Abstract
Background
Social genomics has demonstrated altered inflammatory and type I interferon (IFN) gene expression among people experiencing chronic social adversity. Adverse social experiences such as discrimination and violence are linked to stimulant misuse and HIV, conditions that dysregulate inflammatory and innate antiviral responses, leading to increased HIV viral replication and risk of chronic diseases.
Purpose
We aimed to determine whether methamphetamine (MA) use, unsuppressed HIV viral load (VL) (≥200 c/mL), and experienced intimate partner violence (IPV) (past 12 months) predicted inflammatory and type I IFN gene expression in HIV-positive Black and Latinx men who have sex with men (MSM).
Methods
Participants were 147 HIV-positive Black and Latinx MSM recruited from the mSTUDY, a cohort of 561 MSM aged 18–45 in Los Angeles, CA, of whom half are HIV-positive and substance-using. Transcriptomic measures of inflammatory and type I IFN activity were derived from RNA sequencing of peripheral blood mononuclear cells and matched to urine drug tests, VL, and survey data across two time points 12 months apart. Analysis used linear random intercept modeling of MA use, unsuppressed VL, and experienced IPV on inflammatory and type I IFN expression.
Results
In adjusted models, MA use predicted 27% upregulated inflammatory and 31% upregulated type I IFN expression; unsuppressed VL predicted 84% upregulated type I IFN but not inflammatory expression; and experienced IPV predicted 31% upregulated inflammatory and 26% upregulated type I IFN expression.
Conclusions
In Black and Latinx MSM with HIV, MA use, unsuppressed VL, and experienced IPV predicted upregulated social genomic markers of immune functioning.
Keywords: Gene expression, Immune system, Transcriptome, Methamphetamine, HIV, Viral load
Men living with HIV who used methamphetamine, were virally unsuppressed, and experienced social adversity showed increased activation of proinflammatory and antiviral genes.
Introduction
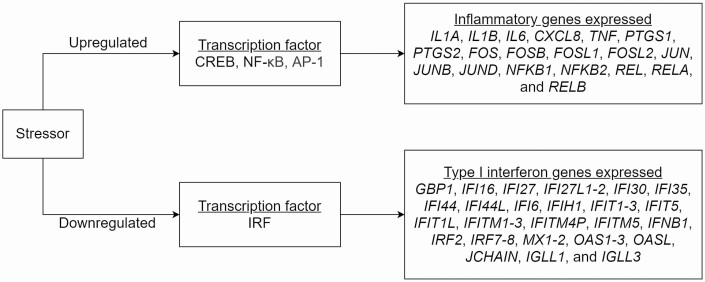
The field of social genomics has investigated ways in which “stress”-mediated alterations in gene expression can play a role in the link between social adversity and pathogenesis of chronic disease, and has recently begun exploring these processes in underserved communities, such as racial/ethnic and sexual minorities (e.g., lesbian, gay, and bisexual people). Past adverse psychosocial experiences have been shown to drive a conserved transcriptional response to adversity (CTRA), a pattern marked by altered expression of 53 leukocyte genes. These include upregulation of 18 proinflammatory genes and downregulation of 25 type I interferon (IFN) genes (as listed in Fig. 1), predominately expressed in myeloid lineage cells comprising up to 10% of circulating leukocytes, including blood monocytes, tissue macrophages, and dendritic cells in both blood and tissue [1]. In the general population, CTRA has been observed in the contexts of experienced racial discrimination [2], experienced homophobia [3], socioeconomic challenges [4], and interpersonal conflict [5].
Fig. 1.
Immune gene expression patterns according to the conserved transcriptional response to adversity.
The empirical literature on diverse men who have sex with men (MSM) has identified syndemic forms of social adversity that increase the risk of methamphetamine (MA) use and poorly controlled HIV infection in those living with HIV. Experiences of intimate partner violence (IPV), homophobic victimization, and housing instability have been shown to increase MA use [6, 7], reduce engagement in care [8] and adherence to antiretroviral therapy (ART) [9], and worsen virologic outcomes [8, 10] in HIV-positive MSM and MSM of color. In addition to driving MA use and hampering access and adherence to ART [8–10], it is possible these adverse social experiences could contribute to the progression of MA-related diseases and HIV infection by dysregulating the body’s inflammatory and innate antiviral responses [11, 12].
There is evidence that comorbid MA use and uncontrolled HIV infection may dysregulate the expression of social genomic markers, possibly in ways that diverge from the conventional CTRA phenotype. HIV infection has been shown to induce systemic inflammation [13–15] and type I IFN production, mediating factors involved in HIV pathogenesis [16–18]. In people with HIV (PWH), MA has shown to further exacerbate HIV disease progression by not only interfering with engagement in care but also driving viral replication via activation of the body’s immune system [19, 20]. MA use influences some of the same immune processes captured in social genomics research [1, 11], including systemic inflammation [21, 22] and type I IFN activity [21, 22], factors which may mediate MA-related pathophysiology. In humans, MA use activates CREB, AP-1, and NF-κB, transcription factors involved in MA-induced inflammation and observed in inflammatory responses to social stress [1, 23, 24]. In PWH, MA use appears to upregulate type I IFN gene expression [25, 26], which contrasts with the conventional CTRA phenotype marked by downregulated type I IFN expression in people without HIV or substance use.
MA-induced inflammation and type I IFN activity are now suspected to increase HIV-1 replication and persistence by increasing immunologic activation [16–18]. This may involve several mechanisms. For example, MA use has been shown to drive spontaneous proliferation of CD4+ and CD8+ T cells [27] and monocyte activation corresponding to elevated sCD14 levels in PWH [28, 29], activity mediated by increased type I IFNs [30, 31]. MA-use has also shown to induce gut dysbiosis, and in turn, increase mucosal inflammatory cytokines and T cell activation in peripheral blood compartments [32, 33]. Sustained elevations in inflammatory and type I IFN gene expression due to persistent MA use and social stress can chronically activate these immune cells [30], and in turn, impair their HIV-specific functional responses [17], reduce T cell proliferative capacity [27], exhaust the immune system [17], and drive viral replication [27, 30]. For these reasons, social genomics assessment of inflammatory and type I IFN expression may offer a useful framework for understanding HIV disease progression in the context of MA use and social adversity.
Based on this premise, our present study aimed to leverage survey and transcriptome data from HIV-positive Black and Latinx MSM to test: (a) whether MA use (urine drug screening) and unsuppressed HIV viral load (VL) (≥200 c/mL) predict both upregulated inflammatory and type I IFN gene expression; and (b) whether experiences of social adversity identified in empirical literature of MSM with HIV—IPV, housing instability, and homophobia—predict upregulated inflammatory gene expression and downregulated type I IFN gene expression based on pre-specified gene sets from social genomics research [1].
Methods
Participants and Procedures
Data were from 147 HIV-positive Black and Latinx sexual minority men from the MSM and Substances Cohort at UCLA Linking Infections Noting Effects (mSTUDY), sponsored by the National Institute on Drug Abuse (NIDA). Briefly, the mSTUDY is a Los Angeles County based cohort study of HIV-positive and HIV-negative MSM from diverse backgrounds, primarily Black and Latinx, who enter the study between the ages of 18 and 45. The mSTUDY aims to serve as a research platform to study factors impacting substance use, immune functioning, HIV transmission and disease progression, and social adversity in this community. Recruitment of participants began July 2014. Participants in the mSTUDY attend study visits every 6 months for laboratory testing, bio-specimen collection, physical examination, and computer-based survey assessments of covering psychosocial, behavioral, and physical health. Greater detail of recruitment and data collection procedures for the mSTUDY are available in previously published works [34, 35]. To minimize confounding by race/ethnicity and HIV status, we only performed transcriptome on participants who identified as Black/African American or Latinx/Hispanic and who were HIV-positive when enrolled in mSTUDY. All study procedures have been approved by our office of human research protection.
Measures
Inflammatory and type I IFN gene expression was assessed using genome-wide transcriptional profiling of leukocytes from peripheral blood mononuclear cells (PBMCs), as established in prior research [3]. The parent mSTUDY collects PBMCs every 6 months from 125 mL of blood drawn via venipuncture and stored in the mSTUDY biorepository at −70°C. We analyzed a total of 210 PBMC samples from 147 participants, of whom 63 had PBMCs over two visits (12 months apart) and 84 only had PBMCs at their first visit. Total RNA (Qiagen RNeasy) was extracted from PBMCs, tested for suitable mass using RiboGreen and integrity using Agilent TapeStation. Samples meeting quality criteria were converted to cDNA using a high-efficiency mRNA targeted library preparation (Lexogen QuantSeq 3′ FWD) and sequenced on Illumina HiSeq 4000 instrument, with assays targeting 10 million single-strand 65 nt sequence reads. Sequencing was performed in the UCLA Neuroscience Genomics Core Laboratory per the manufacturers’ standard protocols. Sample averaged 14.3 million reads, each of which were mapped to the RefSeq consensus human transcriptome sequence and quantified at the gene level using the STAR aligner, normalized to transcripts per million mapped reads, and log2 transformed per standard practice.
Of the 53 CTRA a priori genes identified by the social genomics field (Fig. 1) [1], we only included those in our analyses that had adequate variability for quantifying differences in expression, namely those with ≥1 SD in expression values as per standard practices [36]. A total of 25 genes total met this statistical criterion and were therefore factored in analyses. These included 9 inflammatory genes (CXCL8, IL1A, IL1B, TNF, PTGS2, FOS, FOSB, JUN, and RELB) and 16 type I IFN genes (IFI6, IFI27, IFI30, IFI35, IFI44, IFIH1, IFIT1-3, IFIT5, IFITM, IRF7, MX1, OAS3, OASL, and JCHAIN) as classified in the CTRA literature. We examined immune activation via these two gene sets, inflammatory gene expression and type I IFN gene expression. Additionally, we computed a full CTRA contrast score where the nine inflammatory genes expressed were positively scored and 16 type I IFN genes were sign-inverted (−) to reflect the inverse contribution of downregulated type I IFN expression in the conventional CTRA profile previously observed in general population [1].
Current MA use was assessed by qualitative detection of MA in urine using the Fastect II 4-Panel Drug Test (detection period up to 3–5 days) [37]. Detectable cannabis in urine (detection period up to 14 days) [37] was also assessed as a potential covariate due to research suggesting its link to reduced inflammation [38, 39]. Having an unsuppressed VL was based on viral quantification from polymerase chain reaction and the standard threshold of greater than or equal to 200 counts per mL of blood. Unsuppressed VL at ≥200 c/mL was the selected threshold for this study given extant research validating this cutoff as clinically significant for risk of transmission [40, 41] and disease progression [42], as well as to account for the possibility of a clinically nonsignificant viral blip that still fell under 200 c/mL [42].
Measures of social adversity—experienced IPV, experienced homophobia, and housing instability—were measured using computer-assisted self-interview (CASI). Experienced IPV in the past 12 months was a dichotomous measure, with responses “No” or “Yes” to the question “Have you been hit, kicked or slapped by a lover, boyfriend or girlfriend in the last 12 months?” Experienced homophobia in the past 12 months was measured over five items from the National Health, Aging, and Sexuality/Gender Study [43], such as “I was threatened with physical violence because I am, or was thought to be, gay or bisexual.” Responses were “No” or “Yes” and summed across all five items to make a composite score. Participants were coded as having housing stability if their responses to the question “Approximately how many days have you not had a regular place to stay in the last 6 months?” were 1 or more days. CASI also captured age (in years), race (Black or Latinx), current smoking status (no or yes), and weekly alcohol use (three or less times per week, four or more times per week), which were tested separately for associations with immune gene expression as candidates for inclusion as covariates in multivariable modeling.
Analysis
Statistical analyses used linear random intercept modeling to test independent variables on expression of the prespecified immune response gene transcripts—9 proinflammatory, 16 type I IFN, or 25 total CTRA indicator genes—quantified in count per million. Linear random intercept modeling was conducted using the Stata “mixed” command, handling genes and time as repeated measures and accounting for intra-individual correlations with a fully unsaturated covariance matrix. We conducted bivariate tests on whether our main independent variables of interest—MA use, unsuppressed VL, and three indicators of social adversity (experienced IPV, housing instability, and experienced homophobia)—were associated with expression of inflammatory and type I IFN gene sets, as well as with the conventional CTRA contrast (proinflammatory − type I IFN). We also conducted bivariate tests of candidate variables considered for inclusion as covariates in subsequent adjusted mixed regression models—age, race, current smoking status, cannabis urine test, and weekly alcohol consumption—on expression of these gene sets and the CTRA contrast. These models only adjusted for time.
We used a data-driven approach to select predictor variables in our multivariable models. Specifically, variables that were significantly associated with immune response gene expression in the previous bivariate regressions at p < .05 were included in the two final, mutually adjusted mixed regression models. These included MA use, unsuppressed VL, and two social adversity factors—experienced IPV and housing instability. Models 1 and 2 included these factors with mutual adjustment, predicting inflammatory and type I IFN gene expression, respectively. Significant regression estimates and 95% confident intervals (CIs) from Models 1 and 2 were then exponentiated from log2 units into percent upregulated gene expression.
Results
Participant Characteristics
Baseline descriptive statistics for our sample of Black and Latinx MSM (N = 147) are displayed in Table 1. The mean age for participants was 34 years. The majority of participants identified as Latinx (55%) and the remainder were as Black (45%). At baseline, 41% of participants were virally unsuppressed, 29% of participants tested positive for MA, and 30% tested positive for cannabis. Twenty-nine percent of participants were unstably housed in the past 6 months, 18% reported experienced IPV in the past 12 months, and 45% reported experienced homophobic victimization in the past 12 months. Forty-four percent reported current cigarette smoking, and 12% reported drinking alcohol 4 or more times per week.
Table 1.
Baseline descriptive statistics (N = 147)
| Variable | M | SD |
|---|---|---|
| Age | 34.0 | 6.5 |
| n | % | |
| Race | ||
| Latinx | 81 | 55.1 |
| Black | 66 | 44.9 |
| HIV viral load | ||
| <200 c/mL | 86 | 59.3 |
| ≥200 c/mL | 59 | 40.7 |
| MA urine test | ||
| Negative | 105 | 71.4 |
| Positive | 42 | 28.6 |
| Housing instability (past 6 months) | ||
| No | 105 | 71.4 |
| Yes | 42 | 28.6 |
| Intimate partner violence (past 12 months) | ||
| No | 121 | 82.3 |
| Yes | 26 | 17.7 |
| Homophobic victimization (past 12 months) | ||
| No | 81 | 55.1 |
| Yes | 66 | 44.9 |
| Cannabis urine test | ||
| Negative | 103 | 70.1 |
| Positive | 44 | 29.9 |
| Current cigarette smoking | ||
| No | 82 | 55.9 |
| Yes | 65 | 44.2 |
| Alcohol | ||
| <4 drinks per week | 127 | 88.2 |
| >4 drinks per week | 17 | 11.8 |
MA methamphetamine.
Bivariate Relationships With Inflammatory and Type I IFN Gene Expression
Bivariate analyses indicated that having a positive MA test (B = 0.38, 95% CI [0.10 to 0.66], p < .008), and experienced IPV (B = 0.43, 95% CI [0.12 to 0.74], p = .007) separately predicted upregulated inflammatory gene expression in Black and Latinx MSM. Testing positive for MA (B = 0.72, 95% CI [0.54 to 0.90], p < .001), unsuppressed VL (B = 0.96, 95% CI [0.82 to 1.10], p < .001), experienced IPV (B = 0.35, 95% CI [0.15 to 0.55], p < .001), and housing instability (B = 0.39, 95% CI [0.20 to 0.57], p < .001) separately predicted upregulated type I IFN expression. Experienced homophobic victimization, testing positive for cannabis, age, race, current smoking status, and weekly alcohol consumption were not associated with immune activation and therefore excluded from subsequent mutually adjusted models. Additional analyses found the conventional CTRA contrast score (proinflammatory—type I IFN) was negatively associated with MA use (B = −0.39, 95% CI [−0.55 to −0.22], p < .001), unsuppressed VL (B = −0.65, 95% CI [−0.78 to −0.52], p < .001), and housing instability (B = −0.21, 95% CI [−0.37 to −0.04], p = .014). This paradoxical result was due to the marked upregulation of type I IFN-related expression—and its inverse contribution to the CTRA contrast—which exceeded the positively weighted upregulation of inflammatory genes. Neither IPV nor homophobic victimization was significantly associated with the CTRA contrast score.
Mutually Adjusted Models
Table 2 displays mutually adjusted mixed linear regression models of MA-positive urine test, unsuppressed VL, experienced IPV, and housing instability on gene sets of immune activation—inflammatory gene expression (Model 1) and type I IFN gene expression (Model 2), each adjusting for time. Non-significant variables from the previous analyses were not included in these mutually adjusted models.
Table 2.
Mutually adjusted linear regression models of methamphetamine use, HIV viral load, intimate partner violence, and housing instability on inflammatory (Model 1) and type I interferon expression (Model 2)
| Gene set | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: inflammatory gene expression | Model 2: type I interferon gene expression | |||||||
| Predictors | B a | 95% CI | z | p | B a | 95% CI | z | p |
| Methamphetamine-positive urine test | 0.35 | 0.05 to 0.65 | 2.29 | .022 | 0.39 | 0.21 to 0.57 | 4.25 | <.001 |
| Unsuppressed viral load (≥200 c/mL) | −0.04 | −0.29 to 0.21 | −0.29 | .774 | 0.88 | 0.73 to 1.02 | 11.81 | <.001 |
| Intimate partner violence (past 12 months) | 0.39 | 0.08 to 0.71 | 2.47 | .014 | 0.33 | 0.14 to 0.52 | 3.48 | .001 |
| Housing instability (past 6 months) | 0.01 | −0.28 to 0.29 | 0.04 | .969 | 0.02 | −0.15 to 0.19 | 0.22 | .829 |
aLinear regression estimates are in log2 units, mutually adjusted with time.
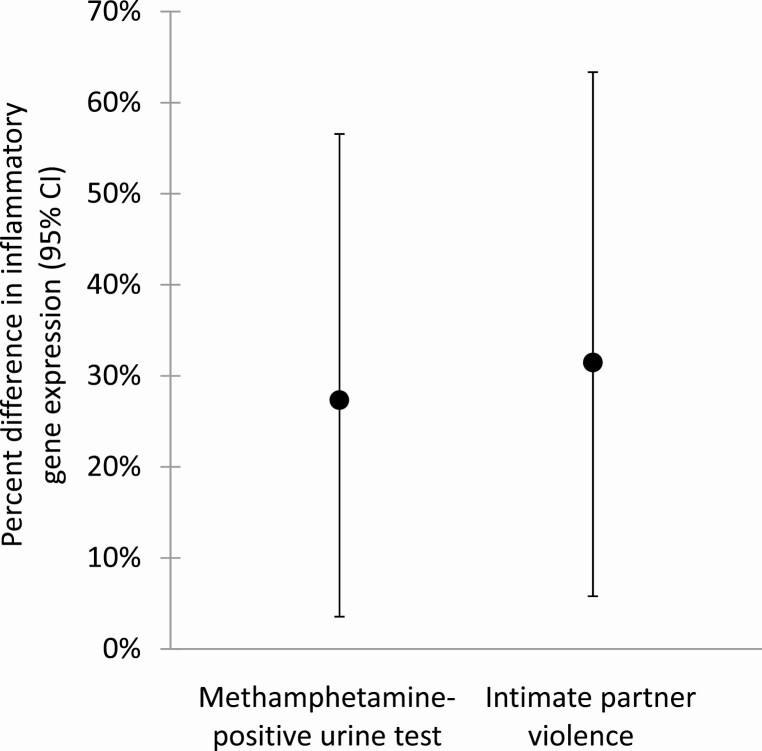
In Model 1, testing positive for MA predicted 27% upregulation (B = 0.35, 95% CI (0.05 to 0.65), p = .022) and experienced IPV predicted 31% upregulation (B = 0.39, 95% CI [0.08 to 0.71], p = .014) of inflammatory gene expression (Table 2). Figure 2 illustrates percent differences with 95% CIs in inflammatory gene expression by MA-positive urine test and experienced IPV, derived from exponentiating the significant estimates in Model 1 (originally in log2 units).
Fig. 2.
Percent difference in inflammatory gene expression associated with having a positive urine test for methamphetamine (MA) and reporting experiences of intimate partner violence (IPV) in the past 12 months. Note: Values are derived from estimates from the mutually adjusted mixed regression Model 1.
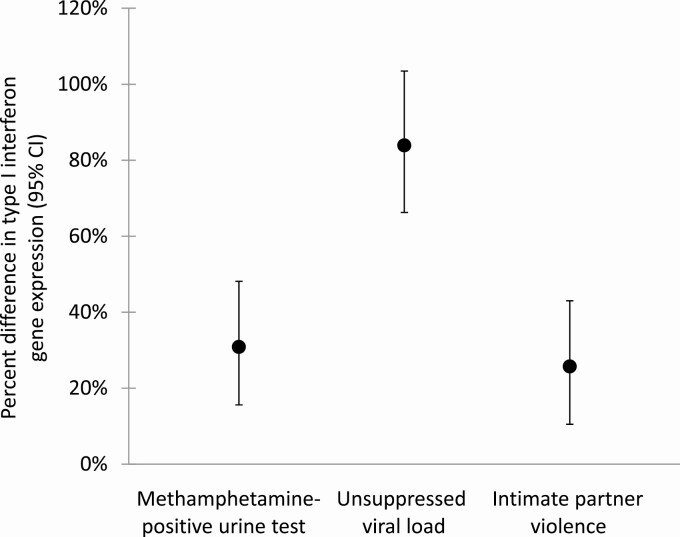
In Model 2, testing positive for MA predicted 31% upregulation (B = 0.39, 95% CI [0.21, 0.57], p < .001), unsuppressed VL predicted 84% upregulation (B = 0.88, 95% CI [0.73 to 1.02], p < .001), and IPV predicted 26% upregulation (B = 0.33, 95% CI [0.14 to 0.52], p = .001) of type I IFN expression (Table 2). Figure 3 illustrates percent differences with 95% CIs in type I IFN gene expression by MA-positive urine test, unsuppressed VL, and experienced IPV, derived from these significant estimates in Model 2.
Fig. 3.
Percent difference in type I interferon gene expression (with 95% confidence intervals) associated with having a MA-positive urine test, having an unsuppressed HIV viral load (VL) (≥200 c/mL), and reporting experienced IPV. Note: Values are derived from estimates from the mutually adjusted mixed regression Model 2.
Discussion
The present study demonstrates that MA use, unsuppressed HIV VL, and experienced IPV—conditions that are often comorbid—additively predicted inflammatory and type I IFN gene expression in Black and Latinx MSM with HIV. These findings suggest that assessing comorbid psychosocial and physical health problems is important for capturing the scope of factors related to immune activation in the forms of inflammatory and type I IFN gene expression, especially in MSM of color living with HIV.
The link between experienced IPV and upregulated inflammatory gene expression is consistent with prior literature demonstrating greater systemic inflammation in people reporting experiences of interpersonal conflict or hostile social environments [3, 5]. Interestingly, type I IFN was also upregulated with experienced IPV rather than the typical downregulated pattern seen in studies of social adversity in the general population [1, 5] and HIV-negative samples of minority MSM [3]. This difference in transcriptome profile was also observed in a prior study of Black and White men of whom a large proportion were HIV-positive, which showed greater IFN response factor activity was associated with both past experiences of racial discrimination and HIV-positive serostatus [44]. Considering this together, it is possible that social adversity upregulates rather than downregulates type I IFN in the context of HIV infection (e.g., possibly due to chronic inflammatory stimulation of HIV-1 replication, which then evokes greater type I IFN activity in response to viral replication).
Housing instability predicted increased type I IFN expression in the absence of other covariates in our sample of PWH, also contrasting with the conventional CTRA profiles showing reduced type I IFN expression in people of low socioeconomic status but without MA use or HIV [4]. However, housing instability no longer predicted type I IFN expression when accounting for IPV, MA use, and unsuppressed VL. It is possible that there exists an indirect relationship between housing instability and upregulated type I IFN expression, but that this relationship is explained by increased risk of MA use, viremia, and IPV in HIV-positive Black and Latinx MSM who are unstably housed.
The association between MA use and upregulated inflammatory genes demonstrated in this study suggests that MA use activates some of the same immune gene regulatory pathways that respond to social adversity. This is consistent with clinical and pre-clinical research showing that MA administration induces peripheral inflammation [21, 22, 45]. MA use activates transcription factors CREB, NF-κB, and AP-1 [19, 23, 24], all of which have also been identified by promoter-based bioinformatics to be involved in the inflammatory component of CTRA [1, 22] and may play a role in MA-related pathophysiology [22, 23] and HIV disease progression [19, 20, 46]. Furthermore, MA use is known to drive some of the psychosocial problems associated with CTRA, such as anxiety and depressed mood [47–49], factors that may play a mediating role in the link between MA use and enhanced inflammatory gene expression. Upregulated type I IFN expression was also observed in those with positive MA urine tests. Although this differs from the conventional CTRA phenotype of downregulated type I IFN within the general population, the pattern detected in our study aligns with previous studies on the role of MA use on upregulated type I IFN and eventual T cell exhaustion and viral replication among PWH [25, 26, 28, 30]. A previous transcriptome-wide study in 55 PWH has also explored MA use and differential leukocyte gene expression [50], but the a priori genes we tested were different from the empirically derived gene sets they identified. Still, the expression patterns identified in their study appeared to involve genes with similar functions as those examined our study, such as innate immune activation and inflammation (e.g., TNF family activation) [50].
Unsuppressed HIV VL was strongly associated with upregulated type I IFN gene expression (+84%), but not with inflammatory gene expression. The relationship between unsuppressed VL and type I IFN activation is likely reciprocal, as HIV is known to increase type I IFN signaling and production, and in turn, type I IFN activity appears to drive CD4+ and CD8+ exhaustion and further viral replication [16–18]. The lack of association between unsuppressed VL and inflammatory gene expression was unexpected given prior research suggesting a role of inflammation in HIV-related pathogenesis [13, 14]. This may be explained by prior research demonstrating that levels of inflammation in PWH remain elevated despite achieving viral suppression [51–53]. As such, our findings indicate that type I IFN activity varies more than inflammation in relation to viremia.
Analyses of the classical CTRA contrast score (proinflammatory − type I IFN) showed unexpected negative associations with MA use, unsuppressed VL, and housing instability, rather than the positive associations that would typically be expected based on previous research. This paradoxical result stemmed from the marked upregulation of type I IFN-related gene expression in association with MA use, unsuppressed VL, and housing instability, leading to marked decrease of the overall CTRA composite score when the IFN component was inversely scored. For this reason, the CTRA contrast score may not be the most appropriate way to quantify social genomic patterns in those living with HIV (where chronic viral replication may stimulate type I IFN activity) and/or using MA. Rather, we recommend testing associations with inflammatory and type I IFN gene sets separately within the context of HIV and MA use.
Our findings should be considered within the scope of this study’s limitations. Limited inferences about the temporal relationship between quantified VL and transcriptome measures, as these were collected at the same study visits. For example, it is possible that the association between VL and type I IFN expression reflects a bidirectional relationship given prior research demonstrating both IFN responses to uncontrolled viremia and involvement of IFNs in HIV pathogenesis [16–18]. Considering that urine tests for MA and collection of PBMCs occurred at the same visit, it is likely that MA use preceded activation of the immune markers measured in this study. However, it is still possible that these immune markers were already elevated and played a role in driving addiction processes in those who tested MA-positive [22, 23]. It is also unclear whether the association between having a positive MA urine test and immune activation is due to mere recency of use or due to stimulant use severity, as having a positive drug test can be indicative of high levels of use [54]. Future research is needed to elucidate the degree to which dysregulated inflammatory and type I IFN expression are linked to severity of MA use. Furthermore, findings from this study reflect data from a limited geographic region, racial/ethnic composition, and timeframe, so it is unclear whether these patterns would be observed outside of Black and Latino MSM in Los Angeles County.
The present study suggests that social genomics research in HIV-positive MSM of color should account for MA use and HIV viremia alongside measures of social adversity, to capture a comprehensive scope of comorbid factors contributing to immune dysregulation. In the social genomics literature, dysregulated inflammatory and type I IFN genes have been linked to the development of various chronic health problems, including psychiatric disorders, cardiovascular disease, metabolic disorders, and some neoplasms [1]. Furthermore, elevated inflammation and type I IFN expression has been linked to T cell exhaustion and HIV viral replication in PWH who use MA [28]. With this in mind, social genomics indicators may serve as a biomarker of chronic disease risk in those with enduring psychosocial problems, disordered MA use, and HIV infection. This observation in turn may help to identify those who might benefit from additional monitoring for chronic diseases or HIV-related immune exhaustion. Furthermore, translational studies are in the nascent stages of demonstrating that dysregulated inflammatory and type I IFN activity can improve with participation in behavioral interventions [1]. Social genomics may help to assess biological responses to novel treatments or comprehensive care programs for substance use disorders, HIV, and social adversity, though this has yet to be tested. Future longitudinal and experimental studies are needed to test the validity of social genomics measures alongside psychosocial assessments and standard clinical endpoints, such as MA urine tests and VL, taken over the course of evidence-based treatment for MA use disorder in PWH.
Contributor Information
Michael J Li, Center for Behavioral and Addiction Medicine, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Center for HIV Identification, Prevention and Treatment Services, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Emily I Richter, Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA, USA.
Chukwuemeka N Okafor, Department of Public Health, Robbins College of Health and Human Sciences, Baylor University, Waco, TX, USA.
Mariah M Kalmin, Center for Behavioral and Addiction Medicine, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Center for HIV Identification, Prevention and Treatment Services, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Shareefa Dalvie, South African Medical Research Council (SAMRC), Unit on Risk & Resilience in Mental Disorders, Department of Psychiatry and Neuroscience Institute, University of Cape Town, Cape Town, South Africa; South African Medical Research Council (SAMRC), Unit on Child & Adolescent Health, Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa.
Sae Takada, Division of General Internal Medicine and Health Services Research, University of California, Los Angeles, Los Angeles, CA, USA.
Pamina M Gorbach, Center for HIV Identification, Prevention and Treatment Services, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA, USA.
Steven J Shoptaw, Center for Behavioral and Addiction Medicine, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Center for HIV Identification, Prevention and Treatment Services, Department of Family Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA.
Steven W Cole, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA.
Funding
This research was supported by the National Institute on Drug Abuse grant (K01DA051329, PI: Li; K01DA047912, PI: Okafor); the MSM and Substances Cohort at UCLA Linking Infections Noting Effects (mSTUDY), National Institute on Drug Abuse grant (U01DA036267; PIs: Gorbach and Shoptaw); the UCLA Postdoctoral Fellowship Training Program in Global HIV Prevention Research, National Institute of Mental Health grant (T32MH080634; PIs: Currier and Gorbach); the USC/UCLA Center on Biodemography and Population Health, National Institute on Aging grant (P30AG017265, PI: Crimmins and Cole); and the Center for HIV Identification, Prevention and Treatment Services, National Institute of Mental Health grant (P30MH058107; PI: Shoptaw).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Michael J. Li, Emily I. Richter, Chukwuemeka N. Okafor, Mariah M. Kalmin, Shareefa Dalvie, Sae Takada, Pamina M. Gorbach, Steven J. Shoptaw, and Steven W. Cole declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1. Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci. 2019;28:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown KM, Diez-Roux AV, Smith JA, et al. . Expression of socially sensitive genes: the multi-ethnic study of atherosclerosis. PLos One. 2019;14:e0214061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li MJ, Takada S, Okafor CN, Gorbach PM, Shoptaw SJ, Cole SW. Experienced homophobia and gene expression alterations in Black and Latino men who have sex with men in Los Angeles County. Brain Behav Immun. 2020;83:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine ME, Crimmins EM, Weir DR, Cole SW. Contemporaneous social environment and the architecture of late-life gene expression profiles. Am J Epidemiol. 2017;186:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li MJ, Okafor CN, Gorbach PM, Shoptaw S. Intersecting burdens: homophobic victimization, unstable housing, and methamphetamine use in a cohort of men of color who have sex with men. Drug Alcohol Depend. 2018;192:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freeman P, Walker BC, Harris DR, Garofalo R, Willard N, Ellen JM; Adolescent Trials Network for HIV/AIDS Interventions 016b Team . Methamphetamine use and risk for HIV among young men who have sex with men in 8 US cities. Arch Pediatr Adolesc Med. 2011;165:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schafer KR, Brant J, Gupta S, et al. . Intimate partner violence: a predictor of worse HIV outcomes and engagement in care. AIDS Patient Care STDS. 2012;26:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman MR, Stall R, Silvestre AJ, et al. . Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. Aids. 2015;29:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li MJ, Su E, Garland WH, et al. . Trajectories of viral suppression in people living with HIV receiving coordinated care: differences by comorbidities. J Acquir Immune Defic Syndr. 2020;84:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cole SW. Psychosocial influences on HIV-1 disease progression: neural, endocrine, and virologic mechanisms. Psychosom Med. 2008;70:562–568. [DOI] [PubMed] [Google Scholar]
- 12. Passaro RC, Pandhare J, Qian HZ, Dash C. The complex interaction between methamphetamine abuse and HIV-1 pathogenesis. J Neuroimmune Pharmacol. 2015;10:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. [DOI] [PubMed] [Google Scholar]
- 14. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bosinger SE, Utay NS. Type I interferon: understanding its role in HIV pathogenesis and therapy. Curr HIV/AIDS Rep. 2015;12:41–53. [DOI] [PubMed] [Google Scholar]
- 17. Zhen A, Rezek V, Youn C, et al. . Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Invest. 2017;127:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng L, Ma J, Li J, et al. . Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J Clin Invest. 2017;127:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wires ES, Alvarez D, Dobrowolski C, et al. . Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol. 2012;18:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasad A, Kulkarni R, Shrivastava A, Jiang S, Lawson K, Groopman JE. Methamphetamine functions as a novel CD4+ T-cell activator via the sigma-1 receptor to enhance HIV-1 infection. Sci Rep. 2019;9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li MJ, Briones MS, Heinzerling KG, Kalmin MM, Shoptaw SJ. Ibudilast attenuates peripheral inflammatory effects of methamphetamine in patients with methamphetamine use disorder. Drug Alcohol Depend. 2020;206:107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. Int Rev Neurobiol. 2014;118:165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krasnova IN, Justinova Z, Cadet JL. Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacology (Berl). 2016;233: 1945–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee YW, Hennig B, Yao J, Toborek M. Methamphetamine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells. J Neurosci Res. 2001;66:583–591. [DOI] [PubMed] [Google Scholar]
- 25. Everall I, Salaria S, Roberts E, et al. ; HNRC Group . Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170:158–171. [DOI] [PubMed] [Google Scholar]
- 26. Coutinho A, Flynn C, Burdo TH, Mervis RF, Fox HS. Chronic methamphetamine induces structural changes in frontal cortex neurons and upregulates type I interferons. J Neuroimmune Pharmacol. 2008;3:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massanella M, Gianella S, Schrier R, et al. . Methamphetamine use in HIV-infected individuals affects T-cell function and viral outcome during suppressive antiretroviral therapy. Sci Rep. 2015;5:13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrico AW, Cherenack EM, Roach ME, et al. . Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. Aids. 2018;32:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grosgebauer K, Salinas J, Sharkey M, et al. . Psychosocial correlates of monocyte activation and HIV persistence in methamphetamine users. J Neuroimmune Pharmacol. 2019;14:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Catalfamo M, Wilhelm C, Tcheung L, et al. . CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rempel H, Sun B, Calosing C, Pillai SK, Pulliam L. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. Aids. 2010;24:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fulcher JA, Hussain SK, Cook R, et al. . Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J Infect Dis. 2018;218:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fulcher JA, Shoptaw S, Makgoeng SB, et al. . Brief report: recent methamphetamine use is associated with increased rectal mucosal inflammatory cytokines, regardless of HIV-1 serostatus. J Acquir Immune Defic Syndr. 2018;78:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aralis HJ, Shoptaw S, Brookmeyer R, Ragsdale A, Bolan R, Gorbach PM. Psychiatric Illness, substance use, and viral suppression among HIV-positive men of color who have sex with men in Los Angeles. AIDS Behav. 2018;22:3117–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okafor CN, Gorbach PM, Ragsdale A, Quinn B, Shoptaw S. Correlates of preexposure prophylaxis (PrEP) use among men who have sex with men (MSM) in Los Angeles, California. J Urban Health. 2017;94:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fredrickson BL, Grewen KM, Coffey KA, et al. . A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110:13684–13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fastect II Drug Screen Dipstick Test Package Insert. Irvine, CA: Branan Medical Corporation; 2005. [Google Scholar]
- 38. Maayah ZH, Takahara S, Ferdaoussi M, Dyck JRB. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165771. [DOI] [PubMed] [Google Scholar]
- 39. Okafor CN, Li M, Paltzer J. Self-reported cannabis use and biomarkers of inflammation among adults in the United States. Brain Behav Immun Health. 2020;7:100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321:451–452. [DOI] [PubMed] [Google Scholar]
- 41. Bavinton BR, Pinto AN, Phanuphak N, et al. ; Opposites Attract Study Group . Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5:e438–e447. [DOI] [PubMed] [Google Scholar]
- 42. McCluskey SM, Siedner MJ, Marconi VC. Management of virologic failure and HIV drug resistance. Infect Dis Clin North Am. 2019;33:707–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fredriksen-Goldsen KI, Kim HJ. The science of conducting research with LGBT older adults—an introduction to aging with pride: National Health, Aging, and Sexuality/Gender Study (NHAS). Gerontologist. 2017;57:S1–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thames AD, Irwin MR, Breen EC, Cole SW. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology. 2019;106:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X, Wang Y, Ye L, et al. . Modulation of intracellular restriction factors contributes to methamphetamine-mediated enhancement of acquired immune deficiency syndrome virus infection of macrophages. Curr HIV Res. 2012;10:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antoni MH, Lutgendorf SK, Blomberg B, et al. . Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zweben JE, Cohen JB, Christian D, et al. ; Methamphetamine Treatment Project . Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. [DOI] [PubMed] [Google Scholar]
- 50. Carrico AW, Flentje A, Kober K, et al. . Recent stimulant use and leukocyte gene expression in methamphetamine users with treated HIV infection. Brain Behav Immun. 2018;71:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team . Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. Plos Pathog. 2017;13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neuhaus J, Jacobs DR Jr, Baker JV, et al. . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. [DOI] [PubMed] [Google Scholar]
- 54. Ehrman RN, Robbins SJ, Cornish JW. Results of a baseline urine test predict levels of cocaine use during treatment. Drug Alcohol Depend. 2001;62:1–7. [DOI] [PubMed] [Google Scholar]