Abstract
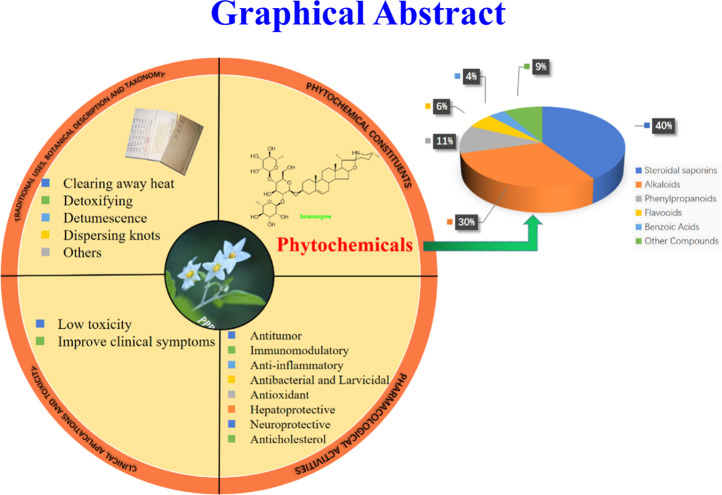
Solanum nigrum Linn., is a common edible medicinal herb of the Solanaceae family which is native to Southeast Asia and is now widely distributed in temperate to tropical regions of Europe, Asia, and America. Traditionally, it has been used to treat various cancers, acute nephritis, urethritis, leucorrhea, sore throat, toothache, dermatitis, eczema, carbuncles, and furuncles. Up to now, 188 chemical constituents have been identified from S. nigrum. Among them, steroidal saponins, alkaloids, phenols, and polysaccharides are the major bioactive constituents. Investigations of pharmacological activities of S. nigrum revealed that this edible medicinal herb exhibits a wide range of therapeutic potential, including antitumor, anti-inflammatory, antioxidant, antibacterial, and neuroprotective activities both in vivo and in vitro. This article presents a comprehensive and systematic overview of the botanical, traditional uses, phytochemical compositions, pharmacological properties, clinical trials, and toxicity of S. nigrum to provide the latest information for further exploitation and applications of S. nigrum in functional foods and medicines.
Keywords: traditional use, phytochemistry, pharmacology, toxicology Taylor and Francis, Solanum nigrum Linn.
Graphical Abstract
Introduction
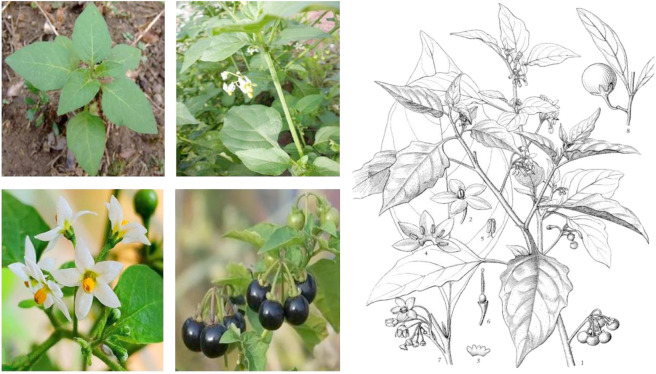
The genus Solanum (Solanaceae family) consists of more than 2,000 species, which are distributed worldwide in tropical and subtropical regions. They mostly have beautiful flowers and fruits. In China, 39 species and 14 varieties of Solanum exist (Editorial Committee of Flora of China, 1979). Solanum nigrum Linn. (Figure 1), also known as Solanum nigrum var. virginicum L. and “龙葵” (in Chinese). S. nigrum is distributed in almost every province in China and is commonly found near the fields, wastelands, and villages. It is also widely distributed in the temperate to tropical regions of Europe, Asia, and America (The Plant List, 2013; Xiang et al., 2019). In China, the plant is known under local names, such as “Yelahu”, “Yehaijiao”, “Heixingxing”, “Heitiantian”, “Kukui”, “Kucai”, “Heidoudou”, and “Yesanzi” (Flora of China, 2007).
FIGURE 1.
Leaves (A); stems and leaves (B); flowers (C); fruits (D); illustration of S. nigrum (E) (1, Upper portion of plant with flowers and fruits; 2, Flower; 3, Opened calyx adaxial view; 4, Opened corolla showing stamens; 5, Stamen; 6, Pistil; 7, Flowering branch; 8, Fruiting branch).
S. nigrum can be used as a medicine and tastes bitter, is of cold property and slightly toxic, and belongs to the lung and kidney meridians. In Chinese folk medicine and traditional Chinese medicine (TCM), people have accumulated rich clinical experience in the use of S. nigrum. The whole plant of S. nigrum has good effects of dispersing blood stasis and detumescence, clearing away heat, as well as detoxification and has been commonly used for the treatment of canker sores, skin eczema, urinary tract infections, bacterial dysentery, prostate, and chronic bronchitis, etc. for thousands of years (Gao et al., 2021). In addition, in modern clinical practice, S. nigrum is commonly combined with other drugs for the treatment of cancers, such as lung cancer, cervical cancer, breast cancer, esophageal cancer, stomach cancer, liver cancer, and bladder cancer. In other Asian countries, such as Japan and India, it has also been documented for the treatment of tumors. Ripe berries of S. nigrum are sweet and salty and were reported to have been used as a famine food in China in the 15th century. In India, the leaves and berries of this plant are commonly consumed as food or vegetable after cooking (Wang, 2007; Zhao, 2010; Wang et al., 2017). In the past few decades, phytochemical research has confirmed that the whole S. nigrum herb contains steroidal saponins, steroidal alkaloids, flavonoids, coumarin, lignin, organic acids, volatile oils, polysaccharides, and other ingredients. The crude extract of S. nigrum and some of the above-mentioned compounds have been confirmed to have various effects, including antitumor, antioxidative, anti-inflammatory, hypotensive, neuroprotective, immunomodulatory, antibacterial, and liver protective effects. Especially the antitumor effect of steroidal saponins and steroidal alkaloids is a research hotspot, and drug researchers expect to find antitumor lead compounds from these components.
In view of the increasing interest in steroid derivatives obtained from S. nigrum due to their significant pharmacological activity, this review provides a systematic summary and criticism of the traditional uses, phytochemistry, pharmacological activity, and safety of S. nigrum based on the literature obtained from databases, with emphasis on the pharmacological activity and potential applications of S. nigrum in TCM. We believe that this review is of great significance for the further research or development of antioxidant functional foods, and novel antitumor drugs based on S. nigrum and its active compounds.
Research Methodology
The literature of this review (until March 2022) was obtained from various important databases, such as Google Scholar, Baidu Scholar, Web of Science, SciFinder Scholar, PubMed, published classic texts of Chinese herbal medicines (e.g., Sheng Ji Zong Lu), the China Knowledge Resource Integrated Database from the China National Knowledge Infrastructure (CNKI), publications in peer-reviewed journals, Ph.D. and M.Sc. theses, as well as other web sources, such as Flora of China, the Plant List, and YaoZh website (https://db.yaozh.com). Keywords used in the literature search were: “Solanum nigrum Linn.”, “Long Kui/龙葵”, “phytochemistry”, “pharmacology”, “biological activity”, “traditional uses”, “secondary metabolites”, “safety”, “toxicology”, and “clinical trial”. After reviewing a total of 576 scientific publications on S. nigrum, excluding some irrelevant content, we mainly focused on 120 documents. ChemDraw Ultra 15.0 software was used to draw the chemical structures.
Botanical Description and Taxonomy
Botanical Description
S. nigrum is an annual erect herbaceous plant with 0.25–1 m in high. It has a taproot system with a well-developed main root and is often lignified. The stem has no inconspicuous edges, is green or purple in color, and nearly glabrous or puberulent. The leaf is ovate, 2.5–10 cm long, and 1.5–5.5 cm wide, and its apex is shortly pointed. The cuneate is base wedge shaped to broad and descending to the petiole, with irregular wavy coarse teeth throughout or on each side and smooth or sparse, soft, and hairy on both sides with five to six veins on each side, and the petiole is about 1–2 cm long. The scorpion-tailed inflorescence is extra-axillary and composed of 3-6-(10) flowers. The total pedicel is about 1–2.5 cm long, and the pedicel is about 5 mm long and nearly glabrous or pubescent. The calyx is small, shallow cup shaped, and about 1.5–2 mm in diameter, and the teeth are oval, the tip is round, and the junction between the two teeth at the base is angled. The corolla is white, the tube is hidden in the calyx and less than 1 mm in length, and the 5-parted crown is about 2.5 mm in length. The lobes are ovoid and about 2 mm long. The filaments are short, the anthers are yellow, about 1.2 mm long, and about four times the length of the filaments, and the apical hole is inward. The ovary is ovoid and about 0.5 mm in diameter, and the style is about 1.5 mm long. The lower part of the middle part is covered with white hairs, the stigma is small, and the head is shaped. The berry is spherical, about 8 mm in diameter, and black when ripe. The seeds are mostly nearly ovoid, about 1.5–2 mm in diameter, and compressed on both sides (Flora of China, 2007).
Taxonomy
S. nigrum in botanical classification as Plantae, Angiospermae, Magnoliopsida, Solanales, Solanaceae, Solanum. Solanaceae is a family of plants with about 80 genera and 3,000 species, widely distributed in tropical and temperate regions, mainly in tropical America. Solanum occupies an important weight in the Solanaceae family, with about 2,000 species, among which the well-known varieties are Solanum melongena L., Solanum tuberosum L., S. nigrum, etc. (The Plant List, 2013; Flora of China, 2007). The spherical berries of S. nigrum are dark purple when ripe. Both the berries and the leaves are edible, but the leaves contain high amounts of alkaloids that must be cooked to detoxify.
Traditional Uses
The first known record describing the medicinal use of S. nigrum was found in Yao Xing Lun (药性论, Tang Dynasty) (Editorial Committee of State Administration of traditional Chinese Medicine, 1999). Since then, the medicinal use of S. nigrum was increasingly reported in many other well-known classical TCM monographs, including Xin Xiu Ben Cao (新修本草, Tang Dynasty), Shi Liao Ben Cao (食疗本草, Tang Dynasty), Ben Cao Tu Jing (本草图经, Song Dynasty), Jiu Huang Ben Cao (救荒本草, Ming Dynasty), Dian Nan Ben Cao (滇南本草, Ming Dynasty), Ben Cao Gang Mu (本草纲目, Ming Dynasty), Dian Nan Ben Cao Tu Shuo (滇南本草图说, Ming Dynasty), and Ben Cao Gang Mu Shi Yi (本草纲目拾遗, Qing Dynasty). In these classical books of TCM, it is recorded that S. nigrum has the functions of clearing away heat, detoxification, detumescence, and dispersion of knots (Editorial Committee of Nanjing University of Chinese Medicine, 2006). In all of these major TCM monographs, S. nigrum has different medicinal properties, including the treatment of sore carbuncle swelling poison, skin eczema, poor urination, chronic bronchitis, excessive leucorrhea, prostatitis, and dysentery. Many TCM herbs or classical prescriptions containing S. nigrum have been used in the form of decoction, powders, granules, tablets, and pills. The traditional and modern prescriptions of S. nigrum commonly used in China are presented in Table 1. For example, according to Sheng Ji Zong Lu, S. nigrum (30 g) is compatible with other herbs such as Hylotelephium erythrostictum (Miq.) H. Ohba (30 g), Coptis chinensis Franch. (30 g), Momordica cochinchinensis (Lour.) Spreng. (15 g), and Abelmoschus manihot (Linn.) Medicus (15 g) for the treatment of malignant sores (https://db.yaozh.com). For the treatment of carbuncles, swelling, and poisoning, S. nigrum can be externally applied for washing and smashing. It can also be combined with TCM, such as Corydalis bungeana Turcz., Chrysanthemum indicum L., and Taraxacum mongolicum Hand.-Mazz., for decoction and subsequent oral administration to treat sore throats. In addition, S. nigrum has a diuretic effect and can be used together with Alisma plantago-aquatica Linn., Akebia quinata (Houttuyn) Decaisne, and other drugs to treat edema, adverse urination and other diseases (Editorial Committee of Nanjing University of Chinese Medicine, 2006). In recent years, it has been commonly used together with Duchesnea indica (Andr.) Focke, Hedyotis diffusa Willd., Solanum lyratum Thunberg, etc. in clinic for the treatment of cancer (Liu et al., 2019a).
TABLE 1.
The traditional and modern prescriptions of S. nigrum in China.
| Preparation name | Composition | Route of administration | Dosage form | Indications for use | References |
|---|---|---|---|---|---|
| Longkui san | S. Nigrum , Hylotelephium erythrostictum (Miq.) H. Ohba, Coptis chinensis Franch., Os Draconis (FossiliaOssiaMastodi), Boswellia carterii Birdw., Momordica cochinchinensis (Lour.) Spreng., Abelmoschus manihot (Linn.) Medicus | External use | Making into powder | Malignant sores | “Sheng Ji Zong Lu” (Song Dynasty, A.D. 1117) |
| Longkui san | S. Nigrum , Boswellia carterii Birdw., Prunus armeniaca L., Coptis chinensis Franch | External use | Making into powder | Malignant sores | “Sheng Ji Zong Lu” (Song Dynasty, A.D. 1117) |
| Longkuigeng san | S. Nigrum , Moschus berezovskii Flerov | External use | Making into powder | Back sore | “Sheng Ji Zong Lu” (Song Dynasty, A.D. 1117) |
| Longkui gao | S. Nigrum , Hyoscyamus niger Linn., Allium sativum L., Coriandrum sativum Linn., Prunus armeniaca L. | External use | Making into cream | Malignant prickling sore pain | “Tai Ping Sheng Hui Fang” (Song Dynasty, A.D. 992) |
| Xinlikang capsules | Scutellaria barbata D. Don, S. Nigrum , Duchesnea indica (Andr.) Focke, Astragalus membranaceus (Fisch.)Bge., Panax ginseng C. A. Meyer, Saussurea involucrata (Kar. et Kir.) Sch.-Bip., Angelica sinensis (Oliv.) Diels, Curcuma longa Linn., Salvia miltiorrhiza Bunge | Take orally | Decocting with water, and then making into granules | Invigorate qi and nourish blood, remove blood stasis and detoxify, cancer | Liu et al. (2019a) |
| Compound Tianxian capsules | Trichosanthes kirilowii Maxim., Clematis chinensis Osbeck, Hedyotis diffusa Willd., Bos taurus domesticus Gmelin, S. Nigrum , Arisaema heterophyllum Blume, Boswellia carterii Birdw., Commiphora myrrha (Nees) Engl., Panax ginseng C. A. Meyer, Astragalus membranaceus (Fisch. )Bge., Polyporus umbellatus (Pers.) Fr., Elphe taeniura Cope, Cinnamomum camphora (L. ) Presl, Moschus berezovskii Flervo | Take orally | Making into capsules | Supervened after esophageal cancer and stomach cancer, peptic ulcer | Kang et al. (2014) |
| Loulian capsules | Hedyotis diffusa Willd., Semiaquilegia adoxoides (DC.) Makino, Polygonum onrientale L., Paris polyphylla Smith. var. yunnanensis (Franch.) Hand.-Mzt., Amyda sinensis (Wiegmann), Curcuma zedoaria (Christm.) Rosc., Lobelia chinensis Lour., Steleophaga Plancyi (Boleny), Whitmania pigra Whitman, Panax ginseng C. A. Meyer, Fallopia multiflora (Thunb.) Harald., S. Nigrum | Take orally | Making into capsules | Hepatitis, liver cirrhosis, liver cancer, breast cancer, hemangioma | Zhang, (2015) |
| Baiying qinghou decoction | Solanum lyratum Thunberg, S. Nigrum , Duchesnea indica (Andr.) Focke, Scutellaria barbata D. Don, Armeniaca mume Sieb | Take orally | Decocting with water | Laryngeal cancer | “Qiu Yuan Ying Fang” |
| Kaizhi longgu san | Os draconis (FossiliaOssiaMastodi), Asarum sieboldii Miq., Gypsum Fibrosum, Ligusticum sinense Oliv., S. Nigrum , Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f., Gypsum Rubrum, Salt | External use | Making into powder | Bad breath | “Pu Ji Fang” (Ming Dynasty, A.D. 1390) |
| Lingxian longcao decoction | Clematis chinensis Osbeck, S. Nigrum , Prunella vulgaris Linn., Smilax glabra Roxb., Trichosanthes kirilowii Maxim., Clematis terniflora DC., Iphigenia indica Kunth, Wikstroemia indica (Linn.) C. A. Mey | Take orally | Decocting with water | Phlegm scrofula, breast mass, wheezing, vomiting | “Yan Fang Xuan Bian” (A.D. 1959) |
In Libya, S. nigrum is often used as folk medicine, and its berries are used as diuretics, antispasmodic, and emetics, and to treat diarrhea, fever, and eye problems, as well as bleeding. In addition, S. nigrum leaves are used as a sedative, cholagogic, and anesthetic for the treatment of insomnia, convulsions, and dysentery as well as the external treatment of wounds and itching. In Italy, the aboveground part of S. nigrum is used as an antispasmodic, sedative, and analgesic drug. In Yemen, S. nigrum is used to dispel phlegm and treat diarrhea and bleeding. In Jordan, S. nigrum fruit is used as an antispasmodic drug. Moreover, S. nigrum is used as an important plant in traditional Indian medicines for the treatment of dysentery, stomach complaints, and fever (Aburjai et al., 2014).
Phytochemical Constituents
S. nigrum is a rich source of natural compounds with varying structural patterns and beneficial properties. To date, approximately 188 phytochemical compounds have been separated and identified from S. nigrum, containing steroids, alkaloids, organic acids, flavonoids, phenylpropanoids and their glycosides, as well as other compounds. Steroidal compounds consist of steroidal saponins (1–76) and steroidal alkaloids (77–101) and are considered to be the main bioactive components of S. nigrum, exhibiting various pharmacological activities, such as antitumor, anti-inflammatory, and antiviral activities. The compounds isolated from S. nigrum are documented and listed in Table 2, and their chemical structures are drawn and presented in Figure 2. In addition, S. nigrum is rich in a large number of polysaccharides, which is a material basis for its various pharmacological activities, such as immunomodulatory and antitumor activities, which are summarized in Table 3.
TABLE 2.
Chemical components structurally identified from S. nigrum.
| No | Chemical constituents | Molecular formula | CAS | Extracts | References |
|---|---|---|---|---|---|
| Steroidal saponins | |||||
| 1 | Diosgenin | C27H42O3 | 512-04-9 | MeOH | Gao et al. (2021) |
| Tsuyoshi et al. (2003) | |||||
| 2 | Degalactotigonin | C50H82O22 | 39941-51-0 | EtOH | Wang (2007) |
| 3 | Stigmasterol | C29H48O | 83-48-7 | EtOH | Zhao (2010) |
| 4 | Pterosterone | C27H44O7 | 18089-44-6 | EtOH | Zhao (2010) |
| 5 | 12-keto-porrigenin | C27H42O5 | 189014-45-7 | EtOH | Zhao (2010) |
| 6 | 28-O-β- d-glucopyranosyl betulinic acid 3β-O-β-D glucopyranoside | C42H68O13 | EtOH | Yang (2014) | |
| 7 | β-daucosterol | C35H60O6 | 474-58-8 | EtOH | Yang (2014) |
| 8 | (25R)-5α-furost-3β, 22α-diol-12-one-26-carboxylicacid-3-O-β-D-glucopyranosy-(1→4)-[O-β-d-glucopyranosyl-(1→2)]-O- β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C47H76O20 | EtOH | Li (2010b) | |
| 9 | Tigogenin3-O-β-d-glucopyranosyl-(1→2)-[O-β-d-xyloyranosyl-(1→3)]-O-β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C52H86O21 | EtOH | Li (2010b) | |
| 10 | Uttroside A | C57H96O28 | 82003-86-9 | EtOH | He et al. (2015), Zhou (2006) |
| 11 | Uttroside B | C56H94O28 | 88048-09-3 | EtOH | He et al. (2015), Zhou (2006) |
| 12 | (22α, 25R)-26-O-(β-d-glucopyranosyl)-22-methoxy-furost-Δ5-3β, 26-diol-3-O-β-d-glucopyranosyl | C57H94O28 | EtOH | He et al. (2015), Zhou (2006) | |
| -(1→2)-O-[β-d-xylopyranosyl-(1→3)]-O-β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | |||||
| 13 | (22α, 25R)-26-O-(β-d-glucopyranosyl)-22-hydroxy-furost-Δ5-3β, 26-diol-3-O-β-d-glucopyranosyl-(1→2)-O-[β-d-xylopyranosyl-(1→3)]-O-β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C57H92O28 | EtOH | He et al. (2015), Zhou (2006) | |
| 14 | (5α, 22α, 25R)-26-O-(β-d-glucopyranosyl)-22-methoxy-furostan-3β, 26-diol-3-β-d-glucopyranosyl-(1→2)-O-[β-d-glucopyranosyl-(1→3)]- O-β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C58H98O29 | 108886-03-9 | EtOH | He et al. (2015), Zhou (2006) |
| 15 | (5α, 22α, 25R)-26-O-(β-d-glucopyranosyl-22-hydroxy-furost-3β,26-diol-3-β-d-glucopyranosyl-(1→2)-O-[β-d-glucopyranosyl-(1→3)]-O-β-d-glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C57H96O29 | EtOH | He et al. (2015), Zhou (2006) | |
| 16 | Solanigroside I | C62H104O31 | EtOH | He et al. (2015), Zhou (2006) | |
| 17 | Solanigroside J | C61H102O31 | 1354759-80-0 | EtOH | He et al. (2015), Zhou (2006) |
| 18 | Solanigroside K | C57H94O28 | EtOH | He et al. (2015), Zhou (2006) | |
| 19 | Solanigroside L | C57H92O28 | EtOH | He et al. (2015), Zhou (2006) | |
| 20 | Solanigroside M | C57H92O28 | EtOH | He et al. (2015), Zhou (2006) | |
| 21 | Solanigroside N | C57H94O28 | EtOH | He et al. (2015), Zhou (2006) | |
| 22 | Solanigroside R | C50H84O23 | EtOH | He et al. (2015), Zhou (2006), Tai et al. (2018) | |
| 23 | Solanigroside S | C50H86O23 | EtOH | He et al. (2015), Zhou (2006) | |
| 24 | Solanigroside T | C45H84O22 | EtOH | He et al. (2015), Zhou (2006) | |
| 25 | Hypoglaucin H | C39H60O15 | 50773-43-8 | EtOH | Zhou (2006) |
| 26 | 5α-pregn-16-en-3β-ol-20-one-lycotetraoside | C44H70O21 | EtOH | He et al. (2015), Zhou (2006) | |
| 27 | Solanigroside A | C49H78O24 | 1029362-42-2 | EtOH | He et al. (2015), Zhou (2006) |
| 28 | Solanigroside B | C45H72O22 | 1029362-44-4 | EtOH | He et al. (2015), Zhou (2006) |
| 29 | (5α, 20S)-3β, 16β-dihydroxy pregn-22-carboxylic acid (22, 16)-lactone-3-O-β-d-glucopyranosyl-(1→2)-O-[β-D-xylopy ranosyl-(1→3)]-O-β-d- glucopyranosyl-(1→4)-O-β-d-galactopyranoside | C45H72O22 | EtOH | He et al. (2015), Zhou (2006) | |
| 30 | Solanigroside U | C50H80O25 | EtOH | He et al. (2015), Zhou (2006) | |
| 31 | Solanigroside V | C50H80O25 | EtOH | He et al. (2015), Zhou (2006) | |
| 32 | Solanigroside W | C45H70O22 | EtOH | He et al. (2015), Zhou (2006) | |
| 33 | Solanigroside X | C45H72O23 | EtOH | He et al. (2015), Zhou (2006) | |
| 34 | Nigrumnin I | C55H90O25 | EtOH | He et al. (2015), Zhou (2006) | |
| 35 | Solanigroside C | C51H82O26 | 905914-27-4 | EtOH | He et al. (2015), Zhou (2006) |
| 36 | Solanigroside D | C55H88O27 | 905914-28-5 | EtOH | He et al. (2015), Zhou (2006) |
| 37 | Solanigroside E | C55H88O28 | 905914-29-6 | EtOH | He et al. (2015), Zhou (2006) |
| 38 | Solanigroside F | C56H92O28 | 905914-30-9 | EtOH | He et al. (2015), Zhou (2006) |
| 39 | Solanigroside G | C50H82O23 | 905914-31-0 | EtOH | He et al. (2015), Zhou (2006) |
| 40 | Solanigroside O | C51H86O23 | EtOH | He et al. (2015), Zhou (2006) | |
| 41 | Nigroside A | C56H94O29 | 386747-86-0 | EtOH | He et al. (2015), Zhou (2006) |
| 42 | Tigogenin/(25R)-5α-spirostan-3β-ol | C27H44O3 | EtOH | Wu (2011) | |
| 43 | (25R)-26-O-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16,22-dione-3-O-α-l-rhamnopyranosyl | C51H86NO23 | MeOH | Xiang et al. (2018) | |
| -(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-galactopyranoside | |||||
| 44 | (25R)-26-O-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16,22-dione-3-O-α-l-rhamnopyranosyl | C45H76NO18 | MeOH | Xiang et al. (2018) | |
| -(1→4)-β-d-glucopyranoside | |||||
| 45 | (25S)-26-O-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16, 22-dione-3-O-α-l-rhamnopyranosyl | C57H96NO27 | MeOH | Xiang et al. (2018) | |
| -(1→2)-[α-Lrhamnopyranosyl-(1→4)]-[β-d-glucopyranosyl-(1→6)]-β-d-glucopyranoside | |||||
| 46 | (25R)-26-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16, 22-dione-3-O-α-Lrhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→4)]-[β-d-glucopyranosyl-(1→6)]-β-d-glucopyranoside | C63H106NO32 | MeOH | Xiang et al. (2018) | |
| 47 | (25S)-26-O-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16, 22-dione-3-O-β-d-glucopyranosyl | C57H96NO28 | MeOH | Xiang et al. (2018) | |
| -(1→6)-β-d-glucopyranosyl-(1→3)-[α-l-rhamnopyranosyl-(1→2)]-β-d-galactopyranoside | |||||
| 48 | (25R)-26-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranosyl-cholest-5(6)-en-3β, 26-diol-16, 22-dione-3-O-α-l-rhamnopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-galactopyranoside | C57H96NO28 | MeOH | Xiang et al. (2018) | |
| 49 | (25R)-26-O-β-d-glucopyranosyl-cholest-5α-3β, 26-diol-16, 22-dione-3-O-β-d-glucopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside | C57H98NO29 | MeOH | Xiang et al. (2018) | |
| 50 | Stigmast-5, 22-dien-3β-ol | C29H48O | EtOH | Sharma et al. (2012) | |
| 51 | Inunigroside A | C50H82O23 | 1427934-51-7 | MeOH | Ohho et al. (2012) |
| 52 | Solanigroside Y1 | C51H82O26 | 2098576-14-6 | MeOH | Wang et al. (2017) |
| 53 | Solanigroside Y2 | C51H82O26 | 2098576-15-7 | MeOH | Wang et al. (2017) |
| 54 | Solanigroside Y3 | C51H80O26 | 2098576-16-8 | MeOH | Wang et al. (2017) |
| 55 | Solanigroside Y4 | C45H70O21 | 2098576-17-9 | MeOH | Wang et al. (2017) |
| 56 | Solanigroside Y5 | C57H94O28 | 2098576-18-0 | MeOH | Wang et al. (2017) |
| 57 | Solanigroside Y6 | C57H94O27 | 2098576-19-1 | MeOH | Wang et al. (2017) |
| 58 | Solanigroside Y7 | C63H106O34 | 2098576-20-4 | MeOH | Wang et al. (2017) |
| 59 | Solanigroside Y8 | C62H104O33 | 2098576-21-5 | MeOH | Wang et al. (2017) |
| 60 | Solanigroside Y9 | C62H104O33 | 2098576-22-6 | MeOH | Wang et al. (2017) |
| 61 | (25R)-26-O-β-D-glucopyranosylfurost-5(6)-ene-3β, 22α, 26-triol-3-O-β-d-glucopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside | C57H96O30 | MeOH | Wang et al. (2017) | |
| 62 | (25R)-26-O-β-Dglucopyranosylfurost-5(6)-ene-3β, 22α, 26-triol-3-O-α-l-rhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside | C57H92O25 | MeOH | Wang et al. (2017) | |
| 63 | (25R)-26-O-β-D-glucopyranosylfurost-5(6)-ene-16α-methoxy-3β, 26-diol-3-O-α-l-rhamnopyranosyl | C39H60O16 | MeOH | Wang et al. (2017) | |
| -(1→2)-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside | |||||
| 64 | (25R)-26-O-β-d-glucopyranosyl-5α-furost-3β, 22α, 26-triol-3-O-β-d-glucopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside | C57H94O30 | MeOH | Wang et al. (2017) | |
| 65 | (25S)-26-O-β-d-glucopyranosyl-5α-furost-3β, 22α, 26-triol-3-O-β-d-glucopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside | C57H94O30 | MeOH | Wang et al. (2017) | |
| 66 | (25R)-26-O-β-Dglucopyranosyl-22α-methoxy-5α-furost-3β, 26-diol-3-O-β-d-glucopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside | C57H96O30 | MeOH | Wang et al. (2017) | |
| 67 | Uttroside B ((25R)-26-O-β-d-glucopyranosyl-5α-furost-3β, 22α, 26-triol-3-O-β-d-glucopyranosyl | C56H94O28 | 88048-09-3 | MeOH | Wang et al. (2017) |
| -(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside) | |||||
| 68 | β-sitosterol | C29H50O | 83-46-5 | EtOH | Yang (2014) |
| 69 | β-carotene glycosides | C35H58O6 | EtOH | Yang (2014) | |
| 70 | Tigogenin | C27H44O3 | 77-60-1 | EtOH | He et al. (2015), Zhou (2006) |
| 71 | Uttronin A | C50H82O22 | 39941-51-0 | EtOH | He et al. (2015), Zhou (2006) |
| 72 | Uttronin B | C39H62O12 | 84955-03-3 | EtOH | He et al. (2015), Zhou (2006) |
| 73 | Dumoside | C40H62O16 | 221526-58-5 | EtOH | He et al. (2015), Zhou (2006) |
| 74 | Nigrumnin II | C55H88O27 | EtOH | He et al. (2015), Zhou (2006) | |
| 75 | Solanigroside H | C51H82O22 | 905914-32-1 | EtOH | He et al. (2015), Zhou (2006) |
| 76 | Cholesterol | C27H46O | 57-88-5 | EtOH | Geng et al. (2020) |
| Alkaloids | |||||
| 77 | β1-solasonine | C39H63NO11 | 73069-18-8 | EtOH | He et al. (2015), Zhou (2006) |
| 78 | β2-solasonine | C39H63NO12 | 73069-19-9 | EtOH | He et al. (2015), Zhou (2006) |
| 79 | Solamargine | C45H73NO15 | 20311-51-7 | EtOH | He et al. (2015), Zhou (2006), Wang (2007) |
| 80 | β2-solamargine | C39H63NO11 | 32449-98-2 | EtOH | He et al. (2015), Zhou (2006) Wang (2007) |
| 81 | Solanigroside P | C39H63NO12 | 1446029-15-7 | EtOH | He et al. (2015), Zhou (2006) |
| 82 | Solanigroside Q | C45H69NO15 | EtOH | He et al. (2015), Zhou (2006), Tai et al. (2018) | |
| 83 | (3β, 12β, 22α, 25R)-3, 12-dihydroxy-spirosol-5-en-27-oic acid | C27H41NO5 | EtOH | He et al. (2015), Zhou (2006) | |
| 84 | Solaoiacid | C44H83NO19 | H2O | Shi et al. (2019) | |
| 85 | (25R)-22αN-4-nor-spirosol-5(6)-en-3β-ol-6-al-3-O-l-rhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside | C45H72NO16 | MeOH | Xiang et al. (2019) | |
| 86 | (25R)-22αN-spirosol-5(6)-en-3β-ol-7-oxo-3-O-l-rhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside | C45H72NO16 | MeOH | Xiang et al. (2019) | |
| 87 | (25R)-22αN-spirosol-4(5)-en-3β-ol-6-oxo-3-O-α-Lrhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl | C45H72NO16 | MeOH | Xiang et al. (2019) | |
| -(1→4)]-β-d-glucopyranoside | |||||
| 88 | Solasodine | C27H43NO2 | 126-17-0 | EtOH | He et al. (2015), Zhou (2006) |
| 89 | N-methylsolasodine | C28H45NO2 | 7604-92-4 | EtOH | He et al. (2015), Zhou (2006) |
| 90 | Tomatidenol | C27H43NO2 | 546-40-7 | EtOH | He et al. (2015), Zhou (2006) |
| 91 | Solanocapsine | C27H46N2O2 | 639-86-1 | EtOH | Liu et al. (2020), Zhou (2006) |
| 92 | Solanaviol | C27H43NO3 | 74131-93-4 | EtOH | He et al. (2015), Zhou (2006) |
| 93 | Solasodine-3-O-β-d-glucopyranoside | C33H53NO7 | EtOH | Chang et al. (2017) | |
| 94 | 12β-hydroxysolasodine β-solatrioside | C45H73NO17 | EtOH | He et al. (2015), Zhou (2006) | |
| 95 | 12β, 27-dihydroxy solasodine β-chacotrioside | C45H73NO17 | EtOH | He et al. (2015), Zhou (2006) | |
| 96 | 23-O-acetyl-12β-hydroxysolasodine | C29H45NO15 | 117803-97-1 | EtOH | He et al. (2015), Zhou (2006) |
| 97 | (3β, 22α, 25R)-spirosol-5-en-3yl-O-α-l-Rhamanopyranosyl-(1-2)-[O-β-d-glucopyranosyl(1–3)]-O-β-d-galactopyranoside | C39H85NO15 | 101009-59-0 | EtOH | Yang (2014) |
| 98 | 15α-hydroxysolasodine | C27H43NO3 | 10009-88-8 | EtOH | Wu (2011) |
| 99 | α-Solanine | C45H73NO15 | 20562-02-1 | EtOH | Huang et al. (2020) |
| 100 | Solasonine | C45H73NO16 | 19121-58-5 | EtOH | He et al. (2015), Zhou (2006) |
| 101 | Leptinine I | C45H73NO15 | EtOH | Gao et al. (2021) | |
| 102 | (7R, 8S)-1-(4-hydroxy-3-methoxyphenyl)-2-{4-{2-[N-2-(4-hydroxyphenyl)ethyl]Carbamoylehenyl | C28H32NO8 | EtOH | Li et al. (2019) | |
| -2-methoxyphenoxyl}}-1, 3-propanodiolnamed | |||||
| 103 | (7S, 8R)-1-(4-hydroxy-3-methoxyphenyl)-2-{4-{2-[N-2-(4-hydroxyphenyl)ethyl]Carbamoylehenyl | C28H32NO8 | EtOH | Li et al. (2019) | |
| -2-methoxyphenoxyl}}-1, 3-propanodiolnamed | |||||
| 104 | (7R, 8R)-1-(4-hydroxy-3-methoxyphenyl)-2-{4-{2-[N-2-(4-hydroxyphenyl)ethyl]Carbamoylehenyl | C28H32NO8 | EtOH | Li et al. (2019) | |
| -2-methoxyphenoxyl}}-1, 3-propanodiolnamed | |||||
| 105 | (7S, 8S)-1-(4-hydroxy-3-methoxyphenyl)-2-{4-{2-[N-2-(4-hydroxyphenyl)ethyl]Carbamoylehenyl-2-methoxyphenoxyl}}-1, 3-propanodiolnamed | C28H32NO8 | EtOH | Li et al. (2019) | |
| 106 | 7′S, 8′R-7-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-N2, N3-bis(4-hydroxyphenethyl)-6-methoxy | C36H37N2O8 | EtOH | Li et al. (2019) | |
| -1, 2-dihydronaphthalene-2, 3-dicarboxamide | |||||
| 107 | 7′R, 8′S-7-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-N2, N3-bis(4-hydroxyphenethyl)-6-methoxy | C36H37N2O8 | EtOH | Li et al. (2019) | |
| -1, 2-dihydronaphthalene-2, 3-dicarboxamide | |||||
| 108 | 7′R, 8′S-7-(4-hydroxy-3, 5-dimethoxyphenyl)-3′-hydroxymethyl-1′-[N-7″-(4″-hydrxyphenyl)ethyl] carbamoylethenyl-3′-methoxybenzodihydrofuran | C29H31NO8 | EtOH | Li et al. (2019) | |
| 109 | 7′S, 8′R-7-(4-hydroxy-3, 5-dimethoxyphenyl)-3′-hydroxymethyl-1′-[N-7″-(4″-hydrxyphenyl)ethyl] carbamoylethenyl-3′-methoxybenzodihydrofuran | C29H31NO8 | EtOH | Li et al. (2019) | |
| 110 | (7′R, 8′R)-2-(4-Hydroxy-3-methoxyphenyl)-3-[N-2-(4-hydroxyphenyl)ethyl]carbamoyl-5-[N-2-(4-hydroxyphenyl)ethyl]carbamoylethenyl-7-methoxybenzodihydrofurn | C36H36N2O8 | EtOH | Li et al. (2019) | |
| 111 | (7′S, 8′S)-2-(4-Hydroxy-3-methoxyphenyl)-3-[N-2-(4-hydroxyphenyl)ethyl]carbamoyl-5-[N-2-(4-hydroxyphenyl)ethyl]carbamoylethenyl-7-methoxybenzodihydrofurn | C36H36N2O8 | EtOH | Li et al. (2019) | |
| 112 | Cannabisin F | C36H36N2O8 | 163136-19-4 | EtOH | Li et al. (2019) |
| 113 | Adenine | C5H5N5 | 73-24-5 | MeOH | Gao et al. (2021) |
| 114 | Pyroglutamic acid | C5H7NO3 | 98-79-3 | MeOH | Gao et al. (2021) |
| 115 | Nicotinic acid | C6H5NO2 | 59-67-6 | MeOH | Gao et al. (2021) |
| 116 | 9-aminononane-1,3,9-tricarboxylic acid | C12H21NO6 | MeOH | Gao et al. (2021) | |
| 117 | Glutarylcarnitine | C12H21NO6 | 102636-82-8 | MeOH | Gao et al. (2021) |
| 118 | (6S)-3-((1H-imidazol-4-yl)methyl)-6-amino-1, 4-diazocane-2, 5, 8-trione | C10H13N5O3 | MeOH | Gao et al. (2021) | |
| 119 | 3-Indoleacrylic acid | C11H9NO2 | MeOH | Gao et al. (2021) | |
| 120 | 6-Hydroxypurine | C5H4N4O | 68-94-0 | MeOH | Gao et al. (2021) |
| 121 | Uridine | C9H12N2O6 | 58-96-8 | MeOH | Gao et al. (2021) |
| 122 | Ethyl 4-glycylbenzoate | C11H13NO3 | MeOH | Gao et al. (2021) | |
| 123 | Adenosine | C10H13N5O4 | 58-61-7 | MeOH | Gao et al. (2021), Wang (2007) |
| 124 | Dihydrocapsaicin | C18H29NO3 | 19408-84-5 | MeOH | Gao et al. (2021) |
| 125 | Choline | C5H14NO | 62-49-7 | MeOH | Gao et al. (2021) |
| 126 | Betaine | C5H11NO2 | 107-43-7 | MeOH | Gao et al. (2021) |
| 127 | Allantoin | C4H6N4O3 | 97-59-6 | MeOH | Gao et al. (2021) |
| 128 | Uracil | C4H4N2O2 | 66-22-8 | MeOH | Gao et al. (2021) |
| 129 | Trigonelline | C7H7NO2 | 535-83-1 | MeOH | Gao et al. (2021) |
| 130 | (2-acetoxyethyl)trimethylammonium | C7H16NO2 + | MeOH | Gao et al. (2021) | |
| 131 | Glycyl-l-leucine | C8H16N2O3 | MeOH | Gao et al. (2021) | |
| 132 | GABA | C4H9NO2 | 56-12-2 | MeOH | Gao et al. (2021) |
| 133 | FMoc-Asn(Trt)-OPfp | C44H31F5N2O5 | MeOH | Gao et al. (2021) | |
| Phenylpropanoids | |||||
| 134 | Trans-4-Hydroxycinnamic acid | C9H8O3 | 501-98-4 | EtOH | Liu et al. (2019b) |
| 135 | Cis-4-Hydroxycinnamic acid | C9H8O3 | 4501-31-9 | EtOH | Liu Z et al. (2019) |
| 136 | Ethyl 3, 4-dihydroxycinnaMate | C11H12O4 | 102-37-4 | EtOH | Liu et al. (2019a) |
| 137 | Cis-caffeic acid ethyl ester | C11H12O4 | 74257-25-3 | EtOH | Liu et al. (2019b) |
| 138 | Trans ferulic acid | C10H10O4 | 537-98-4 | EtOH | Liu Z et al. (2019) |
| 139 | Cis-ferulic acid | C10H10O4 | 1014-83-1 | EtOH | Liu et al. (2019a) |
| 140 | Caffeic acid | C9H8O4 | 331-39-5 | MeOH | Gao et al. (2021) |
| 141 | 4-(4-hydroxyphenyl)-2-methylenebutyrolactone | C11H10O3 | EtOH | Liu et al. (2019b) | |
| 142 | Chlorogenic acid | C16H18O9 | 327-97-9 | MeOH | Gao et al. (2021) |
| 143 | 3-caffeoylquinic acid methyl ester | C17H20O9 | 123483-19-2 | MeOH | Xiang et al. (2019) |
| 144 | Scopoletin | C10H8O4 | 92-61-5 | EtOH | Zhao (2010), Wang et al. (2007), Wang (2007) |
| 145 | (−)-5′-methoxyisolariciresinol-3α-O-β-d-glucopyranoside | C27H36O12 | MeOH | Xiang et al. (2019) | |
| 146 | (+)-isolariciresinol-3α-O-β-d-glucopyranoside | C26H34O11 | MeOH | Xiang et al. (2019) | |
| 147 | Cinnacassoside A | C26H36O12 | 1691248-24-4 | MeOH | Xiang et al. (2019) |
| 148 | Pinoresinol | C20H22O6 | 81446-29-9 | EtOH | Zhao (2010) |
| 149 | Pinoresinol-4-O-β-d-glucopyranoside | C26H32O11 | 69251-96-3 | EtOH | Wang et al. (2007), Wang (2007) |
| 150 | Syringaresinol | C22H26O8 | 487-35-4 | EtOH | Zhao (2010) |
| 151 | Syringaresinol-4-O-β-d-glucopyranoside | C28H36O13 | 137038-13-2 | EtOH | Wang et al. (2007), Wang (2007) |
| 152 | Medioresinol | C21H24O7 | 40957-99-1 | EtOH | Zhao (2010) |
| 153 | Acanthoside D | C34H46O18 | 573-44-4 | MeOH | Xiang et al. (2019) |
| 154 | (+)-medioresonol-di-O-β-d-glucopyranoside | C33H44O17 | MeOH | Xiang et al. (2019) | |
| Flavonoids | |||||
| 155 | Quercetin | C15H10O7 | 117-39-5 | EtOH | Yang (2014) |
| 156 | Quercitrin | C21H20O11 | 522-12-3 | EtOH | Yang (2014) |
| 157 | Isoquercitrin | C21H20O12 | 21637-25-2 | EtOH | Yang (2014) |
| 158 | Quercetin-3-O-β-d-glucopyranosyl(1-2)-β-d-glucopyranoside | C27H30O17 | EtOH | Yang (2014) | |
| 159 | Quercetin-3-O-β-d-galactopyranosyl-(1→6)-β-d-glucopyranoside | C28H32O17 | MeOH | Xiang et al. (2019) | |
| 160 | Quercetin-3-gentiobioside | C27H30O17 | 7431-83-6 | EtOH | Wu (2011) |
| 161 | Quercetin-3-O-α-l-rhaopyranosyl(1→4)-O-β-d-glucopyranosyl-(1→6)-O-β-d-glucopyranoside | C28H32O17 | EtOH | Li (2010b) | |
| 162 | 6-Hydroxyluteolin 7-sophoroside | C27H30O17 | MeOH | Gao et al. (2021) | |
| 163 | Kaempferol | C15H10O6 | 520-18-3 | EtOH | Liu Z et al. (2019) |
| 164 | (8-hydroxy-3′-β-d-galactosyl-isoflavone)-2′-8″-(4‴-hydroxy-flavone)-biflavone | C36H28O11 | EtOAc | Sabudak et al. (2017) | |
| 165 | 2′, 3′, 5-trihydroxy-5″-methoxy-3″-O- α-glucosyl-3-4‴-O-biflavone | C36H30O15 | EtOAc | Sabudak et al. (2017) | |
| Benzoic acids | |||||
| 166 | Gallic acid | C7H6O5 | 149-91-7 | MeOH | Gao et al. (2021) |
| 167 | 2, 4-Dihydroxybenzoic acid | C7H6O4 | 89-86-1 | MeOH | Gao et al. (2021) |
| 168 | Protocatechuic acid | C7H6O4 | 99-50-3 | EtOH | Zhao (2010) |
| 169 | Vanillic acid | C8H8O4 | 121-34-6 | EtOH | Zhao (2010) |
| 170 | 4-Hydroxybenzoic acid | C7H6O3 | 99-96-7 | EtOH | Liu et al. (2019a) |
| 171 | Salicylic acid | C7H6O3 | 69-72-7 | EtOH | Liu et al. (2019b) |
| 172 | 2, 5-Dihydroxybenzoic acid | C7H6O4 | 490-79-9 | MeOH | Gao et al. (2021) |
| Other compounds | |||||
| 173 | Galacturonic acid | C6H10O7 | 14982-50-4 | MeOH | Gao et al. (2021) |
| 174 | Pyruvic acid | C3H4O3 | 127-17-3 | MeOH | Gao et al. (2021) |
| 175 | Formic acid | CH2O2 | 64-18-6 | MeOH | Gao et al. (2021) |
| 176 | Succinic acid | C4H6O4 | 110-15-6 | MeOH | Gao et al. (2021) |
| 177 | Fumaric acid | C4H4O4 | 110-17-8 | MeOH | Gao et al. (2021) |
| 178 | Ursolic acid | C30H48O3 | 77-52-1 | EtOH | Zhao (2010) |
| 179 | Linolenic acid | C18H30O2 | 463-40-1 | MeOH | Gao et al. (2021) |
| 180 | Oleic acid | C18H34O2 | 112-80-1 | EtOH | Wang (2007) |
| 181 | Linoleic acid | C18H32O2 | 60-33-3 | EtOH | Wang (2007) |
| 182 | Palmitic acid | C16H32O2 | 57-10-3 | EtOH | Wang (2007) |
| 183 | 1-monolinolenin | C21H36O4 | 75685-85-7 | EtOH | Zhao (2010) |
| 184 | Lignoceric acid | C24H48O2 | 557-59-5 | EtOH | Zhao (2010) |
| 185 | (E)-docosyl-3-(4-hydroxy-3-methoxyphenyl)acrylate | C32H54O4 | EtOH | Zhao (2010) | |
| 186 | α-carotene | C40H56 | 432-70-2 | EtOH | Wu (2011) |
| 187 | β-carotene | C40H56 | 7235-40-7 | EtOH | Wu (2011) |
| 188 | Xanthophyll | C40H56O2 | 127-40-2 | EtOH | Wu (2011) |
FIGURE 2.
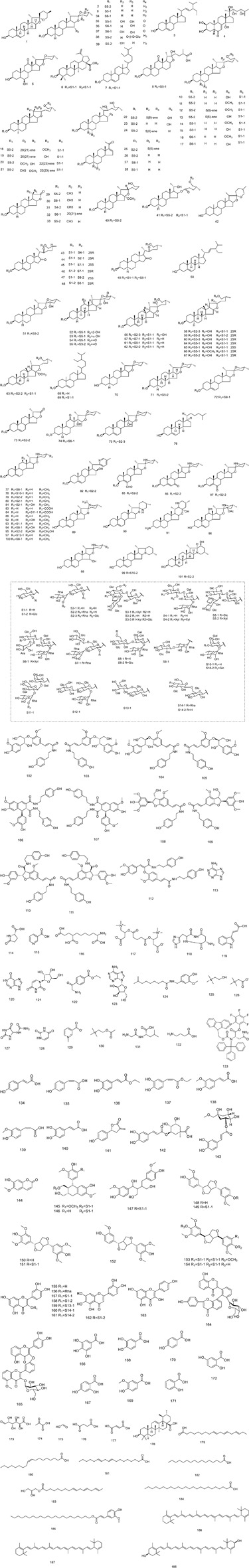
Chemical structures of compounds isolated from S. nigrum.
TABLE 3.
Monosaccharides composition, molecular weight, structures, and bioactivities of polysaccharides purified from S. nigrum.
| No. | Polysaccharides | Monosaccharide composition | M.W. (Da) | Structures | Bioactivities | References |
|---|---|---|---|---|---|---|
| 1 | S1 | mannose, glucose, galactose, arabinose in a ratio of 1.00: 8.32: 6.72: 2.90 | 5.44 × 104 | ND | Prebiotic effects | Yao et al. (2020) |
| 2 | S2 | rhamnose, galacturonic acid, glucose, galactose, arabinose in a ratio of 1.00: 0.76: 4.57: 7.25: 4.49 | 7.22 × 104 | ND | Prebiotic effects | Yao et al. (2020) |
| 3 | SNL-1 | rhamnose, xylose, arabinose, glucose in a ratio of 4.9: 1: 2. 4: 13 | 5.30 × 103 | α-glycosidic and β- glycosidic linkages | ND | Xiao and Zeng (1998) |
| 4 | SNL-2 | glucose, arabinose in a ratio of 13.3: 1 | 1.12 × 104 | α-glycosidic and β- glycosidic linkages | ND | Xiao and Zeng (1998) |
| 5 | SNL-3 | xylose, mannose, glucose, galactose in a ratio of 75.7: 9.2: 4.9: 10.2 | 2.37 × 104 | 1, 3 xyl residue linkage | Immunomodulatory activity | Xiao and Zeng (2000) |
| 6 | SNL-4 | xylose, mannose galactose in a ratio of 89.4: 1.7: 8.9 | 4.77 × 104 | 1, 3 xyl residue linkage | Immunomodulatory activity | Xiao and Zeng (2000) |
| 7 | SNL-WP-1 | galactose, glucose, mannose, arabinose in a ratio of 1.8: 1: 1.2: 2 | 1.41 × 104 | β-glycosidic linkages | Antitumor activity | Li (2010a) |
| 8 | SNL-WP-2 | galactose, glucose, mannose, xylose, arabinose in a ratio of 5.4:10.4: 1.4: 1: 1.6 | 9.12 × 103 | ND | Antitumor activity | Li (2010a) |
| 9 | SNL-AP-1 | galactose, glucose, mannose, xylose, arabinose in a ratio of 28: 1: 6: 51: 13 | >1.6× 106 | β-glycosidic linkages | Antitumor activity | Li (2010a) |
| 10 | SNL-AP-2 | galactose, glucose, mannose, xylose, arabinose in a ratio of 5.8: 1: 1.3: 2.6: 2 | >1.6× 106 | β-glycosidic linkages | Antitumor activity | Li (2010a) |
| 11 | SNLBP | xylose, mannose, glucose, galactose in a ratio of 82.2: 7.4: 3.8: 6.6 | ND | β-glycosidic linkages | Immunomodulatory activity | Liu (2003) |
| 12 | SNL-P1a | rhamnose, xylose, arabinose, glucose | ND | β (1→3) glycosidic bond | Immunomodulatory and antitumor activity | Li (2009) |
Steroidal Saponins
Steroidal saponins are an important class of secondary metabolites and pharmaceutical resources distributed in higher plants and some marine organisms, showing good pharmacological activities. Modern research suggests that steroidal saponins are the major pharmacologically active constituents of S. nigrum. Until now, 76 steroidal saponins (1–76) have been isolated and identified. Current research on the pharmacological activity of S. nigrum is mainly focused on the antitumor and anti-inflammatory activities, and research on the corresponding chemical compositions is mainly focused on the various types of steroidal saponins. In 2006, 22 new steroidal saponins (Solanigroside A-O, Solanigroside R-X) were isolated from the whole plant of S. nigrum, and structure-activity analysis of the steroidal saponins in S. nigrum showed that the cytotoxic activities of spirostanol saponins and progesterone saponins were stronger than those of furostanol saponins and cholesteric saponins (Zhou, 2006). In 2017, Wang et al. (2017), isolated nine new steroidal saponins from the berries of S. nigrum of which Solanigroside Y1 showed significant anti-inflammatory activity. Subsequently, Xiang et al. isolated seven new steroidal saponins (61–67) with a new cholestane 16, 22-dione skeleton from immature S. nigrum berries, and some of these compounds exhibited moderate anti-inflammatory activity (Xiang et al., 2018). The contents of the steroidal saponins are shown in Table 2, and their structures are presented in Figure 2.
Alkaloids
Until now, the alkaloids contained in S. nigrum reported in the literature are mainly steroidal alkaloids, and most of them are present in the form of glycosides in the fruits, stems, leaves, and roots of the plant. The immature fruit of S. nigrum has the highest content of steroidal alkaloids of up to 4.2%, which gradually decreases as the plant grows. This phenomenon may explain the self-protective effect of the plant, as the toxicity of S. nigrum alkaloids prevents the young leaves and fruits from being eaten by other animals and promotes the survival of the species. The steroidal alkaloids contained in S. nigrum are also the basis of the antitumor activity of S. nigrum. Among the steroidal alkaloids contained in S. nigrum, solasonine (100) and solamargine (79) make up to 0.2% and 0.25%, respectively, and the glycoside of solasonine and solamargine formed after alkaline hydrolysis is solasodine (88) (He et al., 2015). Solamargine (79) is the main component of the total alkaloids of S. nigrum, and pharmacological studies have shown that solamargine (79) has strong inhibitory activity against liver cancer, cervical cancer, lung cancer, laryngeal cancer, cholangiocarcinoma, esophageal cancer, etc (Wang 2007; Sun et al., 2010; Ding et al., 2012a; Zhang, 2018; Xiang et al., 2019). In addition, β 2 -solasonine (78), solaoiacid (84), (25R)-22αN-4-nor-spirosol-5(6)-en-3β-ol-6-al-3-O-l-rhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (85), and solasonine (100) showed antitumor and anti-inflammatory activities (Xiang et al., 2019; Li et al., 2020; Zhang et al., 2021).
In addition to steroidal alkaloids found in S. nigrum, other types of alkaloids have also been identified in S. nigrum. Lignanamides are a rare kind of natural product defined as blignans bearing amide groups, displaying diverse biological activities, including neuroprotective, anti-inflammatory, and insecticidal effects. Findings indicated that cannabisin F (112) isolated from the above-ground parts of S. nigrum has significant neuroprotective activity against MPP+-induced SH-SY5Y cell injury models at doses of 12.5, 25, and 50 μM (Li et al., 2019). In 2021, Gao et al. analyzed the chemical constituents of S. nigrum using LC-MS and NMR, and identified 89 compounds, mainly including adenine (113), nicotinic acid (115), 9-aminononane-1, 3, 9-tricarboxylic acid (116), adenosine (123), allantoin (127), and dozens of alkaloids (Gao et al., 2021).
Phenylpropanoids
Phenylpropanol is a naturally occurring compound composed of a benzene ring connected to three straight-chain carbons (C6-C3 groups). It has a phenol structure and is a phenolic substance. In the biosynthesis, most of these compounds are formed by a series of reactions such as deamination and hydroxylation of shikimic acid through aromatic amino acids, such as phenylalanine and tyrosine. Until now, 21 phenylpropanoids (134–154), including 10 phenylpropionic acid and their esters (134–143), 1 coumarin (144), and 10 lignans (145–154), have been successfully separated and chemically identified by spectroscopic analysis, including 1H-NMR and 13C-NMR, of the whole plants of S. nigrum. Scopoletin (144) is widely distributed in S. nigrum, and current studies have shown that it has various pharmacological activities, such as antitumor, anti-inflammatory, hypoglycemic, hypotensive, and anti-neurodegenerative effects (Chu et al., 2019).
Flavonoids
Flavonoids are a class of secondary metabolites that account for over half of the plant phenolics, with various pharmacological activities such as antioxidant and anti-inflammatory activities. In 2014, Yang et al. isolated and identified one flavone (155) and three flavone glycosides (156–158) from the whole plant of S. nigrum (Yang, 2014). In 2017, compound (164) and (165) were isolated and identified from S. nigrum, showing that their cholinesterase inhibitory activity was weaker than that of their ethyl acetate extract of S. nigrum, presumably due to the synergistic effect between these compounds (Sabudak et al., 2017). The antioxidant activity of S. nigrum is closely related to its flavonoid content, and the research on the flavonoids of S. nigrum should be increased to provide the basis for the development and utilization of functional foods of S. nigrum.
Benzoic Acids
In addition, seven benzoic acids with phenolic hydroxyl substituents have been identified from S. nigrum, including gallic acid (166), 2, 4-dihydroxybenzoic acid (167), protocatechuic acid (168), vanillic acid (169), 4-hydroxybenzoic acid (170), salicylic acid (171), and 2, 5-dihydroxybenzoic acid (172) (Wang et al., 2007; Liu et al., 2019b; Gao et al., 2021). Most of these compounds have anti-inflammatory, antioxidant, antibacterial, and antiviral activities, providing broad application prospects and important pharmaceutical intermediates for disease treatment.
Polysaccharides
Polysaccharides are one of the four substances that form the basis of life activities. More and more research results show that some plant polysaccharides have many special biological activities, such as immune regulative, antifatigue, antioxidative, antiradiative, blood sugar-lowering, antiviral, antitumor, and liver-protective effects (Yang, 2014). At present, 12 kinds of polysaccharides have been successfully isolated and purified from S. nigrum, which are reported to have antitumor, immunomodulatory, and liver-protective activities (Liu, 2003; Liu, 2005; Yao et al., 2020). The monosaccharide composition, molecular weight, structural characteristics, and biological activities of the polysaccharides purified from S. nigrum are summarized and presented in Table 3.
Other Compounds
In addition to the above-mentioned compounds, a few compounds (173–188) have been identified from S. nigrum until now, and the corresponding chemical structures are shown in Figure 2. Compounds (173–177) were identified as organic acids. Ursolic acid (178) is a famous antitumor triterpene, and compounds (179–184) are aliphatic compounds. Compound (185) is a ferulic acid ester, which are compounds that are, in addition to α-carotene (186), β-carotene (187), and xanthophyll (188), essential nutrients for people and of great significance to the health of human eyes and skin (Wang, 2007; Zhao, 2010; Gao et al., 2021).
Pharmacological Activities
Numerous studies have reported the pharmacological activities of S. nigrum in the past decades. Various solvent extracts and isolated bioactive compounds of S. nigrum have exhibited many pharmacological properties including antitumor, antioxidant, anti-inflammatory, immunomodulatory, antihypertensive, antimicrobial, and antiviral activities (Yang et al., 2016; Veerapagu et al., 2018; Tian et al., 2019; Guo et al., 2020; Sivaraj et al., 2020). These pharmacological studies have been summarized in Table 4, and the reported effects and mechanisms will be discussed in detail in the following paragraphs. A variety of proprietary Chinese medicines in which S. nigrum extract is one of the medicinal ingredients have been widely used in clinical practice. Some of the patents containing S. nigrum can be found in Table 5, the pharmacological activities are mostly found in the field of treatment of tumors and skin diseases, mostly in the form of combination with other herbs.
TABLE 4.
Biological activity of bioactive compounds and extracts of S. nigrum.
| Biological activities | Extracts/compounds | Types | Testing subjects | Doses/duration | Mechanisms/effects | References |
|---|---|---|---|---|---|---|
| Antitumor activity | ||||||
| SNPE | In vitro | HepG2 cells | 0.5, 1.0 and 2.0 mg/ml for 24 h | IC50 value was 0.75 mg/ml, arrested the cell cycle at the G2/M phase and CDK1, Bcl-2 and Bid protein expression levels ↓ | Wang et al. (2010) | |
| SNPE | In vivo | HepG2 tumor-bearing mice | 1 or 2 µg/ml for 35 days | Tumor weight and tumor volume ↓ | Wang et al. (2011) | |
| SNWE | In vitro | HepG2 cells | 0.05–2 mg/ml for 24 h | The IC50 of SNWE and SNPE was 2.18 and 0.86 mg/ml, respectively, inhibited TPA-induced HepG2 migration, TPA-induced PKCα and p38 protein expression levels ↓ | Yang et al. (2010) | |
| SNPE | ||||||
| SNWE | In vitro | HUVEC and HepG2 cells | 0.1–2 mg/ml for 24, 48, 72, and 96 h | Suppression of the VEGF-induced activation of AKT and mTOR | Yang et al. (2016) | |
| SNPE | ||||||
| SNWE | In vivo | HepG2 tumor-bearing mice | 0–2% for 35 days | Reduced the volume and weight of the tumors, and CD31 protein expression levels ↓ | Yang et al. (2016) | |
| SNPE | ||||||
| SNEE | In vitro | A549 cells | 100 µg/ml for 16 h | Exhibited specifically stat3-suppressing activity in A549 cells through the decrease of Bcl-xL expression | Park et al. (2014) | |
| SNTA | In vitro | RPMI-8226 cells | 12.5, 25, and 50 mg/kg for 14 days | Inhibited I κB-α Phosphorylation and NF-κB/IRF4 signaling pathway to induce apoptosis | Liu et al. (2021) | |
| SNFME | In vitro | C6 cells | 0.025–0.4 mg/ml | IC50 value was 0.23 mg/ml, attenuated cell cloning, migration and invasion | Li et al. (2021) | |
| SNWE | In vitro | TG-elicited peritoneal macrophages | 10–500 mg/ml for 12 h | Decreased NO production and increased the expression of iNOS protein | An et al. (2005) | |
| SNLP-1 | In vivo | Lung Cancer Bearing Mice | 200 mg/kg/day | Played an antitumor role by enhancing the function of the immune system in the body | Pu (2020) | |
| SNCE | In vitro | 786-O cells | 40 mg/ml | Inhibited proliferation and promoted apoptosis by inhibiting the activation of PI3K/Akt signaling pathway | Liao et al. (2020) | |
| 2 | In vitro | Human hepatoma cancer cell line (HepG2 cell) | 3.125, 6.25, 12.5, 25, 50, and 100 µM | IC50 value against HepG2 cell was 0.245 μg/ml | Wang (2007) | |
| 2 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2 Cell) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against four tumor cells were 4.4, 3.1, 1.5, and 0.2 μM, respectively | Zhou (2006) | |
| 26 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2 Cell) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against four tumor cells were 31.8, 34.7, 29.1, and 19.6 μM, respectively | Zhou (2006) | |
| 28 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2 Cell) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against four tumor cells were 52.3, 260.4, 64.7, and 48.6 μM, respectively | Zhou (2006) | |
| 78 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2 Cell) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against four tumor cells were 22.9, 34.2, 42.2, and 19.2 μM, respectively | Zhou (2006) | |
| 79 | In vitro | Human tumor cells lines (K562, KB, K562/A02, and KB/VCR) | NW | IC50 values against four tumor cells were 8.0, 7.8, 5.4, and 7.1 μM, respectively | Zhao (2010) | |
| 79 | In vitro | HepG2 | 3.125, 6.25, 12.5, 25, 50, and 100 µM | IC50 value against HepG2 cell was 19.2 μg/ml | Wang (2007) | |
| 79 | In vitro | Human tumor cells lines (HL-60, U-937, Jurkat, K562, and HepG2) | NW | Exhibited the most potent cytotoxicity to all the cell lines with IC50 values of 3.53, 9.31, 2.72, 8.75, 5.36 μM, respectively | Xiang et al. (2019) | |
| 79 | In vitro | Human tumor cells lines (MDA-MB-231, A549, Hep3B, PC3) | 30 and 100 µM | IC50 values against 4 tumor cells were 1.86, 2.24, 0.78, 5.13 μM, respectively | Tai et al. (2018) | |
| 79 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against 4 tumor cells were 21.4, 25.1, 15.2, and 7.6 μM, respectively | Zhou (2006) | |
| 79 | In vitro | K562 cells | 5, 7.5 and 10 μM for 0, 2, 4, 6, 8, and 24 h | Induced tumor apoptosis by initiating an early lysosomal destabilization pathway | Sun et al. (2010) | |
| 79 | In vitro | SMMC-7721 cells | 0, 5, 10, 15 μg/ml for 72 h | The IC50 values were 9.21 μg/ml | Ding et al. (2012a) | |
| 79 | In vitro | QBC939 cells | 0–10 μΜ | The IC50 value was 9.81 μΜ, Inhibited the metastasis and invasion by inhibiting the expression of PI3K/Akt signal pathway | Zhang (2018) | |
| 84 | In vitro | Lung cancer A549 cells | 1–20 μmol/L for 24 h | IC50 values against A549 cells were 2.36 μM, respectively | Shi et al. (2019) | |
| 99 | In vitro | PANC-1 cells | 0, 2, 4, 6 μg/ml | N-cadherin, vimentin, MMP2 expression level ↓; E-cadherin expression level ↑ | Kong et al. (2020) | |
| 99 | In vitro | RKO and HCT-116 cells | 13–32 µM in RKO cells, 11–28 µM in HCT-116 cells | IC50 were 20.84 and 20.32 µM respectively. The expression levels of cyclin D1 and cyclin-dependent kinase 2 in RKO cells↓; production of ROS in RKO cells ↑; inhibited the migration and invasion of HCT-116 cells | Yan et al. (2020) | |
| 99 | In vitro | HCT-116 cells | 4–32 μmol/L for 48 h | Inhibited proliferation and clone, induced apoptosis by activating Caspase-3 | Hu et al. (2019) | |
| 99 | In vitro | SK-OV3 cells | 5–15 μmol/L for 24, 48, 72 h | Inhibited proliferation and induced apoptosis by regulating the expression of p-Akt, cleaved Caspase-3 and p53 protein | Zhu et al. (2019) | |
| 99 | In vitro | RKO cells | NW | Induced apoptosis by activation of Caspase-3, the increase of intracellular ROS level and the inhibition of FAK phosphorylation | Yan and Hu (2019) | |
| 99 | In vitro | U87 cells | 2.5–30 μg/μl | Inhibited proliferation and induced apoptosisby by down-regulating the expression of Ki-67, PCNA and Bcl-2 protein and up-regulating the expression of Bax protein | Zhao et al. (2019) | |
| 99 | In vitro | SGC-7901 cells | 25, 50, 100 μg/ml for 48, 72 h | Inhibited proliferation and promoted apoptosis by up-regulating the expression of mir-140 and down-regulating the expression of MACC1 | Huang et al. (2020) | |
| 99 | In vitro | EC9706, KYSE30 cells | 4 μmol/L | Enhanced the drug sensitivity of esophageal cancer cell lines EC9706 and kyse30 to 5-fluorouracil and cisplatin via | Wu (2019) | |
| 99 | In vivo | ACHN-induced tumor-bearing mice | 20 mg/kg for 28 days | Inhibited tumor growth through HIF-1α pathway to affect the expression and activity of key enzymes of glycolysis | Wang et al. (2019) | |
| 100 | In vitro | THP-1, MV4-11, NB-4, HL-60, HEL cells | NW | IC50 were 11.19, 12.50, 15.45, 15.87, 17 mM, promoted apoptosis and caused less cell cycle arrest in the G2/M phase through the activation of the AMPK/FOXO3A Axis | Zhang et al. (2021) | |
| 100 | In vitro | HepG2 and QGY-7703 cells | 0–50 µM for 24, 48, 72 h | The proliferation of hepatoma cells was inhibited by activating mir-375-3p, ccat1, Sp1 and IRF5 protein expression levels ↓ | Zhang et al. (2021) | |
| 100 | In vitro | A549 cells | 0, 15, 20, 25 μmol/L for 24, 48, and 72 h | Induced apoptosis by inhibiting the expression of p65 and Bcl-2 protein, enhancing the expression of bik and Bak protein, and activating Caspase-3 pathway | Li et al. (2020) | |
| 100 | In vitro | Human tumor cells lines (NCI-H460, SF-268, MCF-7, HepG2) | 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | IC50 values against four tumor cells were 97.5, 113.5, 75.7, and 48.9 μM, respectively | Zhou (2006) | |
| 100 | In vitro | Human tumor cells lines (HL-60, U-937, Jurkat, K562, and HepG2) | NW | IC50 values against five tumor cells were 33.32, 39.16, 12.85, 26.83, and 17.33 μM, respectively | Xiang et al. (2019) | |
| 180 | In vitro | HepG2 | 3.125, 6.25, 12.5, 25, 50, and 100 µM | IC50 value was 62.3 μg/ml | Wang (2007) | |
| 181 | In vitro | HepG2 | 3.125, 6.25, 12.5, 25, 50, and 100 µM | IC50 value was 57.5 μg/ml | Wang (2007) | |
| Anti-inflammatory activity | ||||||
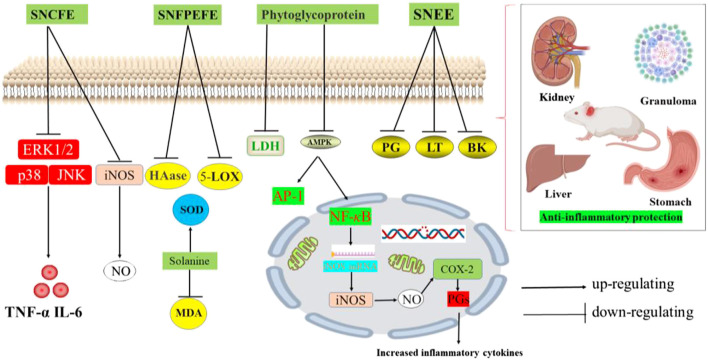
| SNCFE | In vitro | Peritoneal macrophages | 0–200 μg/ml for 4 and 24 h | NO, TNF-α and IL-6 levels ↓; p38, JNK and ERK1/2 expression levels ↓ | Kang et al. (2011) | |
| SNFEE | In vivo | Acute ear edema mouse model | 0.125, 0.250, 0.500, and 1.000 mg/ml | The cell viability below 0.5 mg/ml was about 90%, alleviating edema and decreased thickness of ear tissue | Yeom et al. (2019) | |
| SNEE | In vivo | Acute and sub-acute rat model | 100 and 200 mg/kg | The pathological changes of granuloma, kidney, liver and stomach were lighter than those in the model group | Aryaa and Viswanathswamy (2017) | |
| SNWE | In vitro | Patients with thoracic malignant tumor after radiotherapy | NW | PDGF, TGF-β1, IL-6,TNF-α expression level ↑ | Che (2018) | |
| SNFPEFE | In vitro | Hyaluronidase, lipoxygenase | 100–1,000 µg/ml | The IC50 values of hyaluronidase and lipoxygenase were 810.67 and 781.28 µg/ml | Guo et al. (2020) | |
| 43 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 40.11 μM) | Xiang et al. (2018) | |
| 44 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 72.39 μM) | Xiang et al. (2018) | |
| 45 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 33.00 μM) | Xiang et al. (2018) | |
| 46 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 48.75 μM) | Xiang et al. (2018) | |
| 47 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 50.77 μM) | Xiang et al. (2018) | |
| 48 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 63.66 μM) | Xiang et al. (2018) | |
| 49 | In vitro | LPS-induced RAW 264.7 cells | 2.5, 5, 10, 20, 40, and 50 μM | NO inhibition (IC50 = 11.33 μM) | Xiang et al. (2018) | |
| 52 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 9.7 μM) | Wang et al. (2017) | |
| 53 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 17.8 μM) | Wang et al. (2017) | |
| 54 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 14.0 μM) | Wang et al. (2017) | |
| 56 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 38.3 μM) | Wang et al. (2017) | |
| 57 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 41.0 μM) | Wang et al. (2017) | |
| 59 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 48.5 μM) | Wang et al. (2017) | |
| 60 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 44.0 μM) | Wang et al. (2017) | |
| 63 | In vitro | LPS-induced RAW 264.7 cells | 12.5 and 25.0 μM for 24 h | NO inhibition (IC50 = 22.1 μM) | Wang et al. (2017) | |
| 85 | In vitro | LPS-induced RAW 264.7 cells | NW | NO inhibition (IC50 = 23.42 μM) | Xiang et al. (2019) | |
| 164 | In vitro | BChE assay | NW | moderate BChE inhibitory activity (IC50 = 195.2 µg/ml) | Sabudak et al. (2017) | |
| 165 | In vitro | BChE assay | NW | Moderate BChE inhibitory activity (IC50 = 299.1 µg/ml) | Sabudak et al. (2017) | |
| Antioxidant activity | ||||||
| SNFME | In vitro | DPPH and hydrogen peroxide radicals | 25, 50,100, 150, and 200 μg/ml | The IC50 value of 70.73 μg/ml for DPPH radical scavenging and IC50 59.72 μg/ml for hydrogen peroxide scavenging activity | Veerapagu et al. (2018) | |
| SNFEE | In vitro | DPPH and hydroxyl radical | 0–2.4 mg/ml | The scavenging rate on DPPH, hydroxyl radical scavenging assay were 68.45% and 49.12%, respectively | Teng et al. (2014) | |
| SNFP | In vitro | DPPH and hydroxyl radicals | 0–1.2 mg/ml | The IC50 values were 65.43 μg/ml and 0.33 mg/ml for DPPH, hydroxyl radical scavenging assay | Chen et al. (2020) | |
| SNFEAE | In vitro | FRAP and DPPH· scavenging assays | 100–2,500 μg/ml for FRAP 50–1,000 µg/ml for DPPH | The IC50 values were 119.43 µg/ml and 2.674 µg/ml, FeSO4/L for DPPH and FRAP scavenging activity | Guo et al. (2020) | |
| SNFEE | In vitro | DPPH and ABTS radical | 0–120 μg/ml | Showed moderate free radical scavenging activity against DPPH and ABTS+ free radical with the IC50 were 81.02 and 35.56 μg/ml, respectively | Sivaraj et al. (2020) | |
| Immunoregulatory activity | ||||||
| SNLWP-1 SNLAP-1 SNLAP-2 | In vivo | H22-bearing mice | 50, 100, and 200 mg/kg for 10 days | IL-2, IFN-c levels ↑; IL-10 levels↓ | Ding et al. (2012b) | |
| SNCP | In vivo | Male BALB/C mice | 200, 400, 800 mg/kg for 28 days | B.T, NK cell activity ↑ | Tian et al. (2019) | |
| SNLP-1 | In vivo | Lung Cancer Bearing Mice | 200 mg/kg/day | CD4+/CD8+of T lymphocytes levels ↑; Th1 cytokines levels ↑ | Pu (2020) | |
| Hepatoprotective activity | ||||||
| SNWE | in vivo | CCl4-induced chronic hepatotoxicity in rats | 0.2, 0.5, and 1.0 g/kg for 6 weeks | GOT, GPT, ALP, total bilirubin, superoxide , hydroxyl radical levels↓; GSH, SOD, GST Al, GST Mu levels ↑ | Lin et al. (2008) | |
| SNFBFE | in vivo | D-GalN-induced hepatic fibrosis rats | 16 and 25 mg/kg for 10 days | ALT, AST, ALP enzymes, GSH, SOD, and CAT levels↓ | Chester et al. (2019) | |
| SNWSP | in vivo | CCl4-induced acute injury in rats | 100, 200, 400 mg/kg for 7 days | ALT, AST, ALP, MDA levels↓; SOD, GSH-Px, CAT levels ↑ | Yang et al. (2014) | |
| SNWE | in vivo | Ethanol-induced liver injury in rats | 100, 150, 200 mg/kg for 7 days | ALT, AST, GSTA1, MDA levels↓; SOD, GSH, GSH-Px ↑ | Han (2014) | |
| Antibacterial activity | ||||||
| SNFEE | in vitro | Aspergillus’s Niger, Fusarium oxysprum | 250–1,000 µg/ml for 24 h | Highest antifungal zone was 32.42 and 28.16 mm against Aspergillus’s Niger and Fusarium oxysprum | Mazher et al. (2017) | |
| SNFEE | in vitro | Escherichia coli | 250–625 µg/ml | The maximum zone of inhibition was 25 mm for Escherichia coli at 625 µg/ml concentration | Sivaraj et al. (2020) | |
| SNEE | in vitro | Staphylococcus aureus | 12.5–200 mg/ml | The maximum zones of inhibition were 16.88, 11.33, and 19.25 mm for Staphylococcus aureus, Escherichia coli, Aeromona sobria at 200 mg/ml concentration | Ge (2019) | |
| Escherichia coli | ||||||
| Aeromona sobria | ||||||
| SNEE | in vitro | Alternaria solani | NW | The EC50 values of Rhizoctonia solani and Fusarium oxysporun were 1,629 and 1,262 ppm | Cai (2003) | |
| Cladosporium cucumerinum | ||||||
| Fusarium oxysporun | ||||||
| Rhizoctonia solani | ||||||
| 93 | in vitro | Candida albicans | 0, 8, 16, 32, and 64 mg/L for 12, 24, 36, 48 h | Inhibited the activity of Candida albicans via regulating Ras-cAMP-PKA signaling pathway and reducing the intracellular cAMP content | Li et al. (2015) | |
| 93 | in vitro | Candida albicans | 32, 64 µg/ml | Alkalizing the intracellular vacuole of Candida albicans and causing hyper-permeability of the vacuole membrane | Chang et al. (2017) | |
| Insecticidal activity | ||||||
| SNLME | in vitro | 2nd instar larvae of CPB | 5, 10,15, 20, 25, 30, 35, 40, and 45 mg/ml | Caused 50% mortality for 2nd instar CPB larvae at concentration of 5 ppm and foliar consumption was decreased by 74% | Ben-Abdallah et al. (2019) | |
| SNLCME | in vitro | Cx. vishnui group and An. subpictus | 25, 45, 60 mg/L for 24, 48, and 72 h | Showed 100 percent larval mortality against early 3rd instar of An. subpictus at 60 mg/L | Rawani et al. (2017) | |
| SNFMWE | in vitro | Galba truncatula | NW | The hydro-methanol LC50 = 3.96 mg/L, LC90 = 7.49 mg/L | Hammami et al. (2011) | |
| SNLEAE | in vitro | Culex quinquefasciatus | 10–50 ppm for 24–72 h | LC50 values of ethyl acetate extracts were 17.04 ppm | Rawani et al. (2010) | |
| SNLEE | in vitro | Green Peach Aphid Myzus persicae Sulzer | 4.24 mg/ml for 24, 48, and 72 h | Caused 28.54%, 56.8%, and 57.42% mortality rates after 24, 48, and 72 h exposure | Madanat et al. (2016) | |
| SNLME | in vitro | Culex quinquefasciatus | 6.25–1,000 ppm | Methanol leaves extract causing 90% mortality rate | Rahuman et al. (2009) | |
| Neuroprotective activity | ||||||
| SNL | in vivo | SCOP-induced cognitive impairment rats | 5% and 10% leaf inclusions | ChEs levels↑; restored the impaired memory function | Ogunsuyi et al. (2018) | |
| SNL | in vivo | AlCl3-induced neurodegeneration in Drosophila melanogaster | 0.1% and 1.0% pulverized vegetable for 7 days | GST, MAO, ChE, ROS, TBARS levels ↓; Athletic, memory ability ↑ | Ogunsuyi et al. (2020) | |
| SNL | in vivo | AlCl3-induced neurodegeneration in Drosophila melanogaster | 0.1% and 1.0% pulverized vegetable for 7 days | ROS, GST, Hsp70, Jafrac1, reaper and NF-kҝB/Relish ↓; cnc/Nrf2 and FOXO ↑ | Ogunsuyi et al. (2021) | |
| 112 | In vitro | MPP+-induced SH-SY5Y cells | 12.5, 25, and 50 μM for 1 h | Induced protective autophagy to protect SH-SY5Y cells from MPP+-induced apoptosis, the cell viability of which improved by 12% at 25 μM | Li et al. (2019) | |
| Gastroprotective activity | ||||||
| SNEE | in vivo | Ethanol-induced gastric ulcer mice | 5–500 mg/kg | At dose of 500 mg/kg, the extract was as effective as lansoprazole in reducing all parameters of peptic ulcer in both models | El-Meligy et al. (2015) | |
| SNFME | in vivo | Gastric ulcer rats | 200 and 400 mg/kg | Gastric secretory volume, acidity, pepsin secretion ↓ | Jainu and Devi (2006) | |
| Hypoglycemic activity | ||||||
| SNFWE | in vivo | Streptozotocin-induced Diabetic rats | 1 g/L for 8 weeks | Ca/Mg ratio, plasma glucose, HDL, LDL, VLDL, cholesterol, triglyceride ↓ | Sohrabipour et al. (2013) | |
| Antimalarial activity | ||||||
| 79 | in vivo | Plasmodium yoelii-infected mice | 7.5 mg/kg for 4 days | At a dose of 7.50 mg/kg, the parasitemia suppressions of solamargine were 64.89%, respectively | Chen et al. (2010) | |
| 100 | in vivo | Plasmodium yoelii-infected mice | 7.5 mg/kg for 4 days | At a dose of 7.50 mg/kg, the parasitemia suppressions of solasonine were 57.47%, respectively | Chen et al. (2010) | |
| CNS-depressant activity | ||||||
| SNFEE | in vivo | Wistar rats and CD1 mice | 51, 127.5, and 255 mg/kg | Exploratory and aggressive behavior↓; locomotor activity↓; pentobarbital-induced sleeping time ↑ | Perez et al. (1998) | |
| Hypolipidemic activity | ||||||
| SNWE | in vitro | 3T3L1 cells model | 0.3, 0.4, 0.5 mg/ml | PPARα, CPT-1 ↑; FaS, HMG-CoR↓; amount and lipid content of adipocytes ↓; inhibiting lipogenesis | Peng et al. (2020) | |
| SNSEE | in vivo | Triton-induced hyperlipidemic rats | 200 and 400 mg/kg | Total cholesterol, triglycerides, LDL cholesterol ↓; HDL cholesterol ↑ | Sohrabipour et al. (2013) | |
Note: NM, not mentioned; SNWE, water extracts of S. nigrum; SNPE, polyphenol extracts of S. nigrum; SNEE, ethanol extracts of S. nigrum; SNTA, total alkaloids of S. nigrum; SNFME, Methanol extracts of S. nigrum fruits; SNCE, chloroform Extracts of S. nigrum; SNFEE, ethanol extracts of S. nigrum fruits; SNFP, Polysaccharide from S. nigrum fruit; SNFEAE, Ethyl acetate extracts of S. nigrum fruit; SNCFE, chloroform Fraction extracts of S. nigrum; SNFPEFE, Petroleum ether fraction extracts of S. nigrum fruit; SNCP, Crude Polysaccharides from S. nigrum; SNFBFE, n-butanol fraction extracts of S. nigrum fruit; SNWSP, water-soluble polysaccharides from S. nigrum; SNLME, methanol extracts of S. nigrum leaves; SNLCME, chloroform: methanol (1:1 v/v) extracts of S. nigrum leaves; SNFMWE, methanol-water (8:2 v/v) extracts of S. nigrum fruit; SNLEAE,Ethyl acetate extracts of S. nigrum leaves; SNLEE, ethanol extracts of S. nigrum leaves; SNFWE, water extracts of S. nigrum fruits; SNL, S. nigrum leaves; SNSEE, ethanol extracts of S. nigrum seeds.
TABLE 5.
Patents list of products containing S. nigrum and their claimed pharmacological properties.
| Application | Main composition | Pharmacological properties | Publish number |
|---|---|---|---|
| Herbal preparation | Fritillaria thunbergii Miq., Hedyotis diffusa Willd., S. nigrum , Paris polyphylla Smith, Scutellaria barbata D. Don, Adenophora stricta Miq., Prunella vulgaris Linn., etc. | Treating pulmonary fibrosis | CN111991507A |
| Herbal preparation | Coix lacryma-jobi Linn., Amygdalus persica Linn., Carthamus tinctorius Linn., Scutellaria baicalensis Georgi, Sophora flavescens Alt., Rehmannia glutinosa Libosch., S. nigrum , etc. | Treating lung and colon cancer | CN113398215A |
| Bacteriostatic agent | S. nigrum , Artemisia argyi Levl. et Van., Syzygium aromaticum (L. ) Merr. Et Perry, Cynanchum paniculatum (Bunge) Kitagawa, Carbomer, Salicylic acid, etc. | Treating skin diseases | CN113368193A |
| Herbal preparation | Cordyceps Sinensis (Berk.) Sacc., S. nigrum extract, Taxus chinensis (Pilger) Rehd. extract | Treating lung cancer | CN113332358A |
| Herbal preparation | S. Nigrum , Pteris multifida Poir., Lithospermum erythrorhizon Sieb. et Zucc., Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f., Dioscorea polystachya Turczaninow | Treating psoriasis | CN110368445A |
| Herbal preparation | S. Nigrum , Astragalus membranaceus (Fisch. )Bge., Panax ginseng C. A. Meyer, Atractylodes macrocephala Koidz., Hedyotis diffusa Willd., Curcuma zedoaria (Christm.) Rosc., etc | Treating stomach cancer | CN112717097A |
| Herbal preparation | S. Nigrum , Cinnamomum cassia Presl, Dioscorea polystachya Turczaninow, Asarum sieboldii Miq., Scrophularia ningpoensis Hemsl., Cornus officinalis Sieb. et Zucc., etc | Treating glaucoma | CN108210683A |
| Herbal preparation | S. Nigrum , Hedyotis diffusa Willd., Verbena officinalis Linn., Lithospermum erythrorhizon Sieb. et Zucc., Reynoutria japonica Houtt., Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f., etc | Treating vaginitis and cervicitis | CN111991481A |
| Herbal preparation | S. Nigrum , Bidens pilosa Linn., Hedyotis diffusa Willd., Verbena officinalis Linn., Taraxacum mongolicum Hand.-Mazz., Plantago asiatica L., etc | Treating cholecystitis | CN111529631B |
| Herbal preparation | S. Nigrum , Gentiana scabra Bunge, Gardenia jasminoides Ellis, Paeonia suffruticosa Andr., Angelica sinensis (Oliv.) Diels, Pinellia ternata (Thunb.) Breit., etc | Treating leukemia | CN106822558B |
Abbreviation: A549, human alveolar basal epithelial cells; ABTS, 2, 2′-azino-bis-(3-ethylbenzenthiazoline-6-sulphonic) acids; AChE, acetylcholinesterase; AFP, Alpha-FetoProtein; AKT, proteinkinase B; ALP, alkaline phosphatase; AMPK, 5-AMP activated protein kinase; AP-1, activator protein-1; Bax, bcl-associated X protein; Bcl-2, B-cell CLL/lymphoma 2; Caspase 3, cysteinyl aspartate-specific proteinase-3; Caspase 7, cysteinyl aspartate-specific proteinase-7; CAT, catalase; CDC25, recombinant cell division cycle protein 25; CDK1, recombinant cyclin dependent kinase 1; c-JUN, c-Junamino-terminalkinase; COX-2, cyclooxygenase-2; DLD-1, human colorectal adenocarcinoma epithelial cells; DPPH, 1,1-Diphenyl-2-picrylhydrazyl free radical; GOT, glutamic-oxal(o)acetic transaminase; GPT, glutamic pyruvate transaminase; GPx, glutathione peroxidase; GSH, l-glutathione; GST-α, glutathione S-transferase-α; GST-μ, glutathione S-transferase-μ; H22, mouse H22 hepatocellular carcinoma cells; HAase, Human Hyaluronidase; HCT-116, human colorectal adenocarcinoma cells; HDL, high-density lipoprotein; HEL, human erythorleukemia cell line; HepG2, liver hepatocellular cells; HL-60, Leukemia Myeloidcells; HT-29, human conlon carcinoma cells; IC50, half maximal inhibitory concentration; IFN-γ, Interferon-gamma; IL-17, interleukin-17; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-6, interleukin- 6; iNOS, inducible nitric oxide synthase; c-JNK, Jun N-terminal kinase; LC3, microtubule associated proteins 1A/1B light chain 3; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; LT, leukotriene; MACC1, human metastasis associated in colon cancer 1; MCF-7, human breast adenocarcinoma cell line; MDA, malondialdehyde; MIC, minimum inhibitory concentration; mTOR, molecular target of rapamycin; MV4-11, Human acute monocytic Leukemia cells; MyD88, myeloid differentiation primary response protein; NB4, acute promyelocyte cells; NF-κB, nuclear factor kappa-B; NO, nitric oxide; Nrf2, Nuclear Factor erythroid 2-Related Factor 2; PDTC, pyrrolidine dithiocarbamate; PG, prostaglandin; PKCα, recombinant protein kinase C Alpha; ROS, reactive oxygen species; SGC-7901, human gastric cancer cells; SMMC-721, human hepatocellular carcinoma cells; SOD, superoxide dismutase; TG, thioglycollate; THP-1, human monocytic leukemia cells; TLR4, toll like receptor 4; TNF, tumor necrosing factor; TNF-α, tumor necrosis factor -α; TRAF-6, TNF receptor associated factor 6; VEGF, vascular endothlial growth factor; XTT, 2, 3- bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide; γ-GT, γ-glutamy transpeptidase.
Antitumor Activity
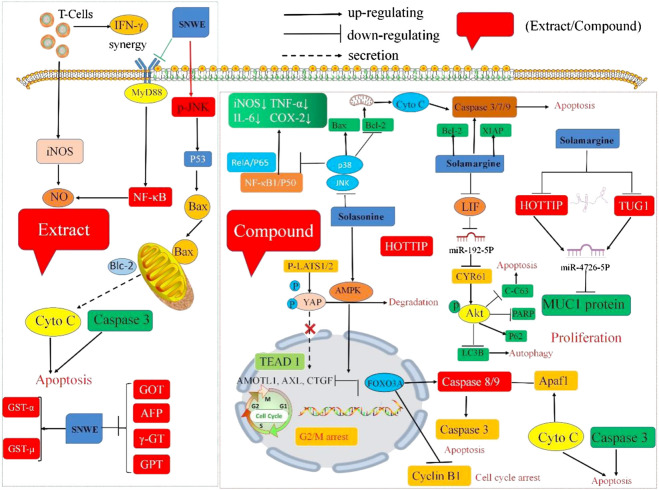
Crude extracts and isolated compounds of S. nigrum have exhibited significant antitumor potential in vitro and in vivo. The underlying mechanism of the antitumor activity of the crude extracts or bioactive substances of S. nigrum is presented in Table 4 and Figure 3.
FIGURE 3.
Schematic representation of the molecular mechanism of anti-tumor activities of crude extracts or isolated compounds from S. nigrum. (SNWE, water extracts of S. nigrum).
Crude Extract
In vitro studies showed that different solvent extracts of S. nigrum significantly inhibited the growth of various cancer cell lines, such as human breast cancer cell line MCF-7, renal cell carcinoma cell line 786-O, esophageal cancer cell line ECA-109, human liver cell lines SMMC-721 and HepG2, gastric cancer cell line MGC-803, human colorectal carcinoma cell lines HT-29, HCT-116, and DLD-1, and human lung cancer cell line A549 (He et al., 2015). Specifically, the treatment with the water extract of S. nigrum (SNWE) induced apoptosis in HepG2 cells by increasing the mitochondrial release of cytochrome C, and activating Caspase-3, and inducing autophagy through implicating the levels of LC3, Bcl-2, and Akt (Lin et al., 2007). Nitric oxide (NO) is an antitumor molecule produced by activated macrophages. Harvesting thioglycollate (TG)-elicited peritoneal macrophages from mice followed by incubation with different concentrations of SNWE (10–500 mg/ml) alone or with recombinant interferon-γ (rIFN-γ) (20 U/ml) for 6 h showed that the extract dose-dependently induced NO production and iNOS expression, which was highly strengthened in combination with rIFN-γ. Further mechanism research demonstrated that pyrrolidine dithiocarbamate (PDTC), an NF-κB inhibitor, inhibited the synergistic effect of S. nigrum and rIFN-γ on the NO production and iNOS expression. These results suggested that S. nigrum increased the NO production through NF-κB activation (An et al., 2005). Furthermore, 1% and 2% SNWE prominently reduced hepatic carcinogenesis to 40% and 20% and significantly increased the survival rate to 90% and 100%, respectively, in AAF/NaNO2-induced hepatoma rats (Hsu et al., 2009). SNWE also caused 43% cytotoxicity, inhibited migration, and suppressed the activities of hexokinase and pyruvate on the of human breast cancer cell line (MCF-7) by about 30% and 40% at a concentration of 10 g/L, respectively (Ling et al., 2019).
Wang et al. reported that the IC50 value of the polyphenolic extract of S. nigrum (SNPE) was 0.75 mg/ml and the cell viabilities of HepG2 cells were 85%, 27%, and 6% at concentrations of 0.5, 1.0, and 2.0 mg/ml, respectively. Furthermore, SNPE arrested the cell cycle at the G2/M phase (21.13%, 24.53%, and 31.62% at 0.5, 1.0, and 2.0 mg/ml, respectively) by regulating the activity of the CDC25 family and CDK1 and reactivated apoptosis via decreasing protein expression of Bcl-2 and Bid. Moreover, SNPE decreased the tumor weight and tumor volume after feeding HepG2 tumor-bearing mice daily with 5 g basal diet containing 1 or 2 µg/ml (w/v) SNPE for 35 days (Wang et al., 2010). Yang et al. (2010) also revealed that SNPE significantly reduced the viability of HepG2 cells (IC50 = 0.86 mg/ml). The mechanism of action study showed that SNPE inhibited TPA-induced HepG2 migration and invasion via blocking the expression of PKCα and attenuated p38 and p38/ERK activation (Yang et al., 2010). Moreover, SNPE inhibited the viability of HepG2 cells through the suppression of the VEGF-induced activation of AKT and mTOR in vitro and reduced the volume and weight of the tumors in the HepG2 tumor-bearing mouse model (Yang et al., 2016).
According to relevant literature reports, different solvent extracts (water, ethanol, chloroform, and n-Butanol) extracts of S. nigrum show strong broad-spectrum antitumor activity. It has been demonstrated that the ethanol extract of S. nigrum fruit (SNCE) could arrest the cell cycle in the S phase and continue to the G2/M phase, inhibiting MCF-7 proliferation (IC50 = 40.77 μg/ml) and inducing apoptosis 43.31% (Churiyah et al., 2020). The n-Butanol extract of S. nigrum inhibited the growth of human colorectal cancer SW480 cells in a dose-dependent manner via blocking cells in the G2/M phase and increasing the expression of Caspase-3 (Ye and Gao, 2019). These studies indicated that different solvent extracts of S. nigrum could be promising candidates for the treatment of cancer.
Isolated Compounds
Furthermore, compounds isolated from S. nigrum also displayed multiple antitumor effects. α-solanine (99) is a chemical component that widely exists in potatoes, tomatoes, eggplants and other Solanaceae plants. Pharmacological studies have shown that it has antitumor and insecticidal activities, but it also showed toxic effects on humans in overdose. Treatment of the human gastric cancer cell line (SGC-7901) with different concentrations of α-solanine (25, 50, 100 μg/ml) for 24 or 48 h inhibited proliferation and promoted apoptosis of cells by upregulating the expression of miR-140 and downregulating the expression of MACC1 (Huang et al., 2020). Furthermore, α-solanine exhibited antitumor properties by regulating the expression of p-Akt, cleaving Caspase-3 and p53 protein, increasing the intracellular ROS level, and inhibiting FAK phosphorylation and the expression of miR-138 and the survivin protein (Wu, 2011; Zhu et al., 2019). The IC50 values of solasonine (100) against five tumor cells (THP-1, MV4-11, HL-60, NB4, HEL) were 11.19, 12.50, 15.87, 15.45, and 17.00 mM, respectively. Moreover, solasonine promoted apoptosis and caused less cell cycle arrest in the G2/M phase through the activation of the AMPK/FOXO3A Axis. In the THP-1-induced xenograft model, all the mice were intraperitoneally injected with 4, 8, and 16 mg/kg solasonine once a day for 14 days, and the pharmaceutical and immunoblotting results showed that solasonine inhibited tumor growth with increasing solasonine concentration and induced the nuclear translocation of FOXO3A, upregulated the expression of Bax and P-CDK1, and downregulated the expression of Bcl-2 and Cyclin B1 (Zhang et al., 2021). Solasonine induced apoptosis by inhibiting the expression of the proteins p65 and Bcl-2, enhancing the expression of the proteins Bik and Bak, and activating the Caspase-3 pathway (Li et al., 2020). Zhang et al. showed that solamargine (79) induced apoptosis by decreasing the mitochondrial membrane potential, upregulating the expression of pro-apoptotic protein, and downregulating the expression of anti-apoptotic protein with an IC50 value of 9.81 μΜ (Zhang, 2018). In 2022, Yin et al. found that solamargine induces apoptosis and autophagy in liver cancer cells by regulating the LIF/miR-192-5p/CYR61/Akt signaling pathway at high doses, thereby inhibiting tumor cell proliferation. Solamargine at low doses regulates the biological function of immune cells by inhibiting the LIF/Stat3 signaling pathway, reshaping the tumor microenvironment, and inhibiting the process of tumor cell heterogeneity, thereby exerting a synergistic antitumor effect. This provides a scientific basis for its wide application in modern clinical treatment (Yin et al., 2022). In the clinic, adjuvant therapies, such as radiotherapy, endocrine therapy, chemotherapy, and targeted therapy, are administered to patients with advanced or metastatic tumors, but subsequent side effects usually result in treatment failure and increased mortality. Hence, the need for drugs with strong efficacy but minimal side effects and toxicity is great. Degalactotigonin (2) isolated from S. nigrum can inhibit the proliferation, invasion, migration and tumorigenicity of renal cell carcinoma cells. It can be used as an effective drug for the treatment of advanced renal cell carcinoma (Wang et al., 2020). This provides strong evidence that TCM can be better applied in the clinic.
Immunomodulatory Activity
S. nigrum crude polysaccharides SNLP-1 displayed obvious immunoregulatory activity for macrophages by promoting the release of NO and the secretion of cytokines (TNF-α and IL-6) in vitro, and further mechanistic studies have indicated that SNLP-1 improves the gene and protein expression levels of TLR4 and its key nodes MyD88, TRAF-6, NF-κB, and c-JUN in the pathway. Establishing a lung cancer mice model and oral administration with 200 mg/kg/day SNLP-1, SNLP-1 not only reduced the tumor weight of lung cancer mice, but also increased the index of thymus and spleen. Flow cytometry revealed that the ratio of T lymphocyte subsets CD4+/CD8+ and the concentrations of serum Th1 cytokines (IFN-γ, IL-2, TNF) in mice were increased by SNLP-1. This indicated that the homogeneous polysaccharide fraction SNLP-1 can enhance the function of the body’s immune system in vivo and in vitro (Pu, 2020). Similarly, prepared and cultured mouse spleen lymphocytes with LPS and ConA, thymus and spleen index, B and T lymphocyte proliferative transformation, and NK cell activity were significantly higher in three groups than in the control group after daily oral administration of 800, 400 or 200 mg/kg crude polysaccharides of S. nigrum for 28 days (Tian et al., 2019). Compared with the control group, S. nigrum water extract polysaccharide (SNLWP-1) and alkali extract polysaccharide (SNLAP-1 and SNLAP-2) significantly increase the weight of immune organs and serum IL-2 and IFN-γ in H22 tumor bearing mice (Ding et al., 2012b). Other extractions protocols, including decoction also exhibited immunomodulatory activity in clinical studies. Treatment with commissioned Longkui Yinxiao Tablet for 8 weeks resulted in higher CD4+/CD8+ and CD4+ levels in the psoriasis treatment group and lower CD8+ and cytokines TNF-α, IL-6, and IL-17 levels compared with the control group (Dai et al., 2018).
The above literature indicated that S. nigrum, especially polysaccharides, has immunomodulatory activity in vitro and in vivo. However the immunomodulatory mechanism of S. nigrum is limited to the level of inflammatory factors, and there is a lack of in-depth exploration of its immunomodulatory mechanism. Research on effective immunomodulatory components mainly focuses on polysaccharides, but more attention should be paid to other effective components.
Anti-Inflammatory Activity
Inflammation is the immune system’s response of the body to pathogenic factors and their damaging effects, and it is a self-protective response to help the body recover and resist infection, disease and pain. Relevant studies have confirmed the anti-inflammatory activity of crude extracts of S. nigrum in various inflammation related models and explained the possible related mechanisms, and a possible mechanism of action is shown in Figure 4. Chloroform extract of S. nigrum showed 80% inhibition of the NO and iNOS production stimulated by LPS at a concentration of 50 μg/ml. TNF-α and IL-6 are cytokines, which are responsible for the pathogenesis of many inflammatory disorders. Thus, the levels of TNF-α and IL-6 measured by ELISA on LPS-induced peritoneal macrophages have been determined, and the results showed that the chloroform fraction reduced the TNF-α and IL-6 levels in a dose-dependent manner and inhibited the phosphorylation of p38, JNK and ERK1/2, which may account for the anti-inflammatory mechanism of the S. nigrum chloroform fraction (Kang et al., 2011). Yeom et al. showed in the TPA-induced acute ear edema mouse model that the cell viability in fruit extract of S. nigrum in 80% ethanol was 51.35% at the concentration of 0.125 mg/ml. The NO production in S. nigrum fruit extract in 80% ethanol decreased to 4.1%, 16.1%, 61.0%, and 79.8% at concentrations of 0.125, 0.250, 0.500, and 1.000 mg/ml, respectively, and could also relieve edema and decrease the thickness of ear tissue in vivo, probably accounting for the abundance of alkaloid and flavonoid (Yeom et al., 2019). Similar results in acute and subacute rat models, and the hydroalcoholic extracts of S. nigrum showed less deposition of macrophages and more deposition of collagen fibres and exhibited organ protection properties (liver, stomach, and kidney), perhaps owing to the presence of steroidal alkaloids and steroidal saponins of S. nigrum (Aryaa and Viswanathswamy, 2017). Self-made S. nigrum suppository promoted the repair of damaged epithelial tissue and restored prostatic secretion through the decrease of the rat prostate wet quality and the white blood cell count and the increase in the density of lecithin corpuscle (Xu et al., 2015). Likewise, self-made S. nigrum could increase the SOD activity and decrease the MDA content to exhibit anti-inflammatory activity in the rabbit synovium after the rabbit knee joint was coated with self-made S. nigrum ointment for 1.5 h per day for 6 days in total in an electroacupuncture-made rabbit knee synovitis model (Li J et al., 2021). In 2017, Wang et al., isolated nine new steroidal saponins from berries of S. nigrum, Among these steroidal saponins, solanigrosides Y1 (52) significantly inhibited the NO production with an IC50 value of 9.7 μM, and some compounds exhibited significant inhibitory effects on the LPS-induced IL-6 and IL-1β production (Wang et al., 2017). In 2018, Xiang et al. isolated seven previously undescribed steroidal glycosides from the unripe berries of S. nigrum, and all seven compounds exhibited inhibitory activities on the NO production with IC50 values ranging from 11.33 to 49.35 µM (Xiang et al., 2018). Overall, the extracts and other preparations from S. nigrum deserve more studies related to inflammatory diseases.
FIGURE 4.
Schematic representation of the molecular mechanism of anti-inflammatory activities of crude extracts or isolated compounds from S. nigrum. (SNCFE, Chloroform fraction extracts of S. nigrum; SNFPEFE, Petroleum ether fraction extracts of S. nigrum fruit; SNEE, Ethanol extracts of S. nigrum).
Antibacterial and Larvicidal Activity
So far, bacterial and fungal infections and multidrug resistance have been great threats to human health and are important challenges that need to be urgently solved. Pharmacological studies have demonstrated that the antifungal activities of different parts of S. nigrum prepared in four solvents (ethanol, chloroform, petroleum ether, and distilled water) to struggle against five fungal strains viz. Aspergillus’s Niger, A. flavors, Saccharomyces cervisae, Alternaria alternate, and Fusarium oxysprum. The MIC was found to be in the range of 250–1,000 μg/ml, and the zone of inhibition was measured in the range of 9.3 mm of stem extract in distilled water against A. flavus and 32.42 mm of fruit extract in chloroform against A. niger (Mazher et al., 2017). The maximum zones of inhibition of ethanol extract of S. nigrum were 16.88, 11.33, and 19.25 mm for Staphylococcus aureus, Escherichia coli, and Aeromona sobria at a concentration of 200 mg/ml, respectively (Ge, 2019). The isolated compound, solasodine-3-O-β-d-glucopyranoside (93) displayed potent fungicidal activity against both azole-sensitive and azole-resistant Candida albicans strains in spider medium with the MIC value of 32 mg/ml, which may be attributed to the essential role of the glucosyl moiety. Subsequent pharmacological mechanism studies found that solasodine-3-O-β-d-glucopyranoside alkalized the intracellular vacuole and elicited vacuole permeability to contribute to cell death in C. albicans (Chang et al., 2017). Another study showed that solasodine-3-O-β-d-glucopyranoside could attenuate the virulence of Candida albicans through inhibiting adhesion and yeast-to-hyphal morphological transformation, and the results of the XTT reduction assay showed that solasodine-3-O-β-d-glucopyranoside could inhibit biofilm formation of C. albicans at concentrations of 16 mg/L or above. Next, downregulation of adhesion-related genes such as ALS3, EAP1, and HWP1 with the addition of 1 mM cAMP rescued the growth rate of solasodine-3-O-β-d-glucopyranoside-treated biofilm from 27.11% to 77.15%. The results suggested that solasodine-3-O-β-d-glucopyranoside inhibits the activity of C. albicans via regulating the Ras-cAMP-PKA signaling pathway and reducing the intracellular cAMP content (Li et al., 2015).
After exposition to graded concentrations (2.5, 5, and 10 ppm) of the synthesized silver nanoparticles (AgNPs) to fight against third instar larvae of C.quinquefasciatus and An. Stephensi, the IC50 and IC90 values of dry leaf, fresh leaf, and berry extracts of S. nigrum for An. Stephensi were 1.33, 1.59, and 1.56 ppm and 3.97, 7.31, and 4.76 ppm, respectively. The IC50 and IC90 values of dry leaf, fresh leaf and berry extracts of S. nigrum for C.quinquefasciatus were 1.26, 1.33, and 2.44 ppm and 14.38, 38.04, and 13.42 ppm, respectively (Rawani et al., 2013). To explore the acaricidal activity of crude extracts of S. nigrum for Tetranychus cinnabarinus, Chen et al. conducted slide-dip and leaf-dip assays and glasshouse experiments, revealing LC50 and LC90 values of 3.50, 3.99, 4.70, 12.15, 13.99, and 14.92 g/L after 1, 3, and 7 days of treatment, respectively. (Chen and Dai, 2016). The methanol leaf extract possessed the highest larvicidal activity (mortality rate of 90%) against the early fourth-instar larvae of C. quinquefasciatus at 1,000 ppm (Rahuman et al., 2009). Leaf extract from S. nigrum caused mortality rates of 28.54%, 56.8%, and 57.42% of the green peach aphid after exposure for 24, 48, and 72 h (Madanat et al., 2016). In addition, the active compounds solamargine and solasonine isolated from S. nigrum were found to fight against Plasmodium yoelii 17XL in mice, and the parasitemia suppressions of solamargine and solasonine were 64.89% and 57.47%, respectively (Chen et al., 2010). However, there is a lack of research on the mechanism underlying the insecticidal effect.
Antioxidant Activity
Excessive accumulation of free radicals in the body may cause aging and tissue damage. Oxidative stress is the pathogen of many human diseases, such as atherosclerosis, ischemic reperfusion injury, inflammation, cancer, aging, and neurodegenerative diseases. Antioxidants are the only weapon against the oxidizing and damaging effects of free radicals on the body’s vital chemicals and cells. Various studies focus on the antioxidant activity of the bioactive compounds or extracts of S. nigrum using in vitro and in vivo assays. Sivaraj et al. carried out DPPH˙ radical, superoxide radical, ABTS˙+ radical cation, phosphomolybdenum reduction, and Fe3+ reducing power assays of the fruit extract of S. nigrum. The maximum DPPH˙ radical scavenging activity was 73.16% at 120 µg/ml (IC50 = 81.02 μg/ml), the maximum superoxide radical scavenging activity was 60.06% at 120 μg/ml (IC50 = 57.82 μg/ml), the maximum ABTS˙+ radical cation scavenging activity was 67.70% at 60 µg/ml (IC50 = 35.56 μg/ml), the maximum phosphomolybdenum reduction was 96.77% at 120 µg/ml (RC50 = 21.25 μg/ml), and the maximum Fe3+ reduction was 72.10% at 120 µg/ml (RC50 = 63.74 μg/ml). These results indicated that two compounds, flavone and oleic acid, may be one of the reasons for the antioxidant property of the fruit extract of S. nigrum (Sivaraj et al., 2020). GSH and ROS levels determined in primary rat astroglial cell cultures showed that the leaf extract of S. nigrum could increase intracellular GSH levels and decrease ROS levels, which indicated that the leaf extract of S. nigrum. could restore the oxidative status by using glutamate as a stressor, prevent the increase in the glutamate uptake, and inhibit the excitotoxicity of glutamate (Campisi et al., 2019). According to the hydroxyl radical scavenging assay, 60% ethanol crude extract of S. nigrum had DPPH scavenging a rates of 68.45% and 49.12% (Teng et al., 2014). Lunasin peptide, purified from S. nigrum, inhibited oxidative DNA damage in a dose dependent manner by blocking the Fenton reaction between Fe2+ and H2O2 by chelating Fe2+ suggesting that lunasin peptide may play an important role in the chemoprevention for oxidative carcinogenesis (Jeong et al., 2010). After the treatment with glucose oxidase or xanthine oxidase alone or in combination with 0.01, 0.1, 1, 10, and 20 μg/ml of glycoprotein for 4 h, the viabilities of the NIH 3T3 cells were 40.4%, 47.3%, 53.0%, 68.2%, and 85.3%, respectively, compared with 33.3% of the control group (glucose oxidase). The viabilities were 75.1%, 79.3%, 83.2%, 86.0%, and 95.1%, respectively, compared with 71.2% of the control group (xanthine oxidase) (Heo and Lim, 2004). Overall, the extracts and compounds from S. nigrum may be suitable antioxidants for the further investigations.
Hepatoprotective Activity
The water extract of S. nigrum (SNWE) demonstrated the potential to protect the liver against diseases. Administration of SNWE at dosages of 0.2, 0.5, and 1.0 g/kg for 6 weeks in CCl4-induced chronic hepatotoxic rats reversed the body and organ weights and caused cloudy swelling, necrosis, cytoplasmic vacuolization and fatty degeneration determined by both qualitative and quantitative histopathological examinations. Furthermore, SNWE also reduced the levels of serum liver enzyme markers (GOT, GPT, ALP, and total bilirubin), superoxide and hydroxyl radicals and restored the GSH and SOD contents to the normal level especially at high doses of 0.5 and 1.0 g/kg (Lin et al., 2008). In AAF-induced liver damage rats, the liver/body weight ratios were 3.1- and 2.9-fold of that of the control group for 1% and 2% SNWE supplement, respectively. SNWE also decreased the levels of the serum biomarkers GOT, GPT, γ-GT, and AFP and induced the expression and the activation of both GSTs (GST-α, and GST-μ), which were responsible for the metabolism of a broad range of xenobiotics and carcinogens. Moreover, the treatment of SNWE regulated the level of Nrf2 and the level of downstream antioxidant enzymes regulated by Nrf2, including GPx, SOD-1, and catalase (Hsu et al., 2009). Chester et al. reported that the hydroalcoholic extract (250 mg/kg) of S. nigrum showed a significant decrease in hepatic GSH, SOD, and CAT and considered this as an index of the antioxidant status of tissues in d-galactosamine-induced hepatic fibrosis rats. Histopathological study also showed that the crude extract had a protective effect on the liver due to the antioxidant properties of the plant (Chester et al., 2019). Polysaccharides, extracted from S. nigrum, alleviated liver swelling, increased the levels of SOD, GSH, and CAT, decreased the content of MDA (Yang et al., 2014).
Neuroprotective Activity
With the development of modern medicine, there are increasing investigations to illustrate the mechanisms of the bioactive constituents isolated from S. nigrum, promoting the research and applications in clinic in turn. Regarding the central nervous system, Ogunsuyi et al. investigated the neuroprotective effect of S. nigrum on an oscopolamine-induced cognitive impairment model in rats. Pretreatment with 10% S. nigrum inclusions could also significantly restore the impaired memory function, decrease the AChE activity, MDA content, and BChE activity, and increase the brain GSH content (Ogunsuyi et al., 2018). In 2019, Ogunsuyi et al. continued to study the neuroprotective effect of S. nigrum in Drosophila melanogaster, and the results also showed that the impaired behavioral physiology and enzyme activities (glutathione-S-transferase, monoamine oxidase, cholinesterase) were ameliorated in Al-treated flies after the daily administration of pulverized vegetables for 7 days (Ogunsuyi et al., 2020). In 2021, Ogunsuyi et al. proved that 1% dietary inclusions of S. nigrum could decrease the survival rate and ROS levels and increase the total thiol contents in vivo (Ogunsuyi et al., 2021).
Anticholesterol Activity
Polyphenols derived from S. nigrum were reported to be an antiobesity agent, which could promote hepatic lipolysis, decrease serum triacylglyceride, cholesterol, and low-density lipoprotein (LDL)-cholesterol and inhibit lipogenesis (Peng et al., 2020). In addition, the oral administration of an ethanolic extract or chloroform fraction of S. nigrum (200 and 400 mg/kg) for 5 days to triton-induced hyperlipidemic rats reversed the elevation of the serum of total cholesterol, triglycerides and LDL cholesterol level and the reduction of the HDL cholesterol level (Sharma et al., 2012).
Clinical Effectiveness in Humans
As a folk medicine widely used around the world, S. nigrum has been widely reported clinically in recent years. The application of decoction of the whole herb or fruit of S. nigrum and its compound preparations for the treatment of liver cancer, lung cancer, cervical cancer, esophageal cancer, breast cancer, nasopharyngeal cancer, and other malignant tumors has attracted the attention of scholars at home and abroad for its remarkable clinical efficacy (Mei et al., 2011).
Liver cancer is currently the fourth most common malignant tumor and the second leading cause of cancer death in China, which seriously threatens the life and health of the Chinese people. In an open, prospective, randomized clinical trial conducted from 2012 to 2015, the clinical efficacy of S. nigrum tablets in the treatment of liver cancer was evaluated. Eighty-two patients with liver cancer were divided into observation group and control group according to the random number table method. The patients in the control group were treated with sorafenib, and the patients in the observation group were treated with S. nigrum tablets on the basis of the control group. The clinical efficacy, liver function recovery, inflammatory factor levels and survival rate were compared between the two groups. Results: The complete remission rate and total effective rate in the observation group were 14.63% (6/41) and 43.90% (18/41), which were significantly higher than those in the control group (2.44% (1/41) and 14.63% (6/41); the number of patients with liver function recovery in the observation group at 3 months, 6 months, and 1 year of treatment were significantly higher than those in the control group; after treatment, the IL-1, IL-6 and TNF-α levels were significantly lower than those in the control group. The 1-year survival rate and 2-year survival rate of the observation group were significantly higher than those of the control group. These research results show that S. nigrum tablet has a significant effect in the treatment of liver cancer, can effectively promote the recovery of liver function, improve the level of inflammatory factors, and improve the survival rate of patients (Yang et al., 2017). In addition, another clinical experimental study showed that the treatment of patients with advanced primary liver cancer with S. nigrum mixture can improve the clinical symptoms, liver function and immune function of the patients, effectively improve the quality of life of the patients, and is expected to prolong the survival period (Huang and Guan, 2013). In view of the diverse in vitro and in vivo pharmacological activities of S. nigrum, larger-scale randomized, double-blind, and controlled trials are needed to verify its clinical efficacy.
Toxicity
Although S. nigrum has been used as a drug or food for thousands of years and a large number of pharmacological activities have been reported, there are limited reports on the safety and side effects of the plant and its bioactive components. In 2005, Lai et al. studied the acute toxicity and genotoxicity assay of S. nigrum. The highest gavage dose of 21.5 g/kg was administered to mice, and no signs of toxicity and death were observed and recorded for 14 days of continuous gavage. In the genotoxicity test, the short-term mutagenicity of S. nigrum juice was investigated by the mouse sperm deformation test, micronucleus test, and Ames test. The results of these three tests were all negative, indicating no genotoxic effect (Lai et al., 2005). In 2014, Mo et al. experimentally confirmed the maximum dose of 494.4 g/kg of aqueous decoction of S. nigrum in mice. When normal people take the commonly used amount of S. nigrum (30–60 g), toxic and side effects are rarely observed. However, overdose can cause poisoning, resulting in symptoms such as headache, abdominal pain, vomiting, diarrhea, pupil dilation, arrhythmia, coma, and other symptoms (Mo et al., 2014).
Modern research showed that S. nigrum mainly contains active chemical ingredients such as alkaloids, saponins, and polysaccharides. Among these active ingredients, steroidal alkaloids have antitumor, liver and kidney protective, antipyretic, analgesic, anti-inflammatory, and expectorant effects. The steroidal alkaloids in S. nigrum not only have a wide range of pharmacological activities but also have some toxicity. Representative compounds are solasonine and solamargine, both of which are steroidal alkaloids composed of solanidine as an aglycon. Solanine causes strong irritation to gastrointestinal mucosa (Li, 2019). In addition, other toxicological tests have also confirmed that solanine can affect the embryonic development, causing miscarriage and stillbirth. The results of solanine on the bone marrow cell cycle of male mice showed that the ratio of cells in G0/G1 phase increased with increasing dose, while the ratios of S phase and G2/M phase decreased, and cells were blocked in G0/G1 phase. Affect the synthesis of DNA, and then affect the G2/M phase, so that the number of cells entering this phase is reduced. The incidence of micronucleus and sperm deformity increased gradually with the dose. Therefore it has potential mutagenic effects and certain genetic toxicity (Ji et al., 2009). However, the content of solanine in the leaves, stems and fruits of S. nigrum will gradually decrease as the plant grows. Preliminary toxicological studies of S. nigrum showed less toxicity and a certain impact on the liver and kidney function. In the future, a large amount of clinical and animal data will be needed to verify the safety of S. nigrum for better use as medicine in the field of clinical and health care and in food homologous products.
Conclusion and Future Perspectives
This review systematically summarizes the latest findings on the botany, traditional uses, phytochemistry, pharmacology, clinical trials, and toxicity of S. nigrum. Regarding the use as a medicinal and edible plant, there are records in the dietary histories of China and India that the leaves and fruits of S. nigrum were cooked as food. S. nigrum has been used to treat various cancers, diarrhea, fever, itchy skin, dysentery, edema, and other diseases in the indigenous peoples of China, India, Italy, Turkey, Yemen, Jordan, and Libya for more than a thousand years. Regarding the research on the phytochemical constituents of S. nigrum, 188 small molecule compounds and dozens of polysaccharides have been isolated and identified. Through systematic analysis, polysaccharides, steroidal saponins, and alkaloids were identified as the representative active components of S. nigrum with numerous pharmacological activities, which have been demonstrated in in vitro and in vivo investigations. Steroidal alkaloids represented by compounds 79 and 100 and polysaccharides represented by SNL-P1a have been considered to be biologically active components with extensive biological properties, including antitumor, anti-inflammatory, immunomodulatory, antimalarial, antibacterial, and antiviral effects. A large number of pharmacological studies have shown that 79 is a very promising candidate for the treatment of cancer. The content of vitamin C in the S. nigrum fruit is quite high, which has good nutritional and health value. The antitumor effect of S. nigrum polysaccharide is mainly through improving the immunity of the body. To sum up, as a food and medicinal resource, S. nigrum has a good health care function and important edible and medicinal value, and deserves more development and research.
However, a number of points also need to be improved: 1) A large number of studies on the in vitro and in vivo activities of S. nigrum extracts are listed in Table 3, but the material basis for the pharmacological activities of these crude extracts is still unknown. A large number of compounds have been isolated from S. nigrum, but current research on these compounds may be just the tip of the iceberg. Research on the chemical composition of S. nigrum mainly focuses on saponins, alkaloids, and polysaccharides, while the research on organic compounds, such as phenolic acids represented by flavonoids, is relatively rare. Therefore, research aimed at clarifying the active ingredients in S. nigrum, and the mechanism of action of the active ingredients should be further elucidated. For the trace active components in S. nigrum, its structure and activity mechanism can be studied through new technologies such as efficient preparation, computer virtual screening, and target fishing. 2) The composition and content of steroidal saponins and steroidal alkaloids in S. nigrum will change with the plant growth. To further explore the material basis of its active components, the composition and content of S. nigrum at different growth stages should be identified, and the biosynthetic pathway should be explored. 3) Toxicological studies are critical to assess the safety of herbal medicines, but data on the toxicology of S. nigrum remain scarce. It has been reported that the unripe S. nigrum fruit has a certain toxicity and can cause human poisoning and harm to human health. Therefore, it is necessary to carry out systematic toxicity and safety assessment studies on S. nigrum extracts and active ingredients to ensure full utilization of drug resources, meet Western standards of evidence-based medicine, and provide accurate evidence for clinical application. By studying the metabolites and metabolic mechanisms of drugs in the body, new drugs with higher biological activity and safer can be found. Therefore, pharmacokinetic, pharmacodynamic, and toxicological research are equally important in the process of drug development. Research on the drug metabolism of S. nigrum should be increased. 4) Since the S. nigrum plant contains potentially toxic compounds such as compound 79, reliable analytical methods are required for proper quality control of product development to ensure that potentially toxic components in S. nigrum products are kept below tolerated levels. 5) S. nigrum fruit is rich in vitamin C and polysaccharides with immunomodulatory effects. The processed products of S. nigrum need further research, exploration, and utilization, especially as a formula for fruit juice, fruit wine, and cosmetics.
Taken together, S. nigrum has attracted great interest as a medicinal and edible herb because of its rich nutrition and wide range of pharmacological activities. It has great development potential in the fields of functional food and TCM, and also provides resources for the lead compound library in the process of new drug development. However, in addition to providing opportunities, the application of S. nigrum also exhibits challenges. Facing the weak links and specific problems of the development of TCM, more time and research efforts need to be devoted to the high-quality development of TCM. We believe that this review can provide a valuable reference for the future development and utilization of S. nigrum.
Author Contributions
YL and LY obtained the literatures. XC, YY, and XD wrote the manuscript and finalized the paper. XH and GG gave ideas and edited the manuscript. All authors approved the paper for publication.
Funding
This work was supported by the Program for the Science and technology projects of Guizhou Province [Qian Kehe foundation-ZK (2021) General-550], Academic New Seedling Cultivation and Innovation Exploration Project’s Cultivation Project of Zunyi Medical University [Qian Kehe Platform Talents (2018)5772-074; Qian Kehe Platform Talents (2019)-017], and the Science and Technology Project of Zunyi [Grant No. ZSKH-HZ-(2020)-78]. Logistics Support Department of the Military Commission, (Grant No.CCD16J001; Grant No.CLB19J051). Project Support of the General Hospital of the Western Theater Command (Grant No. 2021-XZYG-A10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aburjai T. A., Oun I. M., Auzi A. A., Hudaib M. M. (2014). Volatile Oil Constituents of Fruits and Leaves of Solanum nigrumL. Growing in Libya. J. Essent. Oil Bear. Plants 17 (3), 397–404. 10.1080/0972060X.2014.895194 [DOI] [Google Scholar]
- An H. J., Kwon K. B., Cho H. I., Seo E. A., Ryu D. G., Hwang W. J., et al. (2005). Solanum nigrum Produces Nitric Oxide via Nuclear Factor-kappaB Activation in Mouse Peritoneal Macrophages. Eur. J. Cancer Prev. 14 (4), 345–350. 10.1097/00008469-200508000-00006 [DOI] [PubMed] [Google Scholar]
- Aryaa A., Viswanathswamy A. H. (2017). Effect of Solanum nigrum Linn on Acute and Sub-acute Models of Inflammation. J. Young. Pharm. 9 (4), 566–570. 10.5530/jyp.2017.9.108 [DOI] [Google Scholar]
- Ben-Abdallah S., Cáceres L. A., Wang Z., Renaud B. J., Lachâal M., Karray-Bouraoui N., et al. (2019). Host Plant Defenses of Black (Solanum nigrum L.) and Red Nightshade (Solanum Villosum Mill.) Against Specialist Solanaceae Herbivore Leptinotarsa decemlineata (Say). Arch. Insect Biochem. Physiol. 101 (2), e21550. 10.1002/arch.21550 [DOI] [PubMed] [Google Scholar]
- Cai Y. D. (2003). Studies on the fugicidal activity and formulation processing of taraxacum mongolicum. and Solanum nigrum L . Gansu: Master, Gansu Agricultural University. [Google Scholar]
- Campisi A., Acquaviva R., Raciti G., Duro A., Rizzo M., Santagati N. A. (2019). Antioxidant Activities of Solanum nigrum L. Leaf Extracts Determined in In Vitro Cellular Models. Foods 8 (2), 63. 10.3390/foods8020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Li Y., Zhang M., Zheng S., Li Y., Lou H. (2017). Solasodine-3-O-β-d-glucopyranoside Kills Candida Albicans by Disrupting the Intracellular Vacuole. Food Chem. Toxicol. 106, 139–146. 10.1016/j.fct.2017.05.045 [DOI] [PubMed] [Google Scholar]
- Che Y. F. (2018). Effect of Solanum nigrum Fruit on Acute Radiation-Induced Lung Injury after Tumor Radiotherapy and its Effect on Serum Related Inflammatory Cytokines. World Latest Med. Inf. 18 (84), 151–152. 10.19613/j.cnki.1671-3141.2018.84.126 [DOI] [Google Scholar]
- Chen Y. J., Dai G. H. (2016). Effect of the Extract and Compound from Solanum nigrum Linn on Tetranychus Cinnabarinus. J. Appl. Entomol. 141, 458–469. 10.1111/jen.12358 [DOI] [Google Scholar]
- Chen Y., Li S., Sun F., Han H., Zhang X., Fan Y., et al. (2010). In Vivo antimalarial Activities of Glycoalkaloids Isolated from Solanaceae Plants. Pharm. Biol. 48 (9), 1018–1024. 10.3109/13880200903440211 [DOI] [PubMed] [Google Scholar]
- Chen Y., Song Z. K., Zhang H. Y. (2020). Optimization Extraction and Antioxidant Activity of Polysaccharide from Solanum Nigrum Fruit by Response Surface Methodology. Food Sci. Tech-Brazil 45 (07), 209–215. 10.13684/j.cnki.spkj.2020.07.036 [DOI] [Google Scholar]
- Chester K., Zahiruddin S., Ahmad A., Khan W., Paliwal S., Ahmad S. (2019). Bioautography-Based Identification of Antioxidant Metabolites of Solanum nigrum L. and Exploration its Hepatoprotective Potential Against D-Galactosamine-Induced Hepatic Fibrosis in Rats. Pharmacogn. Mag. 15, 104–110. 10.4103/pm.pm_359_18 [DOI] [Google Scholar]
- Chu Z. X., Lu M., Xiong S. (2019). Research Advances on Pharmacological Activities and Pharmacokinetics of Scopoletin. Chem. Res. 30 (14), 434–440. 10.14002/j.hxya.2019.04.017 [DOI] [Google Scholar]
- Churiyah C., Ningsih S., Firdayani F. (2020). The Cytotoxic, Apoptotic Induction, aand Cell Cycle Arrest Activities of Solanum nigrum L. Ethanolic Extract on MCF-7 Human Breast Cancer Cell. Asian Pac J. Cancer Prev. 21 (12), 3735–3741. 10.31557/APJCP.2020.21.12.3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Li M., Fei J. R., Ni H., Huang Z. W., Ding P. J. (2018). Curative Effect of Longkui Yinxiao Tablet Combined With Liangxue Huayu Decoction in the Treatment of Psoriasis and Its Influence on Serum Level of Related Factors. J. Liaoning Univ. Tradit. Chin. Med. 20 (12), 198–201. 10.13194/j.issn.1673-842x.2018.12.057 [DOI] [Google Scholar]
- Ding X., Zhu F. S., Li M., Gao S. G. (2012a). Induction of Apoptosis in Human Hepatoma SMMC-7721 Cells by Solamargine From Solanum nigrum L . J. Ethnopharmacol. 139, 599–604. 10.1016/j.jep.2011.11.058 [DOI] [PubMed] [Google Scholar]
- Ding X., Zhu F., Gao S. (2012b). Purification, Antitumour and Immunomodulatory Activity of Water-Extractable and Alkali-Extractable Polysaccharides From Solanum nigrum L . Food Chem. 131, 677–684. 10.1016/j.foodchem.2011.09.060 [DOI] [Google Scholar]
- Editorial Committee of Flora of China (1979). Chinese Academy of Sciences. Flora of China, 67. Beijing: Science Press, 76. [Google Scholar]
- Editorial Committee of Nanjing University of Chinese Medicine (2006). Dictionary ofChinese Materia Medica. second ed. Shanghai: Science & Technology Press, 873–874. [Google Scholar]
- Editorial Committee of State Administration of traditional Chinese Medicine (1999). Zhonghua Bencao, 7. Shanghai: Science and Technology Press, 309–311. [Google Scholar]
- El-Meligy R. M., Awaad A. S., Soliman G. A., Bacha A. B., Alafeefy A. M., Kenawy S. A. (2015). Prophylactic and Curative Anti-Ulcerative Colitis Activity and The Possible Mechanisms of Action of Some Desert Plants. J. Enzyme Inhib. Med. Chem. 30 (2), 250–258. 10.3109/14756366.2014.915394 [DOI] [PubMed] [Google Scholar]
- Flora of China (2007). Flora of China. Available at: http://www.iplant.cn/info/Solanum%20nigrum?t=z .. [Google Scholar]
- Gao S, H., Su Z. Z., Yang L. J., Li Z. Y. (2021). Chemical Components From Stems of Solanum nigrum by LC-MS and NMR. Chin. Tradit. Herb. Drugs. 52 (5), 1263–1273. 10.7501/J.issn.0253-2670.2021.05.006 [DOI] [Google Scholar]
- Ge J. Q. (2019). Inhibitory Effects of Plants Extract of Trapa pinosa and Solanum Nigrum on Pathogenic Bacteria. J. Jingling, Tech. 35 (1), 88–92. 10.16515/j.cnki.32-1722/n.2019.01.019 [DOI] [Google Scholar]
- Geng Q. S., Zhu Z. J., Wang W. B., Shen Z. B., Li L. F., Xue W. H., et al. (2020). Study on the Biological Mechanism of the Action of Nightshade Against Lung Cancer by Using Network Pharmacology. Chin. J. Clin. Pharmacol. 36 (11), 1588–1591. 10.13699/j.cnki.1001-6821.2020.11.051 [DOI] [Google Scholar]
- Guo R., Li Y., Wang P. (2020). Evaluation on Antioxidant and Anti-Inflammation In Vitro of extracts of three Solanum Nigrum L. Berries. Mod. Food Sci. Technol. 36 (2), 94–101. 10.13982/j.mfst.1673-9078.2020.2.014 [DOI] [Google Scholar]
- Hammami H., Mezghani-Jarraya R., Damak M., Ayadi A. (2011). Molluscicidal Activity of Various Solvent Extracts from Solanum nigrum var. villosum L. aerial Parts Against Galba truncatula . Parasite 18, 63–70. 10.1051/parasite/2011181063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q. (2014). Analysis of GSTA1 on Ethanol Induced Mice Hepatocytes Injury and Hepatoprotective Role of Solanum nigrum Linn . Heilongjiang: Master, Northeast Agricultural University. [Google Scholar]
- He J., Zhou C. D., Ma B. Z., Liu F., Liu X., Zhao T. (2015). Research Progress on Chemical Constituents and Antitumor Pharmacological Activities of Solanum nigrum . China Pharm. 26 (31), 4433–4436. 10.6039/j.issn.1001-0408.2015.31.37 [DOI] [Google Scholar]
- Heo K. S., Lim K. T. (2004). Antioxidative effects of Glycoprotein Isolated from Solanum nigrum L . J. Med. Food 7 (3), 349–357. 10.1089/jmf.2004.7.349 [DOI] [PubMed] [Google Scholar]
- Hsu J. D., Kao S. H., Tu C. C., Li Y. J., Wang C. J. (2009). Solanum nigrum l. Extract Inhibits 2-Acetylaminofluorene-Induced Hepatocarcinogenesis Through Overexpression of Glutathione s-Transferase and Antioxidant enzymes. J. Agric. Food Chem. 57, 8628–8634. 10.1021/jf9017788 [DOI] [PubMed] [Google Scholar]
- Hu B., An H. M., Yan X., Zheng J. L., Huang X. W., Li M. (2019). Effects of Solanine on Proliferation and Apoptosis of Colorectal Cancer HCT116 Cells. Acta Chin. Med. Pharmacol. 47 (1), 61–63. 10.19664/j.cnki.1002-2392.190016 [DOI] [Google Scholar]
- Huang D. B., Guan J. (2013). Clinical Study of Solanum nigrum Mixture on Quality of Life and Immune Function in Patients with Advanced Liver Cancer. Lishizhen Med. Mater. Med. Res. 24 (07), 1676–1678. 10.3969/j.issn.1008-0805.2013.07.058 [DOI] [Google Scholar]
- Huang M. M., Liu M. Y., Li B. H., Li K. (2020). Solanine Regulates Proliferation and Apoptosis of Gastric Cancer Cells By Targeting miR-140/MACC1 pathway. Chin. J. Clin. Pharmacol. 36 (16), 2440–2443. 10.13699/j.cnki.1001-6821.2020.16.017 [DOI] [Google Scholar]
- Jainu M., Devi C. S. (2006). Antiulcerogenic and Ulcer Healing Effects of Solanum nigrum (L.) on Experimental Ulcer Models: Possible Mechanism for the Inhibition of Acid Formation. J. Ethnopharmacol. 104 (1-2), 156–163. 10.1016/j.jep.2005.08.064 [DOI] [PubMed] [Google Scholar]
- Jeong J. B., De Lumen B. O., Jeong H. J. (2010). Lunasin Peptide Purified from Solanum nigrum L. Protects DNA From Oxidative Damage by Suppressing the Generation Of Hydroxyl Radical Via Blocking Fenton Reaction. Cancer Lett. 293, 58–64. 10.1016/j.canlet.2009.12.019 [DOI] [PubMed] [Google Scholar]
- Ji B. Y., Wu P., Lang L. (2009). Progress in Toxicological Research of Solanine. Chin. Tradit. Herb. Drugs. 40, 29–31. [Google Scholar]
- Kang H., Jeong H. D., Choi H. Y. (2011). The Chloroform Fraction of Solanum Nigrum Suppresses Nitric Oxide and Tumor Necrosis Factor-α in LPS-Stimulated Mouse Peritoneal Macrophages Through Inhibition of p38, JNK and ERK1/2. Am. J. Chin. Med. 39 (6), 1261–1273. 10.1142/S0192415X11009548 [DOI] [PubMed] [Google Scholar]
- Kang M., Wang R. S., Liu W. Q., Tang A. Z. (2014). Research on the Inhibition of Nasopharyngeal Carcinoma Cells Growth by Compound Tianxian Capsule Drug-Containing Serum In Vitro . Chin. J. Mod. Med. 24 (1), 5–9. [Google Scholar]
- Kong H. R., Dai S. J., Chen H., Lou B., Ni X. F., Sun H. W. (2020). α-Solanine Inhibits Epithelial-Mesenchymal Transition in Trichostatin A-Resistant Human Pancreatic Cancer Cells Through The WNT Signaling Pathway. J. Hepatopancreatobiliary Surg. 32 (2), 89–96. 10.11952/j.issn.1007-1954.2020.02.006 [DOI] [Google Scholar]
- Lai Y. H., Ma Z. C., Yan H. Y., Mao H. X. (2005). Acute Toxicity and Genetic Toxicity Tests of Solanum Nigrum L Juice. Carcinog. Teratog. Mutagen. 17 (3), 54–58. 10.3969/j.issn.1004-616X.2005.01.018 [DOI] [Google Scholar]
- Li Y., Chang W., Zhang M., Ying Z., Lou H. (2015). Natural Product Solasodine-3-O-β-D-Glucopyranoside Inhibits the Virulence Factors of Candida Albicans. Fems Yeast Res. 15. 10.1093/femsyr/fov060 [DOI] [PubMed] [Google Scholar]
- Li C. X., Song X. Y., Zhao W. Y., Yao G. D., Lin B., Huang X. X., et al. (2019). Characterization of Enantiomeric Lignanamides From Solanum Nigrum l. And their Neuroprotective Effects Against MPP+-Induced SH-SY5Y Cells Injury. Phytochemistry 161, 163–171. 10.1016/j.phytochem.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Li L. M., Huang J. P., He W. F., Li Q. P., Wu W. G., Long S. K. (2020). Molecular Mechanism of Apoptosis Induced by Solasonine Extracted from Solanum nigrum in A549 Cells. Tradit. Chin. Drug Res. Clin. Pharmacol. 31 (12), 1422–1427. 10.19378/j.issn.1003-9783.2020.12.006 [DOI] [Google Scholar]
- Li J. H., Li S. Y., Shen M. X., Qiu R. Z., Fan H. W., Li Y. B. (2021). Anti-Tumor Effects of Solanum Nigrum l. Extraction on c6 High-Grade Glioma. J. Ethnopharmacol. 274 (1), 114034. 10.1016/j.jep.2021.114034 [DOI] [PubMed] [Google Scholar]
- Li N., Li D. P., Ding J. X., Xie X. W., Zheng X. L., Yao X. Q., et al. (2021). Effects of External Application of Solanum nigrum ointment on Serum SOD, MDA and Synovial Morphology in Rabbits With Knee Synovitis. J. Gansu Univ. Med. 38 (1), 16–20. 10.16841/j.issn1003-8450.2021.01.04 [DOI] [Google Scholar]
- Li J. (2009). Study on Anti-Cervical Cancer And Immunomodulating Effects of Sub-Fraction 1a of Polysaccharides From solanum nigrum linne . Hebei: Doctor, Yanshan University. [Google Scholar]
- Li G. Y. (2010a). Studies on Isolation, Purification, Structural Identification And Anti-H22 Tumor Activity of Polysaccharides From Salanum nigrum L . Jiangsu: Master, Nanjing Agriculture University. [Google Scholar]
- Li X. C. (2010b). Studies on chemical constituents of Solanum nigrum L . Jilin: Master, Jilin University. [Google Scholar]
- Li C. (2019). Effect of α-Solanine on Migration and Invasion of Human Trophoblast Cell. Changsha: Master, Hunan Agriculture University. [Google Scholar]
- Liao C., Liang H., Zhang R. M., Sun C., Wu X. L., Li L., et al. (2020). Effect of chloroform extract from Solanum nigrum L on clear renal cell carcinoma. J. Clin. Urol. 36 (6), 454–457. 10.13201/j.issn.1001-1420.2020.06.008 [DOI] [Google Scholar]
- Lin H. M., Tseng H. C., Wang C. J., Chyau C. C., Liao K. K., Peng P. L., et al. (2007). Induction of autophagy and apoptosis by the extract of Solanum nigrum Linn in HepG2 cells. J. Agric. Food Chem. 55, 3620–3628. 10.1021/jf062406m [DOI] [PubMed] [Google Scholar]
- Lin H. M., Tseng H. C., Wang C. J., Lin J. J., Lo C. W., Chou F. P. (2008). Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem. Biol. Interact. 171, 283–293. 10.1016/j.cbi.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Ling B., Xiao S., Yang J., Wei Y., Sakharkar M. K., Yang J. (2019). Probing the antitumor mechanism of Solanum nigrum l. Aqueous extract against human breast cancer mcf7 cells. Bioeng. (Basel) 6 (4), 112. 10.3390/bioengineering6040112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Song Y. J., Wang W. W., Zou S. H., Li H. J., Wang C. H., et al. (2019a). Phenols from Solanum nigrum . Chin. Tradit. Pat. Med. 41 (4), 828–831. 10.3969/j.issn.1001-1528.2019.04.023 [DOI] [Google Scholar]
- Liu S., Hu X. S., Lin H. S. (2019b). Mechanism of Xinlikang Capsule inhibiting the proliferation of lung cancer A549 cells and the analysis of its effective componets. Chin. J. Cancer Prev. Treat. 26 (9), 613–618. 10.16073/j.cnki.cjcpt.2019.09.003 [DOI] [Google Scholar]
- Liu J. H., Lyu D. Y., Zhou H. M., Kuang W. H., Chen Z. X., Zhang S. J. (2020). Study On Molecular Mechanism Of Solanum Nigrum In Treatment Of Hepatocarcinoma Based On Network Pharmacology And Molecular Docking. Zhongguo Zhong Yao Za Zhi 45 (1), 163–168. 10.19540/j.cnki.cjcmm.20190807.401 [DOI] [PubMed] [Google Scholar]
- Liu K., Wang Y., Nie T., Hao J. Q., Huang R., Jiang J. B. (2021). Effects of Total alkaloids of Solanum nigrum on mice plasma cell myeloma through NF-κB/IRF4 signaling pathway. West. J. Tradit. Chin. Med. 34 (2), 9–13. 10.12174/j.issn.2096-9600.2021.02.03 [DOI] [Google Scholar]
- Liu Z., Ma C., Tang X., Tang Q., Lou L., Yu Y., et al. (2019). The Reciprocal interaction between LncRNA CCAT1 and miR-375-3p contribute to the downregulation of IRF5 gene expression by Solasonine in HepG2 human hepatocellular carcinoma Cells. Front. Oncol. 9, 1081. 10.3389/fonc.2019.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. (2003). Study on the composition of polysaccharices from Solanum Nigrum L . J. Chang. Univ. Sci. Technol. Nat. Sci. 15 (4), 20–21. [Google Scholar]
- Liu T. (2005). The studies on the extraction, purifieation and determination of active compositions in solanum nigrum L . Hunan: Master, Central South University. [Google Scholar]
- Madanat H. M., Al Antary A., Abu Zarqa M. H. (2016). Toxicity of six ethanol plant extracts against the green peach aphid myzus persicae sulzer (homoptera: aphididae). Fresen Environ. Bull. 25 (3), 706–718. [Google Scholar]
- Mazher M., Anjum M., Mushtaq W., Noshad Q., Malik Z. N. (2017). Antifungal assay of Solanum nigrum Linn. fruit, leaves and stems extracts in different solvents. Int. J. Biosci. 10 (4), 380–385. 10.12692/ijb/10.4.380-385 [DOI] [Google Scholar]
- Mei Q. X., Zhang Z. Q., Lin H., Guan J., Jiang Q. M., Li H. N. (2011). Research progress on the pharmacological effects and clinical application of Solanum nigrum in the treatment of tumors. China Pharm. 23 (39), 3735–3737. 10.6039/j.issn.1001-0408.2012.39.31 [DOI] [Google Scholar]
- Mo L. J., He D., Zhou C. R., Ling L. L. (2014). Experimental study on acute toxicity of raw Solanum nigrum and Solanum nigrum juice. China health care Nutr. 5, 2889–2890. 10.3969/j.issn.1004-7484(x).2014.05.610 [DOI] [Google Scholar]
- Ogunsuyi O. B., Ademiluyi A. O., Oboh G., Oyeleye S. I., Dada A. F. (2018). Green leafy vegetables from two Solanum spp. (Solanum nigrum L and Solanum macrocarpon L) ameliorate scopolamine-induced cognitive and neurochemical impairments in rats. Food Sci. Nutr. 6, 860–870. 10.1002/fsn3.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunsuyi O. B., Oboh G., Özek G., Göger F. (2020). Solanum vegetable-based diets improve impairments in memory, redox imbalance, and altered critical enzyme activities in Drosophila melanogaster model of neurodegeneration. J. Food Biochem. 45 (3), e13150. 10.1111/jfbc.13150 [DOI] [PubMed] [Google Scholar]
- Ogunsuyi O. B., Olagoke O. C., Afolabi B. A., Oboh G., Ijomone O. M., Barbosa N. V., et al. (2021). Dietary inclusions of Solanum vegetables mitigate aluminum-induced redox and inflammation-related neurotoxicity in drosophila melanogaster model. Nutr. Neurosci., 1–15. 10.1080/1028415X.2021.1933331 [DOI] [PubMed] [Google Scholar]
- Ohno M., Murakami K., El-Aasr M., Zhou J.-R., Yokomizo K., Ono M., et al. (2012). New spirostanol glycosides from Solanum nigrum and S. jasminoides . J. Nat. Med. 66, 658–663. 10.1007/s11418-012-0637-z [DOI] [PubMed] [Google Scholar]
- Park J. S., Bang O. S., Kim J. (2014). Screening of Stat3 inhibitory effects of Korean herbal medicines in the A549 human lung cancer cell line. Integr. Med. Res. 3, 67–73. 10.1016/j.imr.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. H., Cheng J. J., Yu M. H., Chung D. J., Huang C. N., Wang C. J. (2020). Solanum nigrum polyphenols reduce body weight and body fat by affecting adipocyte and lipid metabolism. Food Funct. 11, 483–492. 10.1039/c9fo02240f [DOI] [PubMed] [Google Scholar]
- Perez R. M., Perez J. A. J. A., Garcia L. M., Sossa H. H. (1998). Neuropharmacological activity of Solanum nigrum fruit. J. Ethnopharmacol. 62, 43–48. 10.1016/s0378-8741(98)00059-2 [DOI] [PubMed] [Google Scholar]
- Pu Y. W. (2020). Extraction and isolation of Solanum nigrum polysaccharide and its immunomodulatory mechanism. Chongqing: Master, Chongqin Medical University. [Google Scholar]
- Rahuman A. A., Bagavan A., Kamaraj C., Vadivelu M., Zahir A. A., Elango G., et al. (2009). Evaluation of indigenous plant extracts against larvae of Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 104, 637–643. 10.1007/s00436-008-1240-9 [DOI] [PubMed] [Google Scholar]
- Rawani A., Ghosh A., Chandra G. (2010). Mosquito larvicidal activities of Solanum nigrum L. leaf extract against Culex quinquefasciatus Say. Parasitol. Res. 107, 1235–1240. 10.1007/s00436-010-1993-9 [DOI] [PubMed] [Google Scholar]
- Rawani A., Ghosh A., Chandra G. (2013). Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop. 128, 613–622. 10.1016/j.actatropica.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Rawani A., Ray A. S., Ghosh A., Sakar M., Chandra G. (2017). Larvicidal activity of phytosteroid compounds from leaf extract of Solanum nigrum against Culex vishnui group and Anopheles subpictus. BMC Res. Notes 10 (1), 135. 10.1186/s13104-017-2460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabudak T., Ozturk M., Alpay E. (2017). New bioflavonoids from Solanum nigrum L. by anticholinesterase and anti-tyrosinase activities-guided fractionation. Rec. Nat. Prod. 11 (2), 130–140. [Google Scholar]
- Sharma B. K., Iyer D., Patil U. K. (2012). Bioactivity guided fractionation in experimentally induced hyperlipidemia in rats and characterization of phytoconstituent from Solanum nigrum . J. Herbs, Spices Med. Plants 18, 257–267. 10.1080/10496475.2012.688933 [DOI] [Google Scholar]
- Shi F., Wang C., Wang L., Song X., Yang H., Fu Q., et al. (2019). Preparative isolation and purification of steroidal glycoalkaloid from the ripe berries of Solanum nigrum L. by preparative HPLC-MS and UHPLC-TOF-MS/MS and its anti-non-small cell lung tumors effects In Vitro and In Vivo . J. Sep. Sci. 42, 2471–2481. 10.1002/jssc.201801165 [DOI] [PubMed] [Google Scholar]
- Sivaraj C., Yamini S., Yahavi A., Kumar R. P., Arumugam P., Manimaaran A. (2020). Antioxidant, antimicrobial activities and GCMS analysis of fruit extract of Solanum nigrum L . J. Pharmacogn. Phytochem. 9 (4), 1114–1121. [Google Scholar]
- Sohrabipour S., Kharazmi F., Soltani N., Kamalinejad M. (2013). Effect of the administration of Solanum nigrum fruit on blood glucose, lipid profiles, and sensitivity of the vascular mesenteric bed to phenylephrine in streptozotocin-induced diabetic rats. Med. Sci. Monit. Basic Res. 19, 133–140. 10.12659/MSMBR.883892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Zhao Y., Li X., Yuan H., Cheng A., Lou H. (2010). A lysosomal-mitochondrial death pathway is induced by solamargine in human K562 leukemia cells. Toxicol vitro 24, 1504–1511. 10.1016/j.tiv.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Tai B. H., Van Doan V., Yen P. H., Nhiem N. X., Cuc N. T., Trang D. T., et al. (2018). Two new steroidal alkaloid saponins from the whole plants of Solanum nigrum . Nat. Product. Commun. 13 (11), 1934578X1801301–1460. 10.1177/1934578x1801301111 [DOI] [Google Scholar]
- Teng F., Yuan C. P., Wang P. (2014). Study on antioxidant activity of extracts from Solanum nigrum L.berries and analysis of the active ingredients. J. Anhui Agric. Sci. 42 (19), 6217–6219. 10.13989/j.cnki.0517-6611.2014.19.040 [DOI] [Google Scholar]
- Version 1.1 The Plant List (2013). The Plant List. Available At: http://www.theplantlist.org/ .
- Tian H. L., Yu S. W., Pei Y., Shen Z. D., Dong R. J., Kang D. Z. (2019). Effects of crude polysaccharide from Solanum nigrum L. on immune system of mice. J. Yanbian Med. Coll. 42 (1), 8–11. 10.16068/j.1000-1824.2019.01.003 [DOI] [Google Scholar]
- Tsuyoshi I., Hidetsugu T., Takehiko H., Toshihiro N. (2003). Cytotoxic activity of steroidal glycosides from solanum plants (Pharmacognosy). Biol. Pharm. Bull. 26 (8), 1198–1201. [DOI] [PubMed] [Google Scholar]
- Veerapagu M., Jeya K. R., Sankaranarayanan A., Rathika A. (2018). In Vitro antioxidant properties of methanolic extract of Solanum nigrum L. fruit. Pharma Innovation J. 7 (5), 371–374. [Google Scholar]
- Wang L. Y., Wang N. L., Yao X. S. (2007). Non-Saponins from Solanum nigrum L. Zhong Yao Cai 30 (7), 792–794. 10.13863/j.issn1001-4454 [DOI] [PubMed] [Google Scholar]
- Wang H. C., Chung P. J., Wu C. H., Lan K. P., Yang M. Y., Wang C. J. (2010). Solanum nigrum L. polyphenolic extract inhibits hepatocarcinoma cell growth by inducing G2/M phase arrest and apoptosis. J. Sci. Food Agric. 91, 178–185. 10.1002/jsfa.4170 [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiang L., Yi X., He X. (2017). Potential anti-Inflammatory steroidal saponins from the berries of solanum nigrum L. (European black nightshade). J. Agric. Food Chem. 65 (21), 4262–4272. 10.1021/acs.jafc.7b00985 [DOI] [PubMed] [Google Scholar]
- Wang P., Wang Y. D., Zhao W. B., Zhang S. S., Qi X. M. (2019). Effects of Solanum nigrum alkaloids combined with curcumone on energy metabolism of renal cell carcinoma cells. Chin. Rem. Clin. 19 (18), 3213–3215. 10.1186/s12935-019-0981-0 [DOI] [Google Scholar]
- Wang Y., Hong T., Chen L., Chu C., Zhu J., Zhang J., et al. (2020). The natural extract degalactotigonin exerts antitumor effects on renal cell carcinoma cells through repressing YAP. Transl. Cancer Res. 9 (12), 7550–7561. 10.21037/tcr-20-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y. (2007). Continue study on cytotoxic active constituents and study on quality control of Solanum nigrum L . Liaoning: Master, Shenyang Pharmaceutical University. [Google Scholar]
- Wu X. W. (2011). A virtual screening research for the antitumor activity of the ingredients in LSYQD. Henan: Master, Zhengzhou University. [Google Scholar]
- Wu J. B. (2019). Solanine promotes the drug sensitivity of esophageal cancer cells to 5-FU/DDP by inducing the expression of microRNA-138. Henan: MasterZhengzhou University. [Google Scholar]
- Xiang L., Wang Y., Yi X., He X. (2018). Anti-inflammatory steroidal glycosides from the berries of Solanum nigrum L. (European black nightshade). Phytochemistry 148, 87–96. 10.1016/j.phytochem.2018.01.019 [DOI] [PubMed] [Google Scholar]
- Xiang L., Wang Y., Yi X., He X. (2019). Steroidal alkaloid glycosides and phenolics from the immature fruits of Solanum nigrum . Fitoterapia 137, 104268. 10.1016/j.fitote.2019.104268 [DOI] [PubMed] [Google Scholar]
- Xiao G. W., Zeng H. P. (1998). Isolation, purification and identification of polysaccharides from black nightshade (Solanum nigrum). Chin. J. Pharm. Biotechnol. 5 (3), 157–160. 10.19526/j.cnki.1005-8915.1998.03.008 [DOI] [Google Scholar]
- Xiao G. W., Zeng H. P. (2000). Isolation, purification and identification of polysaccharides from black nightshade (Solanum nigrum) (Ⅱ). Chin. Tradit. Herb. Drugs. 31 (3), 162–164. [Google Scholar]
- Xu H. Y., Kong L. X., Ni G. L. (2015). Effects of longkui suppository treating experimental chronic prostatitis rats. Hebei J. Tradit. Chin. Med. 37 (1), 70–72+163. 10.3969/j.issn.1002-2619.2015.01.027 [DOI] [Google Scholar]
- Yan X., Hu B. (2019). “Effect and mechanism of solanine on anoikis and apoptosis of colorectal cancer RKO cells,” in Abstracts of the 17th National Cancer academic conference of integrated traditional Chinese and Western Medicine.17 [Google Scholar]
- Yan X., Li M., Chen L., Peng X., Que Z. J., An H. M., et al. (2020). α-Solanine inhibits growth and metastatic potential of human colorectal cancer cells. Oncol. Rep. 43, 1387–1396. 10.3892/or.2020.7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. Y., Hsu L. S., Peng C. H., Shi Y. S., Wu C. H., Wang C. J. (2010). Polyphenol-rich extracts from Solanum nigrum attenuated PKC alpha-mediated migration and invasion of hepatocellular carcinoma cells. J. Agric. Food Chem. 58, 5806–5814. 10.1021/jf100718b [DOI] [PubMed] [Google Scholar]
- Yang Y., Hu X. X., Zhou L. L., Gao S. L., Ding X. (2014). Protective effect of Solanum nigrum polysaccharide on CCl4 induced acute liver injury in mice. Chin. Tradit. Pat. Med. 36 (12), 2602–2605. 10.3969/j.issn.1001-1528.2014.12.036 [DOI] [Google Scholar]
- Yang M. Y., Hung C. H., Chang C. H., Tseng T. H., Wang C. J. (2016). Solanum nigrum suppress angiogenesis-mediated tumor growth through inhibition of the AKT/mTOR pathway. Am. J. Chin. Med. 44 (16), 1273–1288. 10.1142/S0192415X16500713 [DOI] [PubMed] [Google Scholar]
- Yang X. F., Wen Q. X., Zhang J. R. (2017). To investigate the effect of morel on prevention and treatment of liver cancer. Chin. J. Integr. Tradit. West. Med. Liver Dis. 27 (06), 353–355. 10.3969/j.issn.1005-0264.2017.06.012 [DOI] [Google Scholar]
- Yang Y. (2014). The therapeutic basis and hepatoprotective activities of Solanum nigrum L . Jiangsu: Master, Nanjing agricultural University. [Google Scholar]
- Yao H., Wang L., Tang X., Yang Z., Li H., Sun C., et al. (2020). Two novel polysaccharides from Solanum nigrum L. exert potential prebiotic effects in an In Vitro fermentation model. Int. J. Biol. Macromol. 159, 648–658. 10.1016/j.ijbiomac.2020.05.121 [DOI] [PubMed] [Google Scholar]
- Ye L. F., Gao Z. W. (2019). Study of Solanum nigrum n-butanol extract on the proliferation of human colorectal cancer SW480 and its mechanism. World J. Integr. Tradit. West Med. 14 (3), 356–358+363. 10.13935/j.cnki.sjzx.190315 [DOI] [Google Scholar]
- Yeom Y.-E., Kim M. A., Kim J., Lee C.-M. (2019). Anti-inflammatory effects of the extract of Solanum nigrum L. on an acute ear edema mouse model. Mater. Technol. 34 (14), 851–857. 10.1080/10667857.2019.1638671 [DOI] [Google Scholar]
- Yin S., Jin W., Qiu Y., Fu L., Wang T., Yu H. (2022). Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J. Hematol. Oncol. 15 (32), 1–6. 10.1186/s13045-022-01248-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tian F., Jiang P., Qian S., Dai X., Ma B., et al. (2021). Solasonine suppresses the proliferation of acute monocytic leukemia through the activation of the AMPK/FOXO3A axis. Front. Oncol. 10. 10.3389/fonc.2020.614067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. M. (2015). Clinical observation of entecavir combined with Loulian Capsules in the treatment of HBeAg liver cirrhosis in decompensated stage. Med. Inf. 045, 50–51. [Google Scholar]
- Zhang X. H. (2018). Effect of solamargine extracted from Solanum Nigrum on growth and metastasis on the mechanism of cholangiocarcinoma cells. Jiangsu: Doctor, Nanjing University of Chinese Medicine. [Google Scholar]
- Zhao S. Y., Lei Y. J., Ma S. M., Gao M. (2019). Solanum nigrum combined with KLF16 gene synergistically inhibit glioma cell proliferation and induce apoptosis. J. Mol. Diagn Ther. 11 (4), 303–309. [Google Scholar]
- Zhao Y. (2010). Chemical constituents of two solanum species, microbial transformation and biological activities. Shandong: Doctor, Shandong University. [Google Scholar]
- Zhou X. L. (2006). Study on anticancer active component of Solanum nigrum . Liaoning: Doctor, Shenyang Pharmaceutical University. [Google Scholar]
- Zhu J. Y., Ma F. H., Xu Y. H. (2019). Solanum nigrum inhibits proliferation and promote apoptosis of ovarian cancer cells through regulates the expression of p-AKT, cleaved Caspase-3 and p53 protein. Shaanxi J. Tradit. Chin. Med. 40 (5), 556–560. 10.3969/j.issn.1000-7369.2019.05.004 [DOI] [Google Scholar]