Abstract
The radA gene predicted to be responsible for homologous recombination in a hyperthermophilic archaeon, Desulfurococcus amylolyticus, was cloned, sequenced, and overexpressed in Escherichia coli cells. The deduced amino acid sequence of the gene product, RadA, was more similar to the human Rad51 protein (65% homology) than to the E. coli RecA protein (35%). A highly purified RadA protein was shown to exclusively catalyze single-stranded DNA-dependent ATP hydrolysis, which monitored presynaptic recombinational complex formation, at temperatures above 65°C (catalytic rate constant of 1.2 to 2.5 min−1 at 80 to 95°C). The RadA protein alone efficiently promoted the strand exchange reaction at the range of temperatures from 80 to 90°C, i.e., at temperatures approaching the melting point of DNA. It is noteworthy that both ATP hydrolysis and strand exchange are very efficient at temperatures optimal for host cell growth (90 to 92°C).
RecA protein is a key enzyme of homologous recombination in eubacteria and is also essential to many other aspects of DNA metabolism (for review, see references 3, 11, and 18). To make recombination possible, the RecA protein is polymerized on single-stranded DNA (ssDNA) in the presence of ATP and Mg2+, forming a helical nucleoprotein filament. This presynaptic recombination complex interacts with double-stranded DNA (dsDNA), aligns homologous sequences between the ss- and dsDNA, and promotes the DNA strand exchange by switching DNA pairing within the triple-stranded DNA complex.
Although ATP hydrolysis has been shown to not be required for the presynaptic complex formation (10), the latter possesses Mg2+- and ssDNA-dependent ATPase activity. This activity can easily be used to monitor the RecA protein nucleation as well as saturation of the RecA binding to ssDNA that results in formation of the complex activated for recombination. The in vivo strand exchange process can be modelled in vitro by the transfer of one strand of linear dsDNA to the complementary circular ssDNA (for example, M13 phage); this transfer results in branched DNA recombination intermediates (joint molecules) that are gradually converted into nicked circular heteroduplex dsDNA molecules (final products) and linear ssDNA. As a rule, the circular ssDNA is introduced into the strand exchange reaction in the form of the presynaptic complex. In principle, the strand exchange can proceed in the presence of nonhydrolyzable analogues of ATP, ATPγS, or ADP-A1F4− (12, 14), but the hydrolysis is required to exchange molecules longer than 1,500 bp as well as to dissociate RecA from the heteroduplex products, to facilitate the bypass of heterologous sequences, and to maintain the reaction polarity (for references, see reference 7).
SSB protein is another important component of the strand exchange reaction proceeding under physiological temperature (37°C). This protein affects both the binding of RecA to ssDNA by removing secondary structures from the latter and the completion of strand exchange (2) by covering the linear ssDNA replaced from the reaction (26).
The recA gene of Escherichia coli is a prototypic member of a large family of genes found ubiquitously (18). These recA-like genes have been described in two domains of life other than Bacteria: Eucarya (RAD51 [16]) and Archaea (radA genes [20, 21]). Evolutionary comparisons of the deduced sequences of known RecA-like proteins show that the archaeal proteins are structurally more closely related to their eukaryal analogues (5).
Recently, the archaeal RadA protein from Sulfolobus solfataricus (RadASs) has been characterized (22). Its ssDNA-dependent ATPase has been found to be weak (catalytic rate constant [kcat] of 0.1 to 0.2 min−1 at a temperature range from 65 to 85°C) and more comparable to that observed for the Saccharomyces cerevisiae Rad51 (Rad51Sc) protein (kcat of 0.7 min−1 [25]) or human Rad51 (Rad51Hs) protein (kcat of 0.16 min−1 [1]) than for the E. coli RecA (RecAEc) protein (kcat of 30 min−1 [7]). The strand exchange reaction measured at 65°C for 90 min has demonstrated the ability of the RadA protein alone to convert 13% φX174 dsDNA into final products.
Desulfurococcus amylolyticus (strain Z533) is a hyperthermophilic and obligatory anaerobic archaeon that has been isolated from hot volcanic vents of Kamchatka and Kunashir (4). Like most members of the Crenarchaeota group (9), D. amylolyticus shares an extraordinary resistance to high temperatures. The strain grows at a pH range from 5.7 to 7.5, with an optimum pH of 6.4, at temperatures between 68 and 97°C, with an optimum temperature of 90 to 92°C.
Here, we describe the cloning and sequencing of the recA/RAD51-like gene (named here radADa) from the archaeon D. amylolyticus, the purification of RadA (named here RadADa) protein, and the characterization of its recombination activities. As is shown, the temperature optimum for the two activities analyzed is similar to that described for the host cells.
MATERIALS AND METHODS
Materials.
Restriction endonucleases were obtained from MBI-Fermentas, ATP and ATPγS were obtained from Sigma, [α-32P]ATP was obtained from ICN, isopropyl-β-d-(−)-thiogalactopyranoside (IPTG) was obtained from Wako Pure Chemical, DEAE-cellulose (DE-52) and phosphocellulose (P11) were obtained from Whatman Biochemicals, and proteinase K was obtained from Fisher Biotech. Both supercoiled circular dsDNA (replication form [RFI]) and the circular ssDNA of bacteriophage M13mp18 were obtained from Sigma. Bacteriophage φX174 DNA (RFI and circular ssDNA) was obtained from New England Biolabs. Linear duplex (RFII) DNA was prepared from RFI DNA by digestion with PstI restriction endonuclease.
radADa cloning procedure.
Two degenerative oligonucleotide PCR primers, 5′-TACATCGACAC(GT)GA(AG)GGIAC-3′ and 5′-CT(GT)GCCAT(GT)ACTTGGTTIGT-3′, were used in a PCR with highly purified DNA of the Z533 strain of D. amylolyticus, which resulted in synthesis of a 345-bp DNA fragment. Sequencing of this fragment showed that it was a suitable probe in Southern hybridization of the DNA Z533 fragments digested by different restriction endonucleases to detect those which carried the radADa gene. By Southern hybridization, a 2.8-kb XhoI fragment containing the radADa gene was selected and cloned into the pUC119 vector, and both strands of the insert were sequenced by a standard dideoxy procedure (19).
Protein overproduction and purification.
The RadADa protein was purified from E. coli BL21(DE3) [F− ompT hsdSB (rB− mB−) dcm gal ΔrecA306] carrying the plasmids pLysS and pET21b-radADa in the pET expression system developed by Novagen. The pET21b-radADa plasmid was constructed from the pET21b vector by replacement of a NdeI-BamHI fragment with an entire radADa gene, flanked by NdeI and BglII sites. The inserted radADa gene was created by PCR with the primers 5′-GACATATGAGTGAGGAGAAG-3′ and 5′-ACAGATCTCACCTACTCTTA-3′. The validity of the sequence was confirmed by a standard dideoxy sequencing procedure.
A cell extract in TEMS buffer (50 mM Tris-HCl [pH 8], 5 mM 2-mercaptoethanol, 5 mM EDTA, 25% [wt/vol] sucrose) was prepared by sonication from IPTG-induced BL21ΔrecA306/DE3 cells, harboring the plasmids pET21-radADa and pLysS. Brij 58 was added to final concentration of 0.5% (wt/vol), and the lysate was incubated at 80°C for 15 min, chilled on ice, and centrifuged (30,000 × g) for 60 min at 4°C. The supernatant obtained was applied to a DEAE cellulose column (DE52) and then to a phosphocellulose (P11) column, and RadADa protein was eluted in the range of 200 to 300 mM sodium chloride gradient in both cases. After being heated additionally at 85°C for 15 min, the RadADa-containing fractions were applied to a MonoQ column, and the protein was eluted at approximately 350 mM NaCl. The pool was applied to a Superdex column, and the RadADa protein was eluted near a void volume showing an average oligomer form as a 12-mer. This fraction was dialyzed against storage buffer (20 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 100 mM NaCl, 50% glycerol). All required changes in buffer were performed by dialysis. The protein concentration was determined by the Bio-Rad protein assay kit (Bradford). The N-terminal amino acid sequence of the RadADa protein was determined with a gas protein sequencer (Applied Biosystems, model 473A).
ssDNA-dependent ATP hydrolysis.
The amount of ATP hydrolyzed was determined by thin-layer chromatography with polyethyleneimine cellulose sheets as described earlier (27). Reactions were carried out in the TMD buffer (pH 7.6) (25 mM Tris-HCl, 10 mM MgCl2, 0.1 mM DTT) containing 0.5 mM [α-32P]ATP, 8 μM RadADa, and M13 ssDNA as indicated. The mixture was covered with mineral oil, incubated for a period of 5 to 15 min at the temperature designated, and arrested by stirring on ice.
DNA strand exchange.
For DNA strand exchange, the agarose gel assay (8) was used. This assay was conducted and results were visualized as described previously (15). The standard reaction was carried out in TAcMD buffer (pH 7.9) (20 mM Tris-acetate, 10 mM Mg-acetate, 50 mM K-acetate, 0.1 mM DTT) containing 2.5 mM ATP, 8 μM M13mp18 ssDNA, and 8 μM RadADa. The mixture was covered with mineral oil and preincubated for 5 min, and then the reaction was initiated by addition of 16 μM M13mp18 dsDNA linearized by PstI digestion. Agarose gels stained with ethidium bromide were documented with the EDAS 120 system (Amersham Pharmacia Biotech). The efficiency of each strand transfer was calculated by making use of the program 1D Software (Kodak Digital Science) via the amount of dsDNA converted to the final product.
In order to control RadADa dependence of the strand exchange reaction, 20 μl of RadADa was incubated with 1 μl of proteinase K (10 mg/ml, water solution) for 5 h at 37°C, and this inactivated protein was used in the standard reaction described. To control the homology dependence of the strand exchange reaction, φX174 phage DNA (ss- or dsDNA forms) was used together with M13mp18 DNA (ss- or dsDNA forms) in nonhomologous reactions performed under standard conditions of the strand exchange reaction.
CD measurements.
The circular dichroism (CD) spectra of 1 μM RadADa protein were measured with a Jasco spectropolarimeter, model J-720W, by using a 0.1-cm cuvette in the far-UV region between 200 and 250 nm in a solution containing 20 mM Tris-HCl and 200 mM NaCl. The residue molar ellipticity, [θ], was defined as 100 [θ]obs/(lc), where [θ]obs is the observed molar ellipticity, l is the length of the light path in centimeters, and c is the residue molar concentration of the protein.
Nucleotide sequence accession number.
The sequence data have been submitted to the DDBJ, EMBL, and GenBank databases under accession no. AF145465.
RESULTS AND DISCUSSION
Cloning and sequencing of radADa.
The recA/RAD51-like gene of D. amylolyticus was cloned as follows. Two degenerative oligonucleotide PCR primers, complementary to the regions corresponding, respectively, to the β2 and β5 strands of the E. coli RecA three-dimensional structure (24), were used in the PCR with highly purified DNA of D. amylolyticus that resulted in synthesis of a 345-bp DNA fragment. The latter was used as a probe in Southern hybridization of the D. amylolyticus DNA fragmented by different restriction endonucleases. Thus, a 2.8-kb XhoI fragment containing the complete radADa gene was selected and cloned into the pUC119 vector, and both strands of the insert were sequenced.
As was expected (5, 21), the predicted RadADa amino acid sequence was more closely related to the Rad51 family than to the RecA family. This sequence showed 40% identity and 65% similarity to the Rad51Hs protein (Fig. 1), whereas it showed only 20% identity and 35% similarity to RecA. In addition, the sequence demonstrated all of the particular structural features of crenarchaeotal RadA proteins, including the sizes of the three insertions relative to RecA and the nine signature amino acids that have taxonomic and phylogenetic significance (20). The structural similarity of RadADa to RadA from S. solfataricus RadASs) was maximal among all known archaeal proteins, showing 67% identity and 82% homology to their complete amino acid sequences (Fig. 1).
FIG. 1.
Amino acid sequence of two archaeal RadA proteins (D. amylolyticus RadA [DaRadA] and S. solfataricus RadA [SsRadA]), human Rad51 (Rad51 [HsRad51]), and bacterial RecA (E. coli RecA [EcRecA]). A dash indicates a gap introduced in a sequence to optimize the alignment with the other sequences compared. A period represents a residue identical to that from RadADa. ins1, -2, and -3 and asterisks mark, respectively, the positions of three taxonomic significant insertions relative to RecAEc and nine phylogenetic significant residues described earlier (20). The accession numbers of RadADa, RadASs, Rad51Hs, and RecAEc, respectively, are as follows: AF145465, U45310, D13804, and V00328.
Expression of the radADa gene in E. coli and purification of RadADa protein.
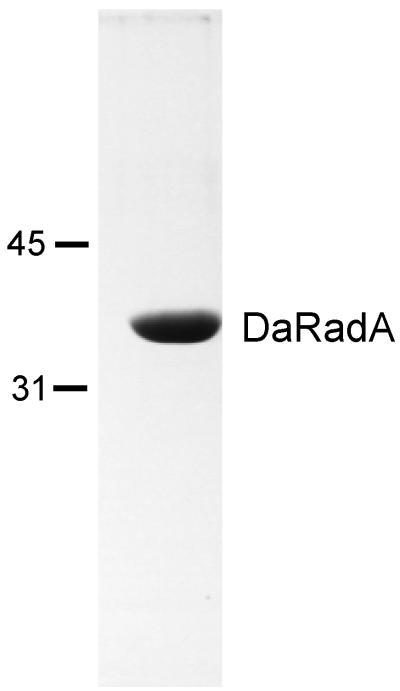
In order to overproduce the RadADa protein, an entire radADa gene was subcloned into the pET21b expression vector (for details, see Materials and Methods). The degree of RadADa protein homogeneity was estimated as more than 98% (Fig. 2). The protein preparations were free from detectable exonucleases, as determined at both 37 and 80°C. The N-terminal amino acid sequence of the RadADa protein was determined as follows: SEEKETIK. This sequence was in accordance with that predicted from the nucleotide sequence.
FIG. 2.
Purity of RadADa. A 10-μg portion of purified RadADa was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (12% polyacrylamide gel), and the gel was stained with Coomassie blue. The molecular mass markers, labeled on the left, are in kilodaltons.
ssDNA-dependent ATPase activity.
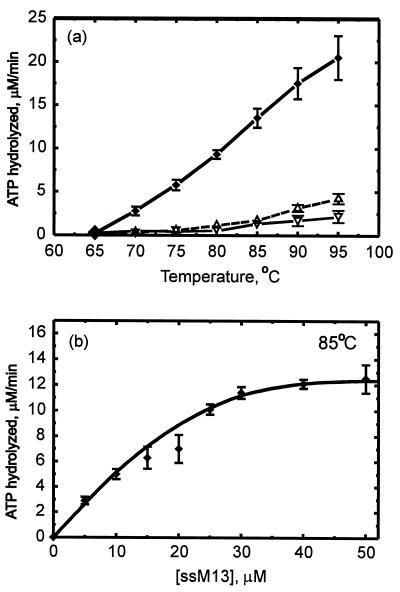
The RadADa ATP hydrolysis activity was tested at a wide range of temperatures. The enzyme required two cofactors—magnesium (data not shown) and ssDNA—and was active exclusively at temperatures above 65°C (Fig. 3a). The temperature restriction is in good accordance with that observed for the activity of ATP-dependent topoisomerase purified from D. amylolyticus earlier (23). The RadADa ATP hydrolysis showed monomer kcat values of 1.2 to 2.5 min−1 at the temperature interval 80 to 95°C, which is more closely related to the ATP hydrolysis catalyzed by the S. cerevisiae Rad51 protein at 37°C than that catalyzed by E. coli RecA. The analysis was stopped at 95°C because of a significant decrease of an accuracy of measurements performed at more higher temperatures. Relative to RadASc, showing a significant ATPase activity at 65°C and only 50% more at 75°C (22), the RadADa protein had negligible ATPase at 65°C and demonstrated a practically linear increase of this activity up to 95°C.
FIG. 3.
ssDNA-dependent ATP hydrolysis. (a) Temperature dependence. (b) ssDNA concentration dependence. The error bars indicate the standard deviations for three sets of experiments. The reaction mixture (20 μl) contained TMD buffer (pH 7.6), 0.5 mM [α-32P]ATP, 50 μM M13 ssDNA (ssM13) (unless indicated otherwise in the figure), and 8 μM RadADa. The mixture was incubated for period of 5 to 15 min at the temperatures indicated. ⧫, ssDNA-dependent ATP hydrolysis after subtraction of both the spontaneous hydrolysis of ATP (▵) and the ssDNA-independent ATP hydrolysis (▿).
At 85°C, the ATP hydrolysis increased with ssDNA concentration, reaching a saturation plateau (Fig. 3b). This dependence allowed us to estimate the apparent binding stoichiometry as 1 RadADa monomer per 3 nucleotides. This ratio is similar to that observed for all three representatives of RecA-like proteins: E. coli RecA, S. cerevisiae Rad51, and S. solfataricus RadA (13, 22, 25).
DNA strand exchange activity.
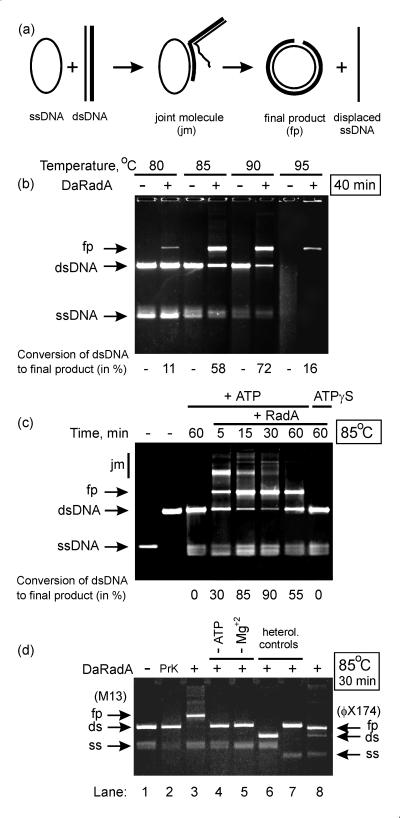
Figure 4 shows characteristics of the strand exchange reaction (schematically presented in Fig. 4a) promoted by the RadADa protein. As can be clearly seen, the reaction was very efficient at temperatures close to 90°C (Fig. 4b). At 85°C, the reaction progressed so rapidly that 90% of the dsDNA was already converted to final products after 30 min (Fig. 4c). However, a certain regression of the reaction was also observed, because after an additional 30 min, the amount of final products decreased slightly, whereas the amount of dsDNA increased. The explanation of the latter effect seems to be obvious. In the experiments described, the RadADa protein catalyzed the reaction in the absence of a thermoresistant SSB protein or its archaeal analogue, RPA protein (6). As mentioned above, the SSB protein is an essential component of the reaction at both early and late phases to remove, respectively, ssDNA secondary structures and the replaced linear ssDNA. Because the activity of RadADa showed a temperature optimum near to the melting temperature of duplex DNA, where ssDNA lacks secondary structures, it appears that the SSB protein is not required for the early phase, but it is still necessary for the late phase in order to arrest the observed regression.
FIG. 4.
DNA strand exchange reaction. A scheme of the reaction (a), the temperature (b) and kinetic (c) characteristics, and control reactions (d) are shown. In panels b and c, the reaction mixture (20 μl) contained TAcMD buffer (pH 7.9), 2.5 mM ATP, 8 μM RadADa, 8 μM M13mp18 ssDNA, and 16 μM linearized M13mp18 dsDNA. The latter was added to initiate the reaction after 5 min of preincubation at the temperature indicated. In panel d (homologous controls, lanes 1 to 5), the reaction mixture (20 μl) was the same as described above, but either without RadADa (lane 1) or with RadADa degraded by proteinase K (PrK) (lane 2); a complete mixture (lane 3); the same mixture as in lane 3, but without ATP (lane 4); or the same mixture as in lane 3, but without Mg+2 (lane 5). In panel d (heterologous controls, lanes 6 to 8), the reaction mixture (20 μl) contained TAcMD buffer (pH 7.9), 2.5 mM ATP, 8 μM RadADa, 8 μM M13mp18 ssDNA, and 16 μM linearized φX174 dsDNA (lane 6) or 8 μM φX174 ssDNA and 16 μM linearized M13mp18 dsDNA (lane 7), or 8 μM φX174 ssDNA and 16 μM linearized φX174 dsDNA (lane 8, showing the strand exchange reaction with φX174 DNA substrates).
All necessary controls are presented in Fig. 4d. They show that the reaction was absolutely dependent on the integrity of RadADa protein (lanes 1 and 2 relative to lane 3), being also ATP dependent (lane 4) and Mg+2 dependent (lane 5). Lanes 6 and 7, relative to lane 8, show the homology dependence of the reaction that could not be performed when one of the DNA substrates was replaced by heterological φX174 DNA.
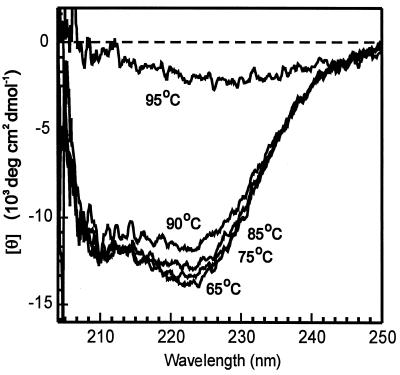
A temperature of 95°C appeared to be critical for two reasons. At this temperature, both the melting of dsDNA and the aggregation of the RadADa protein occurred. The latter was observed by a sharp alteration of the CD spectrum measured for the RadADa protein solution (in the absence of any stabilizing cofactors, ATP or DNA) when the temperature was elevated from 90 to 95°C (Fig. 5). In fact, the far-UV CD spectrum of the RadADa protein measured at 35°C showed negative double maxima at 210 and near 220 nm that characterize the presence of α-helical structures in the protein composition (28). The same character of the RadADa CD spectrum was maintained at a wide range of elevated temperatures from 65 to 90°C, while at 95°C the protein aggregation and/or denaturation was observed (Fig. 5). However, even at 95°C, 16% of the final products were created by RadADa alone (Fig. 4b). As far as we know, this is the first demonstration of RecA- or Rad51-like protein ability to promote strand exchange reaction so efficiently and at such high temperatures as well. For comparison, the RadA protein from S. solfataricus described earlier (22) promotes only 13% of the complete exchange of DNA strands between ssDNA and the homologous linear dsDNA of φX174 bacteriophage at 65°C.
FIG. 5.
Far-UV CD spectra of RadADa protein. Measurements were performed in solution containing 20 mM Tris-HCl (pH 7.5), 200 mM NaCl, and 5 μM RadADa at the temperatures indicated.
ATPγS was found to be an unsuitable cofactor for the strand exchange reaction at 85°C (Fig. 4c) because of its great instability at high temperatures. In fact, the half-life of ATPγS at 85°C has been found to be about 10 min (E. Glazunov, personal communication).
The presented data show that the RadADa protein efficiently promotes two main recombinational reactions—the strand exchange and ATP hydrolysis—at temperatures close to, if not equal to, those found to be optimal for host cell growth (90 to 92°C). These results provide evidence for the structural and functional adaptation of the protein to environmental conditions. The ability of the protein to function only at elevated temperatures (above 65°C) shows that some constituents of its secondary structure appear to be too rigid to function at lower temperatures. We have previously demonstrated the role of the α-helix primary structures of RecA proteins from psychrotrophic, mesophilic, and thermophilic bacteria in the flexibility that allows these proteins to function at 20, 37, and 80°C, respectively (17). These data enable us to suggest that both the stability and high activity of the RadADa protein at 90°C are a result of the particular structural characteristics of its α-helices and β-strands.
ACKNOWLEDGMENTS
This work was supported by an International Research Scholar's award from the Howard Hughes Medical Institute (grant 75195-546101 to V.A.L.), a collaboration grant of the Monbusho International Scientific Research Program of the Ministry of Education, Science, and Culture of Japan (no. 07044199), and the Russian Foundation for Basic Research (grant no. 99-04-49869).
We thank Valery I. Shalguev (PNPI) for help with the preparations of the archaeal biomass, Igor Shevelev (PNPI) for analysis of nuclease activities in RadADa preparations, and Alexander Akhmedov (Institute of Immunology, Basel, Switzerland) for providing some materials and useful advice.
REFERENCES
- 1.Bauman P, Benson F E, West S C. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87:757–766. doi: 10.1016/s0092-8674(00)81394-x. [DOI] [PubMed] [Google Scholar]
- 2.Bedale W A, Cox M M. Evidence for the coupling of ATP hydrolysis to the final (extension) phase of RecA protein-mediated DNA strand exchange. J Biol Chem. 1996;271:5725–5732. doi: 10.1074/jbc.271.10.5725. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P R, Trasy R B, Kowalczykowski S C. DNA strand exchange proteins: a biochemistry and physical comparison. Front Biosci. 1998;3:570–603. doi: 10.2741/a304. [DOI] [PubMed] [Google Scholar]
- 4.Bonch-Osmolovskaya E A, Slesarev A I, Miroshnichenko M I, Svetlichnaya T P, Alexseyev V A. Characteristics of Desulfurococcus amylolyticus—a new extremely thermophilic archaebacterium isolated from thermal springs of Kamchatka and Kunashir island. Microbiologiya. 1988;57:94–101. [Google Scholar]
- 5.Brendel V, Brocchieri L, Sandler S J, Clark A J, Karlin S. Evolutionary comparisons of RecA-like proteins across all major kingdoms of living organisms. J Mol Evol. 1997;44:528–541. doi: 10.1007/pl00006177. [DOI] [PubMed] [Google Scholar]
- 6.Chedin F, Seitz E M, Kowalczykowski S C. Novel homologs of replication protein A in Archaea: implications for the evolution of ssDNA-binding proteins. Trends Biochem Sci. 1998;23:273–277. doi: 10.1016/s0968-0004(98)01243-2. [DOI] [PubMed] [Google Scholar]
- 7.Cox M M. Why does RecA protein hydrolyse ATP? Trends Biochem Sci. 1994;19:217–222. doi: 10.1016/0968-0004(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 8.Cox M M, Lehman I R. RecA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci USA. 1981;78:3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delong E. Archaeal means extreme. Science. 1998;280:542–543. doi: 10.1126/science.280.5363.542. [DOI] [PubMed] [Google Scholar]
- 10.Egelman E H, Stasiak A. Structure of helical recA-DNA complexes. Complexes formed in the presence of ATPγS and ATP. J Mol Biol. 1986;191:677–697. doi: 10.1016/0022-2836(86)90453-5. [DOI] [PubMed] [Google Scholar]
- 11.Kowalczykowski S C, Dixon D A, Eggleston S D, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowalczykowski S C, Krupp R A. DNA-strand exchange promoted by RecA protein in the absence of ATP: implications for the mechanism of energy transduction in protein-protein nucleic acid transactions. Proc Natl Acad Sci USA. 1995;92:3478–3482. doi: 10.1073/pnas.92.8.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauder S D, Kowalczykowski S C. Asymmetry in the recA protein DNA filament. J Biol Chem. 1991;266:5451–5458. [PubMed] [Google Scholar]
- 14.Menetski J P, Bear D G, Kowalczykowski S C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci USA. 1990;87:21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namsaraev E A, Baitin D M, Bakhlanova I V, Alexseyev A A, Ogawa H, Lanzov V A. Biochemical basis of hyper-recombinogenic activity of Pseudomonas aeruginosa RecA protein in Escherichia coli cells. Mol Microbiol. 1998;27:727–738. doi: 10.1046/j.1365-2958.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T, Shinohara A, Nabetani A, Ikeya T, Yu X, Egelman E H, Ogawa H. RecA-like recombination proteins in eukaryotes: function and structures of RAD51 genes. Cold Spring Harbor Symp Quant Biol. 1993;58:567–576. doi: 10.1101/sqb.1993.058.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Petukhov M, Kil Y, Kuramitsu S, Lanzov V. Insights into thermal resistance of proteins from the intrinsic stability of their α-helices. Proteins Struct Funct Genet. 1997;29:309–320. doi: 10.1002/(sici)1097-0134(199711)29:3<309::aid-prot5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Roca A J, Cox M M. RecA protein: structure, function, and the role in recombination DNA repair. Prog Nucleic Acid Res Mol Biol. 1997;56:129–223. doi: 10.1016/s0079-6603(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Sandler S J, Hugenholtz P, Schleper C, DeLong E F, Pace N R, Clark A J. Diversity of radA genes from cultured and uncultured Archaea: comparative analysis of putative RadA proteins and their use as a phylogenetic marker. J Bacteriol. 1999;181:907–915. doi: 10.1128/jb.181.3.907-915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler S J, Satin L H, Sarma H S, Clark A J. recA-like genes from three archaean species with putative protein products similar to Rad51 and Dmc1 proteins of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:2125–2132. doi: 10.1093/nar/24.11.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seitz E M, Brockman J P, Sandler S J, Clark A J, Kowalczykowski S C. RadA protein is an archaeal RecA protein homolog that catalyzes DNA strand exchange. Genes Dev. 1998;12:1248–1253. doi: 10.1101/gad.12.9.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slesarev A I. Positive supercoiling catalysed in vitro by ATP-dependent topoisomerase from Desulfurococcus amylolyticus. Eur J Biochem. 1988;173:395–399. doi: 10.1111/j.1432-1033.1988.tb14012.x. [DOI] [PubMed] [Google Scholar]
- 24.Story R M, Weber I T, Steitz T A. The structure of the E. coli RecA protein monomer and polymer. Nature. 1992;355:318–324. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- 25.Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast Rad51 protein. Science. 1994;259:1892–1896. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- 26.Ullsperger C J, Cox M M. Quantitative RecA protein binding to the hybrid duplex product of DNA strand exchange. Biochemistry. 1995;34:10859–10866. doi: 10.1021/bi00034a019. [DOI] [PubMed] [Google Scholar]
- 27.Weinstock G M, McEntee K, Lehman I R. Hydrolysis of nucleoside triphosphates catalyzed by the recA protein of Escherichia coli: characterization of ATP hydrolysis. J Biol Chem. 1981;256:8829–8834. [PubMed] [Google Scholar]
- 28.Yang J T, Wu C-S C, Martinez H M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]