131.0, 138.0] | 136.2 [131.7, 139.3] | 138.1 [134.6, 140.6] | 138.4 [136.0, 141.0] | 0.098 | 0.002 | 0.087 |
| Albumin (g/L) | 30.0 [26.7, 33.6] | 30.3 [27.7, 34.0] | 30.2 [25.7, 33.5] | 30.8 [26.4, 34.3] | 0.428 | 0.293 | 0.138 |
| Prealbumin (mg/L) | 41.9 [26.0, 56.3] | 47.4 [21.3, 58.5] | 59.5 [38.0, 94.5] | 62.0 [39.0, 89.4] | 0.792 | <0.001 | 0.936 |
| C-reaction protein (mg/L) | 12.5 [6.7, 19.6] | 18.0 [11.5, 28.5] | 9.4 [2.6, 20.3] | 7.4 [3.1, 15.3] | 0.005 | <0.001 | 0.313 |
| Procalcitonin (ng/ml) |
5.0 [3.1, 6.0] |
5.2 [3.7, 7.2] |
3.7 [2.0, 5.7] |
4.3 [2.7, 6.1] |
0.689 |
<0.001 |
0.842 |
| HBV parameters | |||||||
| HBV-DNA (log10 IU/ml) | 5.0 [3.1, 6.0] | 5.2 [3.7, 7.2] | 3.7 [2.0, 5.7] | 4.3 [2.7, 6.1] | 0.013 | <0.001 | 0.031 |
| Antiviral treatment history, n (%) | 130 (66.0) | 57 (60.6) | 135 (53.8) | 331 (65.3) | 0.560 | 0.066 | 0.001 |
| Antiviral naïve | 11 (5.6) | 9 (9.6) | 11 (4.4) | 35 (6.9) | |||
| <6 months | 55 (27.9) | 27 (28.7) | 104 (41.4) | 141 (27.8) | |||
| ≥6 months |
|||||||
| Severity scores, (median [IQR]) | |||||||
| MELD | 31.0 [29.0, 35.0] | 26.00 [23.0, 28.0] | 17.0 [12.0, 22.8] | 16.0 [12.0, 21.0] | <0.001 | <0.001 | 0.234 |
| iMELD | 51.0 [47.0, 57.0] | 45.0 [41.0, 48.8] | 37.0 [30.0, 42.8] | 34.0 [29.0, 39.0] | <0.001 | <0.001 | 0.002 |
| MELD-sodium | 32.0 [30.0, 36.0] | 27.0 [24.25, 30.0] | 19.0 [13.0, 25.0] | 17.0 [12.0, 23.0] | <0.001 | <0.001 | 0.041 |
| Child-Pugh | 12.0 [11.0, 13.0] | 11.0 [10.0, 12.0] | 9.0 [8.0, 11.0] | 9.0 [8.0, 10.0] | <0.001 | <0.001 | 0.462 |
| CLIF-C AD | 61.7 [56.1, 69.7] | 53.8 [47.4, 59.4] | 44.8 [39.8, 52.0] | 43.5 [38.2, 49.3] | <0.001 | <0.001 | 0.006 |
| CLIF-C ACLF | 45.3 [40.9, 49.3] | 39.0 [35.0, 42.0] | 32.1 [28.0, 38.3] | 30.9 [26.8, 35.4] | <0.001 | <0.001 | 0.006 |
| CLIF SOFA | 8.0 [7.0, 9.0] | 7.0 [6.0, 7.0] | 5.00 [3.0, 6.0] | 5.0 [3.0, 6.0] | <0.001 | <0.001 | 0.047 |
| CLIF OF |
10.0 [10.0, 11.0] |
9.00 [8.0, 9.0] |
7.0 [6.0, 8.0] |
6.0 [6.0, 8.0] |
<0.001 |
<0.001 |
0.085 |
| Organ failure | |||||||
| Liver, n (%) | 177 (89.8) | 59 (62.8) | 60 (24.0) | 110 (21.7) | <0.001 | <0.001 | 0.534 |
| Coagulation, n (%) | 139 (70.6) | 9 (9.6) | 5 (2.0) | 5 (1.0) | <0.001 | 0.005 | 0.412 |
| Kidney, n (%) | 38 (19.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.001 | - | - |
| Cerebral, n (%) | 13 (6.6) | 1 (1.1) | 1 (0.4) | 3 (0.6) | 0.077 | 1.000 | 1.000 |
| Circulation, n (%) | 6 (3.0) | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0.205 | 1.000 | 1.000 |
| Lungs, n (%) |
2 (1.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0.825 |
- |
- |
| LT-free mortality (%) | |||||||
| 28-day | 64/175 (37.1%) | 31/90 (34.4%) | 13/243 (5.7%) | 0 (0.0) | 0.145 | <0.001 | <0.001 |
| 90-day | 96/168 (56.0%) | 55/88 (60.3%) | 53/184 (19.6%) | 0 (0.0) | 0.260 | <0.001 | <0.001 |
| 1-year | 110/165 (65.1%) | 61/86 (68.8%) | 76/237 (29.8%) | 13/493 (2.1%) | 0.209 | <0.001 | <0.001 |
ACLF, acute-on-chronic liver failure; ALT alanine aminotransferase; BUN, blood urea nitrogen; CLIF-OF, Chronic Liver Failure-organ failure; CLIF-SOFA, Chronic Liver Failure-sequential organ failure assessment; Cr, creatinine; HE, hepatic encephalopathy; iMELD, integrated MELD; INR, International normalised ratio; LT, liver transplantation; PLT, platelet count; NL ratio, neutrophil-lymphocyte ratio; MELD, model of end-stage liver disease; SDC, stable decompensated cirrhosis; TB, total bilirubin; UDC, unstable decompensated cirrhosis; WBC, white blood cell.
p value for comparisons between patients with ACLF and pre-ACLF.
p value for comparisons between patients with pre-ACLF and UDC.
p value for comparisons between patients with UDC and SDC.
Consistent with the PREDICT study, patients with pre-ACLF and ACLF shared similar 90-day (61.63% vs. 57.14%) and 1-year mortality rates (70.93% vs. 67.07%) (Fig. S2), and they initially displayed higher levels of serum ALT, aspartate aminotransferase (AST), bilirubin, and international normalized ratio (INR) and higher grades of systemic inflammation as indicated by the white blood cell (WBC) count, neutrophil-lymphocyte ratio (NL ratio), and C-reactive protein (CRP) than patients with UDC. Further analysis on bacterial infection types indicated that pneumonia, spontaneous bacterial peritonitis and bacteremia were high prevalence in patients with ACLF and pre-ACLF (Table S1). In addition, serum HBV-DNA was significantly higher in the pre-ACLF group, suggesting an involvement of the HBV-specific mechanism in the progression from pre-ACLF to ACLF. The risk of ACLF development appeared to peak within the first 4 days after enrollment and gradually decline with hospitalization prolongation (Fig. S3). The evolving disease course of ACLF development was exhibited by deteriorating parameters related to multiple intrahepatic and extrahepatic systems, including coagulation dysfunction, renal dysfunction, hematological disorder, and electrolyte disturbance (Table S2).
Compared with patients with pre-ACLF, patients in the UDC group represented significantly lower inflammatory parameters, including WBC count, CRP, procalcitonin, and NL ratio. However, they exhibited a higher degree of portal hypertension, as shown by frequent episodes of variceal bleeding (21.5% vs. 8.5%, p <0.001) and lower platelet counts (62.00 [42.00, 95.50] vs. 73.00 [47.25, 103.50] 109/L, p = 0.003). Patients in the SDC group were younger, less frequently had previous decompensations, and had more stable liver and renal function than those in the UDC group, with a very low 1-year mortality rate. Overall, our findings recapitulated the 3 distinct clinical trajectories in patients with HBV-related cirrhosis and AD.
Impacts of precipitants on AD-ACLF transition
We next explored the risk factors associated with ACLF development in patients with HBV-related cirrhosis and AD. Previous studies have demonstrated that the patterns of precipitants determined the clinical phenotypes of AD and ACLF. Therefore, we hypothesized that the precipitant type may affect AD-ACLF transition. Patients with pre-ACLF were more likely to be precipitated by hepatitis B flare with HBV reactivation, spontaneous hepatitis B flare with high HBV-DNA load and superimposed infection on HBV when compared with those without progression to ACLF (Table S3). In patients with hepatitis B flare, high viral load predisposed patients to develop ACLF, and HBV-DNA load <2,000 IU/ml was associated with a lower the risk of ACLF development (Fig. S4). In addition, we analyzed the OFs and grade of ACLF at enrollment by precipitants. The prevalence of precipitant events was not significantly different in ACLF grade 1, 2 and 3 (Table S4). As for OFs, spontaneous hepatitis B flare with high HBV-DNA load was more frequent in liver and coagulation failure, while variceal bleeding was significantly higher in extrahepatic OF (Table S5). Also, patients with ACLF precipitated by variceal bleeding had improved ACLF grades at day 4 and day 7, probably due to effective immediate intervention (Table S6). For patients with AD without ACLF, ACLF development was also more prone to present as liver and coagulation failure (Table S7).
Risk factors for ACLF development
Among 970 patients with AD without ACLF, 94 developed ACLF during hospitalization, while 876 did not. Through univariate Cox analysis, 15 variables including 3 HBV-specific precipitants, bacterial infection, and other laboratory parameters were screened as potential risk factors for ACLF development (p <0.1) (Table S8). Neutrophil count was dropped due to high correlation with WBC count and NL ratio (0.9 and 0.62, respectively). The multivariate regression revealed that hepatitis B flare with HBV reactivation (hazard ratio [HR] 2.39; 95% CI 1.34–4.26; p = 0.003), spontaneous hepatitis B flare with high HBV-DNA load (HR 2.09; 95% CI 1.10–3.97; p = 0.024), superimposed infection on HBV (HR 4.19; 95% CI 1.85–9.46; p = 0.001), bacterial infection (HR 3.59; 95% CI 1.31–9.84; p = 0.013) and laboratory tests including ln (TB) (mg/dl) (HR 2.68; 95% CI 1.82–3.94; p <0.001), ln (INR) (HR 38.72; 95% CI 13.92–107.71; p <0.001), and ln (NL ratio) (HR 1.69; 95% CI 1.16–2.45; p = 0.006) were independently associated with ACLF development (Table 2). The effect of these precipitants on ACLF development was shown to be independent of each other by an interaction analysis (Table S9). We further calculated the subhazard risk for ACLF development while using LT as a competing event and the competing risk analysis confirmed the risk factors identified in the prior analysis (Table S10). Collectively, our findings highlight the impact of HBV-specific and non-specific precipitants in driving the AD-ACLF transition.
Table 2.
The multivariate regression for development of ACLF in patients with HBV-related decompensated cirrhosis in the derivation cohort.
| Characteristics | HR (95% CI) | p value |
|---|---|---|
| Demographic data | ||
| ln (Age) | 2.71 (0.89-5.86) | 0.074 |
| Precipitant events | ||
| Hepatitis B flare with HBV reactivation | 2.39 (1.34-4.26) | 0.003 |
| Spontaneous hepatitis B flare with high HBV-DNA load | 2.09 (1.10-3.97) | 0.024 |
| Superimposed infection on HBV | 4.19 (1.85-9.46) | 0.001 |
| Hepatotoxic drugs | 1.60 (0.73-3.51) | 0.245 |
| Bacterial infection | 3.59 (1.31-9.84) | 0.013 |
| Variceal bleeding | 2.18 (0.85-5.57) | 0.103 |
| Disease severity parameter | ||
| ln (TB) | 2.68 (1.82-3.94) | <0.001 |
| ln (INR) | 38.72 (13.92-107.71) | <0.001 |
| ln (Hemoglobin) | 0.79 (0.23-2.68) | 0.706 |
| ln (Sodium) | 0.39 (0-165.7) | 0.759 |
| Ascites | 2.27 (0.84-6.17) | 0.108 |
| AD number | 0.34 (0.05-2.16) | 0.252 |
| Systemic inflammatory | ||
| ln (WBC count) | 0.67 (0.42-1.07) | 0.096 |
| ln (NL ratio) | 1.69 (1.16-2.45) | 0.006 |
HR, hazard ratio; INR, International normalized ratio; NL ratio, neutrophil-lymphocyte ratio; TB, total bilirubin; WBC, white blood cell.
Developing a model to predict HBV-ACLF development
Using a logistic regression approach, a model incorporating 7 predictors was established to predict the probability of ACLF development. Predictors were composed of 3 intrahepatic precipitants (hepatitis B flare with HBV reactivation, spontaneous hepatitis B flare with high HBV-DNA load, and superimposed infection on HBV), one extrahepatic precipitant (bacterial infection), 2 disease severity parameters [ln (TB) and ln (INR)], and 1 systematic inflammatory parameter [ln (NL ratio)]. Their coefficients were used as a relative weight to compute the corresponding score (Table S11). The formula used for calculating the prediction score was as follows:
Score = −7.71 + 1.38 ∗ (Hepatitis B flare with HBV reactivation) + 0.74 ∗ (Spontaneous hepatitis B flare with high HBV load) + 1.50∗ (Superimposed infection on HBV) + 0.91 ∗ (Bacterial infection) + 0.81 ∗ ln (TB) + 4.17 ∗ ln (INR) + 0.63 ∗ ln (NL ratio). The probability of ACLF development equals to logit (Score) and dropped between 0% and 100%. The relative importance of predictors in the derivation model is shown in Fig. S5.
Performance of the new prognostic score
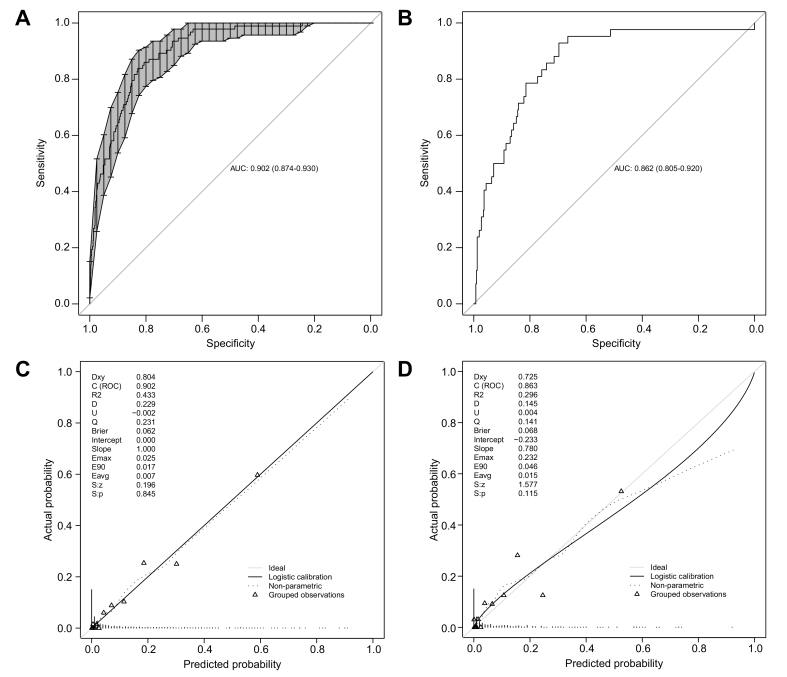
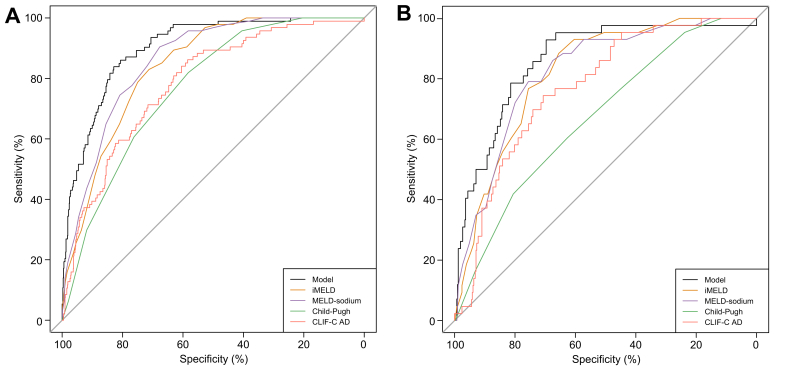
The current model achieved a high discriminative performance, as shown by a high C-index (0.902, 95% CI 0.874−0.930) (Fig. 2A). Moreover, the superiority of the current model was demonstrated by a significant improvement of the C-index over 4 other generic scores (CLIF-C AD: 0.783, p <0.001; iMELD: 0.804 p <0.001; MELD-Na: 0.859, p <0.001; Child-Pugh: 0.783, p <0.001; Fig. 3, Table 3). The calibration plot showed a good agreement between the predicted and actual probabilities in the derivation cohort (intercept: 0.00, slope: 1.00, Brier: 1.062) (Fig. 2C).
Fig. 2.
The discrimination and calibration plot of the ACLF development prediction model.
(A) ROC curve (solid line) and AUC (95%CI) (shadow area) of the model predicting ACLF development in derivation cohort; (B) ROC curve and AUC (95% CI) of the model predicting ACLF development in the validation cohort; (C) Calibration plot in the derivation cohort; (D) Calibration plot in the validation cohort. ACLF, acute-on-chronic liver failure.
Fig. 3.
Predictive ability comparison between ACLF development predicting model and other prognostic scores.
(A) The ROC curves of the new model and CLIF-C AD, iMELD, MELD-sodium, and Child-Pugh score comparison in the derivation cohort. (B) The ROC curves of the new model and CLIF-C AD, iMELD, MELD-sodium, and Child-Pugh score comparison in the validation cohort. ACLF, acute-on-chronic liver failure; AD, acute decompensation; CLIF-C AD, CLIF consortium acute decompensation score; iMELD, integrated MELD; MELD, model for end-stage liver disease.
Table 3.
Predictive performance of models for ACLF development in patients with HBV-related decompensated cirrhosis in the derivation cohort and validation cohorts.
| Derivation cohort (n = 970) |
Validation cohort (n = 458) |
|||
|---|---|---|---|---|
| C-index (95% CI) | p value | C-index (95% CI) | p value | |
| Model | 0.902 (0.874-0.930) | 0.862 (0.805-0.920) | ||
| iMELD | 0.840 (0.807-0.873) | <0.001 | 0.821 (0.767-0.876) | 0.033 |
| MELD-sodium | 0.859 (0.828-0.891) | <0.001 | 0.823 (0.764-0.882) | 0.027 |
| Child-Pugh | 0.769 (0.727-0.810) | <0.001 | 0.762 (0.594-0.750) | <0.001 |
| CLIF-C AD | 0.783 (0.737-0.829) | <0.001 | 0.771 (0.707-0.835) | <0.001 |
ACLF, acute-on-chronic liver failure; AD, acute decompensation; CLIF-C AD, CLIF consortium acute decompensation score; iMELD, integrated MELD; MELD, model for end-stage liver disease.
Validation of the HBV-ACLF prediction model
In the validation group, of 458 patients with HBV-related AD without ACLF, 43 developed ACLF during hospitalization (Table S12). The outcomes and prevalence of pre-ACLF were similar in the derivation and validation cohorts (Table S13). The C-index of the model exceeded 0.85 in the validation cohort (0.862, 95% CI 0.805−0.920) (Fig. 2B) and was superior to 4 other generic scores (CLIF-C AD, iMELD, MELD-Na, and Child-Pugh; Fig. 3, Table 3). The calibration plot showed a good agreement between the predicted and actual probabilities in the validation (intercept: −0.230, slope: 0.78, Brier: 1.083) cohorts (Fig. 2D). Additionally, the C-indexes of the model for 28-day, 3-month and 1-year LT-free mortality were 0.763, 0.781 and 0.702 in the validation cohort, respectively (Table S14). Collectively, the model to predict HBV-ACLF occurrence was proven to be statistically robust.
Utility of prediction model to differentiate patients with high risk of developing ACLF from those with low risk
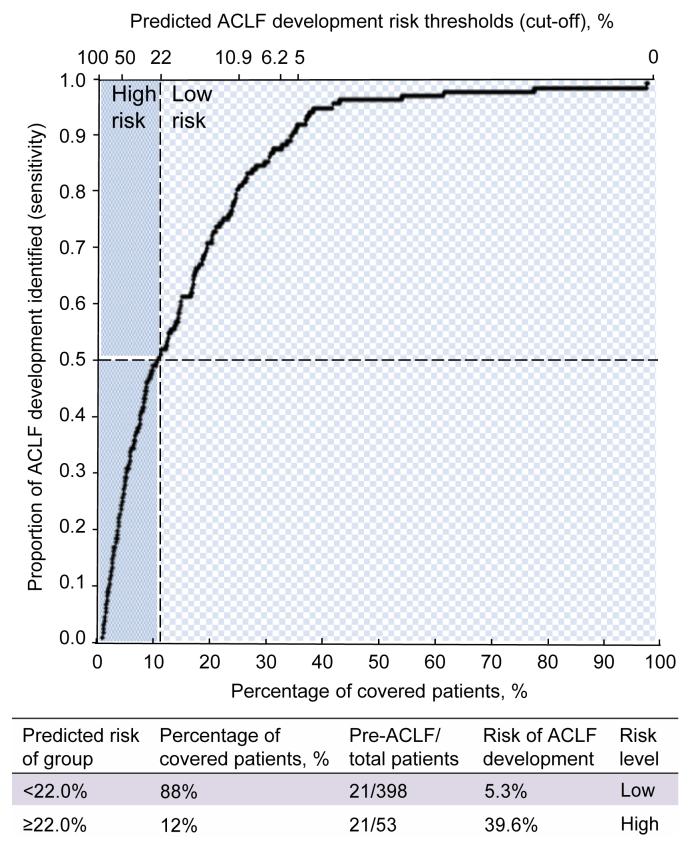
Finally, we stratify patients with different degrees of risk by an optimal cut-off threshold (0.22) based on 90% specificity in the derivation cohort. The negative predictive value (NPV) and positive predictive value (PPV) were 0.95 and 0.4, respectively. In the validation cohort, this cut-off value could facilitate specialists to assign approximately 88% of the patients to the low-risk group, and only 5% of the patients in the low-risk group developed ACLF (Fig. 4). By contrast, 39% of patients in the high-risk group developed ACLF, representing a >7-fold increased risk compared to those in the low-risk group. Overall, applying an optimal cut-off value of the model score simply differentiated patients at a high risk of developing ACLF from those with a low likelihood.
Fig. 4.
Differentiating patients at a high risk and low risk of ACLF development with the selected cut-off in the validation cohort.
The cut-off value of 0.22 covers 12% of patients as the high-risk group and 88% as the low-risk group, with a sensitivity of 0.5 and specificity of 0.92. The risks of ACLF development in the high- and low-risk groups were 39.6% and 5.3%, respectively. ACLF, acute-on-chronic liver failure.
Discussion
Our study validated 3 clinical courses with differential prognosis proposed by the PREDICT study in patients with HBV-related acutely decompensated cirrhosis by using a longitudinal prospective, multicenter cohort. We identified HBV-specific and non-specific precipitants that were associated with ACLF development. More importantly, a precipitant-based model was developed and validated externally; this model could have high clinical significance for predicting ACLF occurrence and initiating early interventions.
According to the study design in our protocol, in accordance with the CANONIC study and other studies,18,20,21 pre-ACLF were classified as patients who developed the condition within 28 days after enrollment. Among all patients with AD without ACLF at enrollment, 9.6% developed ACLF during hospitalization within 28 days, compared with 20.3% in the PREDICT study who developed ACLF within 3 months. The observed difference in the rates of AD-ACLF progression likely cannot be completely explained by the observed period as the majority of ACLF development occurred within 2 weeks, and a gradual time-dependent decline in the progression from AD to ACLF was observed. Moreover, the 3 subtypes, namely pre-ACLF, UDC, and SDC, had 3-month mortality rates comparable with their counterparts in the PREDICT study (our study vs. PREDICT: pre-ACLF, 61.6% vs. 53.7%; UDC, 22.4% vs. 21.0%; SDC, 0% vs. 0%). Thereby, these findings highlight a rapid AD-ACLF transition and a narrow ‘golden window’ to prevent ACLF in patients with HBV etiology, which may be accounted for by HBV-specific characteristics of AD and ACLF. Moreover, we noted a remarkably lower 1-year mortality in the SDC group than in the PREDICT study (2.6% vs. 9.5%). The observed discrepancy in mid-term survival may be affected by etiology-oriented treatment for underlying cirrhosis. Anti-HBV NUCs have been shown to stabilize or improve LT-free outcomes in patients with HBV-related decompensated cirrhosis,22,23 and all patients in our study received antiviral therapies with close monitoring for the interruption. However, long-term complete alcohol abstinence was unlikely for a considerable proportion of patients with alcohol-related cirrhosis because of a lack of discipline, alcohol abuse, or a dependence problem after discharge.19,24 Our results suggested an optimal mid-term outcome for patients with HBV-related stable cirrhosis under viral control, who could be transferred to primary care.
One of the remarkable findings was the identification of specific precipitants that were associated with ACLF development. Considering the chronological order, a causality between a precipitant and ACLF occurrence can be speculated. We noted that a high viral load was a critical determinant in precipitating ACLF in patients with a spontaneous flare-up of hepatitis B. The findings concurred with a prior study that showed a rapid and significant viral reduction following tenofovir disoproxil fumarate treatment, predicting survival benefit in patients with ACLF precipitated by spontaneous hepatitis B flare.25 Furthermore, hepatitis B flare with HBV reactivation was associated with an increased risk of developing ACLF. Actually, it has been reported that HBV reactivation by interruption/discontinuation of antiviral treatments was more likely to cause a high level of HBV replication.26 Thus, our findings suggested a crucial role of hepatic necroinflammation caused by a vigorous immune response against active viral replication in driving HBV-AD to ACLF. Supporting this, a significantly higher level of indicators of liver injury, such as ALT and AST, was observed in pre-ACLF patients compared with the other 2 groups. In addition, we found that superinfection on HBV constituted another influential hepatic precipitant in progression to ACLF, in line with previous studies that reported superinfections frequently led to rapid exacerbation and death in patients with cirrhosis.27,28 Bacterial infection is the most frequent extrahepatic trigger in the development of OF(s) and ACLF in both alcohol- and HBV-related cirrhosis.29,30 A prior study has shown a remarkably higher mortality in ACLF precipitated by bacterial infection than by other insults.31 Our study showed that bacterial infection was the only extrahepatic precipitant associated with progression to ACLF, enforcing the central role of bacterial infection in the extrahepatic precipitating events of ACLF. In addition to precipitants, our study confirmed that the presence of high-grade systematic inflammation was associated with AD-ACLF progression, and it identified the NL ratio as an independent risk factor. Many studies have reported a robust association between NL ratio and death in patients with decompensated cirrhosis and ACLF.[32], [33], [34] Our study revealed that NL ratio was more statistically applicable than other surrogates of systematic inflammation, such as WBCs and CRP, probably affected by liver dysfunction or compartmentalization. Other predictive variables were bilirubin and INR, which are well-recognized indicators of liver and coagulation dysfunction/failures. Based on the precipitant–systemic inflammation–organ dysfunction/failure framework, we developed a model with high statistical accuracy to predict ACLF development. The C-index of our model in the validation cohort was lower than that in the derivation cohort, possibly due to milder disease severity of patients in the validation cohort. Still, it was significantly higher than for the other 4 traditional prognostic scores. The application of an optimal model score was shown to successfully screen patients at high risk of developing ACLF, which would be clinically helpful for the management of patients with AD.
Nevertheless, our study has several limitations. First of all, the follow-up of ACLF development ended at 28 days after enrollment, and the number of patients with pre-ACLF may be underestimated compared with the PREDICT study. However, the bias may be limited, and as indicated earlier, the AD-ACLF transition mainly occurred within 2 weeks and declined with time. Secondly, the relatively low PPV limited the accuracy of prediction that only less than 50% of patients with pre-ACLF could be identified. However, the high NPV enables clinicians to exclude low-risk patients in the clinical scenario, which is meaningful for the allocation of medical resources and the adjustment of management strategy. Third, due to the lack of dynamic HBV-DNA, the impact of viral response on ACLF development was not investigated in this study.
In summary, pre-ACLF in patients with HBV-related cirrhosis and AD followed a distinct clinical course compared to UDC and SDC. Hepatitis B flare with HBV reactivation, spontaneous hepatitis B flare with high HBV-DNA load, superimposed infection on HBV, and bacterial infection are precipitants associated with an increased risk of AD-ACLF progression. A model that incorporated precipitants and indicators of systemic inflammation and organ injury accurately predicted ACLF development. Our findings represent an essential step towards preventing the AD-ACLF transition and provide information on the management of patients with AD and for the design of interventional clinical studies.
Financial support
The study is supported by Shanghai Hospital Development Commission (SHDC2020CR1037B), National Key R&D Program of China (2017YFC0908100), the National Science and Technology Major Project (2018ZX10302206, 2018ZX10723203, 2017ZX10202202), Shanghai Municipal Education Commission–Guofeng Clinical Medicine Grant Support, the National Natural Science Foundation of China (8217032461, 82170629, 81930061, 81900579, 81970550, 82070613, 82070650, 81972265, 81870425, 81774234), the Chongqing Natural Science Foundation (CSTC2019jcyj-zdxmX0004), Beijing Municipal Science & Technology Commission (Z191100006619033), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S131), Clinical Research Program of Nanfang Hospital, Southern Medical University (2018CR037, 2020CR026), Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2019ZD006), President Foundation of Nanfang Hospital, Southern Medical University (2019Z003), the Foundation for Innovative Research Groups of Hubei Provincial Natural Science Foundation (2018CFA031), Hubei Province’s Outstanding Medical Academic Leader Program and Project of Hubei University of Medicine (FDFR201902, 2020XGFYZR05), the Fundamental Research Funds for the Central Universities (2021FZZX001-41), the Guangdong Basic and Applied Basic Research Foundation (2020A1515010052), Natural Fund of Guangdong Province (2016A030313237) and Guangzhou City Science and Technology Project (201607010064).
Authors’ contributions
Concept and design: Hai Li. Acquisition, analysis, or interpretation of data: all authors. Statistical analysis: Tongyu Wang. Original drafting of the manuscript: Tongyu Wang. Reviewing and editing draft for important intellectual content: Hai Li and Yu Shi. Administrative, technical, or material support: Hai Li, Guohong Deng, Xiaobo Wang, Xin Zheng, Yan Huang, Jinjun Chen, Zhongji Meng, Yanhang Gao, Zhiping Qian, Feng Liu, Xiaobo Lu, Yu Shi, Huadong Yan, Yubao Zheng, Wenting Tan and Weituo Zhang. Data collection: Shan Yin, Wenyi Gu, Yan Zhang, Fuchen Dong, Jianyi Wei, Xiaomei Xiang, Yi Zhou, Yixin Hou, Qun Zhang, Shue Xiong, Jing Liu, Liyuan Long, Ruochan Chen, Xiuhua Jiang, Sen Luo, Yuanyuan, Chen, Chang Jiang, Jinming Zhao, Liujuan Ji, Xue Mei, Jing Li, Tao Li, Rongjiong Zheng, Xinyi, Zhou and Haotang Ren.
Obtained funding: Hai Li, Guohong Deng, Xiaobo Wang, Xin Zheng, Yan Huang, Jinjun Chen, Zhongji Meng, Yanhang Gao, Yu Shi, Yubao Zheng.
Data availability statement
The data shown in this article are available from the corresponding authors upon a reasonable request.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100529.
Contributor Information
Yu Shi, Email: zjushiyu@zju.edu.cn.
Hai Li, Email: aclf_group@163.com.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sepanlou S.G., Safiri S., Bisignano C., Ikuta K.S., Merat S., Saberifiroozi M., et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jepsen P., Younossi Z.M. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. J Hepatol. 2021;75(Suppl 1):S3–S13. doi: 10.1016/j.jhep.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Yang Y., Hu Y., Wu W., Yang Q., Zheng M., et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62(1):232–242. doi: 10.1002/hep.27795. [DOI] [PubMed] [Google Scholar]
- 7.Wu T., Li J., Shao L., Xin J., Jiang L., Zhou Q., et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–2191. doi: 10.1136/gutjnl-2017-314641. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Chen L.Y., Zhang N.N., Li S.T., Zeng B., Pavesi M., et al. Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci Rep. 2016;6:25487. doi: 10.1038/srep25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Liang X., You S., Feng T., Zhou X., Zhu B., et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75(5):1104–1115. doi: 10.1016/j.jhep.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Liang X., Jiang J., Yang L., Xin J., Shi D., et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut. 2022;71(1):163–175. doi: 10.1136/gutjnl-2020-323395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Qiao L., Wang X., Deng G., Huang Y., Chen J., Meng Z., et al. Cohort profile: a multicentre prospective validation cohort of the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) study. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-037793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu W.Y., Xu B.Y., Zheng X., Chen J., Wang X.B., Huang Y., et al. Acute-on-Chronic liver failure in China: rationale for developing a patient registry and baseline characteristics. Am J Epidemiol. 2018;187(9):1829–1839. doi: 10.1093/aje/kwy083. [DOI] [PubMed] [Google Scholar]
- 14.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Xu B.Y., Wang X.B., Zheng X., Huang Y., Chen J., et al. Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin Gastroenterol Hepatol. 2020;18(11):2564–2572 e2561. doi: 10.1016/j.cgh.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Mei X., Li H., Deng G., Wang X., Zheng X., Huang Y., et al. Prevalence and clinical significance of serum sodium variability in patients with acute-on-chronic liver diseases: a prospective multicenter study in China. Hepatol Int. 2022;16(1):183–194. doi: 10.1007/s12072-021-10282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau R., Jalan R., Gines P., Pavesi M., Angeli P., Cordoba J., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437 e1421-1429. [DOI] [PubMed] [Google Scholar]
- 19.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustot T., Fernandez J., Garcia E., Morando F., Caraceni P., Alessandria C., et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 21.Klein L.M., Chang J., Gu W., Manekeller S., Jansen C., Lingohr P., et al. The development and outcome of acute-on-chronic liver failure after surgical interventions. Liver Transpl. 2020;26(2):227–237. doi: 10.1002/lt.25675. [DOI] [PubMed] [Google Scholar]
- 22.Jang J.W., Choi J.Y., Kim Y.S., Woo H.Y., Choi S.K., Lee C.H., et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61(6):1809–1820. doi: 10.1002/hep.27723. [DOI] [PubMed] [Google Scholar]
- 23.Das K., Das K., Datta S., Pal S., Hembram J.R., Dhali G.K., et al. Course of disease and survival after onset of decompensation in hepatitis B virus-related cirrhosis. Liver Int. 2010;30(7):1033–1042. doi: 10.1111/j.1478-3231.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 24.Caputo F., Domenicali M., Bernardi M. Diagnosis and treatment of alcohol use disorder in patients with end-stage alcoholic liver disease. Hepatology. 2019;70(1):410–417. doi: 10.1002/hep.30358. [DOI] [PubMed] [Google Scholar]
- 25.Garg H., Sarin S.K., Kumar M., Garg V., Sharma B.C., Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53(3):774–780. doi: 10.1002/hep.24109. [DOI] [PubMed] [Google Scholar]
- 26.Lei J.H., Peng F., Chen Z., Xiao X.Q. Is HBV viral load at admission associated with development of acute-on-chronic liver failure in patients with acute decompensation of chronic hepatitis B related cirrhosis? BMC Infect Dis. 2019;19(1):363. doi: 10.1186/s12879-019-3988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng T.C., Liu C.J., Chang C.T., Su T.H., Yang W.T., Tsai C.H., et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72(6):1105–1111. doi: 10.1016/j.jhep.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Kc S., Sharma D., Basnet B.K., Mishra A.K. Effect of acute hepatitis E infection in patients with liver cirrhosis. JNMA J Nepal Med Assoc. 2009;48(175):226–229. [PubMed] [Google Scholar]
- 29.Fernandez J., Acevedo J., Wiest R., Gustot T., Amoros A., Deulofeu C., et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 30.Wong F., Piano S., Singh V., Bartoletti M., Maiwall R., Alessandria C., et al. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J Hepatol. 2021;74(2):330–339. doi: 10.1016/j.jhep.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Mucke M.M., Rumyantseva T., Mucke V.T., Schwarzkopf K., Joshi S., Kempf V.A.J., et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38(4):645–653. doi: 10.1111/liv.13568. [DOI] [PubMed] [Google Scholar]
- 32.Rice J., Dodge J.L., Bambha K.M., Bajaj J.S., Reddy K.R., Gralla J., et al. Neutrophil-to-lymphocyte ratio associates independently with mortality in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16(11):1786–1791 e1781. doi: 10.1016/j.cgh.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Guo H., Yu X., Zhu H., Zhang X., Yang J., et al. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol. 2021;21(1):422. doi: 10.1186/s12876-021-02007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W., Yan H., Zhao H., Sun W., Yang Q., Sheng J., et al. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: differentiate it from No-ACLF. Liver Int. 2018;38(2):248–257. doi: 10.1111/liv.13504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data shown in this article are available from the corresponding authors upon a reasonable request.