Abstract
Pharmacovigilance (PV) came suddenly into the spotlight when several new vaccines, developed as a response to the COVID-19 pandemic, received emergency authorisation and were rolled out on a large scale in late 2020. The vaccines underwent stringent clinical trials and evaluation from regulatory authorities, but with the use of novel technology and an anticipated rapid and vast deployment of the vaccines, the importance of a well-functioning international post marketing safety surveillance system was stressed. International PV stakeholders were faced with several challenges due to the extent of the global vaccination campaign. The unprecedented volume of reports of suspected adverse events following immunization has led to the development and use of new tools. Furthermore, the collaboration between various PV stakeholders was encouraged and strengthened. PV rose to the challenges posed by the currently ongoing global COVID-19 vaccination campaign and successful adaptations were made in a short period of time. However, the pandemic has not ended yet, the vaccination campaign is far from being completed, and further challenges are anticipated. Advances made during the pandemic will be important to strengthen PV in future and ensure to advance medicines’ safety together.
Plain Language Summary
Global safety monitoring of the COVID-19 vaccines: challenges, preparations, and outlooks
Pharmacovigilance (PV) is the umbrella term for all sciences and activities relating to the detection, assessment, understanding, and prevention of adverse effects relating to medicines or vaccines. PV came into the spotlight when several new vaccines were authorised and rolled out as a response to the COVID-19 pandemic.
The anticipated extent of the global vaccine rollout stressed the importance of a well-functioning safety surveillance system and international collaborations between patients, health care workers, vaccine producers, regulatory authorities, and PV centres.
The identification and communication of potential safety concerns showed that adaptations to PV processes made in a short period of time as well as international collaborations were successful. However, it is important to learn from experiences made so far and to make sure the positive advances are maintained in the future to advance medicines’ safety together.
Keywords: adverse drug reaction reporting systems, COVID-19 vaccines, drug monitoring, pharmacovigilance
Background
‘Almost all fear is fear of the unknown. Therefore, what’s the remedy? To become acquainted with the things you fear’.
Peace Pilgrim (Mildred Lisette Norman)
In 2020 we found ourselves faced with a new unknown causing drastic changes to everyday life worldwide and the rapid dissemination of information caused an infodemic accompanying the pandemic, leading to uncertainties and fear exacerbated by misinformation. Past vaccine misinformation still has an impact on both healthcare professionals and the public today. The infodemic observed during the COVID-19 pandemic and the COVID-19 vaccination campaign undermines the trust in health institutions and programmes. An important difference between the COVID-19 pandemic and previous pandemics with higher mortality rates is the extent and velocity of (mis-)information spreading due to wide mobile device and Internet coverage. The fast and broad distribution of non-verified information as well as the rapid emergence of anti-vaccine accounts on social media facilitated the spreading of false and partly dangerous information, conspiracy theories and more. 1
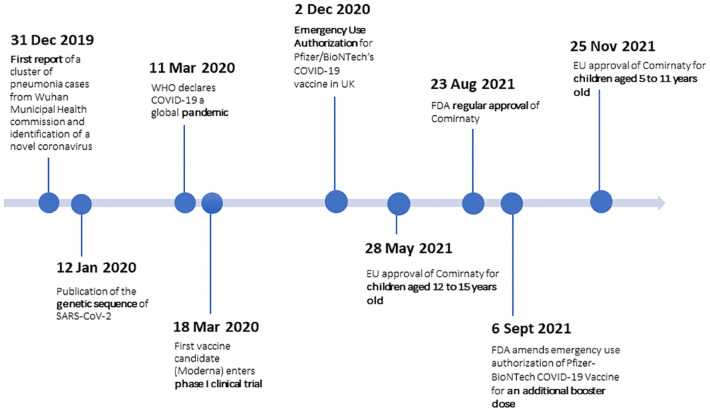
Vaccines are critical to control COVID-19, declared a pandemic by the World Health Organisation (WHO) on March 11th, 2020, and vaccine development started early. This was possible due to rapid gene sequencing of the novel severe acute respiratory syndrome-causing coronavirus 2, known as SARS-CoV-2, which was publicly shared on January 12, 2020. Due to its high infectivity and mortality rates and its strong impact on societies and economies worldwide, governments made immense financial resources available for vaccine research, allowing scientists to work on the development of vaccine candidates. Thanks to advances in viral immunology, structural biology, and novel vaccine platforms capable of eliciting robust immune responses, hundreds of vaccine candidates were developed in an unprecedentedly short time span. Furthermore, scientists could use the prior knowledge of coronaviruses, with the first human coronavirus identified in the 1960s. 2 Utilising findings from prior vaccine studies for previous human coronavirus infections, such as SARS, caused by SARS-CoV-1, between 2002 and 2004, and the Middle East respiratory syndrome (MERS), caused by MERS-CoV, in 2012 enabled a significant jump forward in the vaccine development for COVID-19. 2 Studies investigating immune responses to different gene fragments of SARS-CoV-1, for example, showed that using the full-length spike proteins as vaccine antigens elicited the strongest and longest lasting humoral response when compared to membrane and nucleocapsid proteins. 3 Therefore, most of the COVID-19 vaccine candidates are based on the spike protein. Also, platforms such as viral vectors and protein subunits were already tested for SARS-CoV-1 and MERS. Even though only a few SARS-CoV-1 and MERS vaccines entered clinical trials and none of them obtained market authorisation, findings from those trials were valuable during the development of the COVID-19 vaccines. For the vaccines’ safety surveillance important previous findings were taken into account: the observation of antibody-dependent disease enhancement (ADE) in association with the SARS-CoV-1 and MERS vaccine candidates led to it being included in the list of adverse events of special interest (AESIs) and the early creation of case definitions. 4 On March 16th, 2020, roughly 2 months after publication of the gene sequence of SARS-CoV-2, the first vaccine candidate (Moderna’s mRNA-based vaccine) entered its phase I clinical trial and by November 2020 at least 67 substances were under evaluation in phase I-III clinical trials.5,6 The first vaccine (Pfizer/BioNTech’s mRNA-based vaccine) obtained emergency use authorisation (EUA) on December 2nd, 2020, in the United Kingdom (UK), followed by the United States on December 11, 2020. By September 7th, 2021, 22 vaccines were approved in some form for use in at least one country, 7 114 vaccines were in clinical development and 185 candidates were in pre-clinical development. 8 Many of these vaccines use novel technologies and vaccine platforms, such as mRNA and viral vectors.
The rapid market authorisation of several vaccines was made possible by the simultaneous performance of multiple clinical trial phases as well as the inclusion of large patient cohorts in these studies. Furthermore, regulators deployed so called ‘rolling reviews’ – reviews of clinical data as soon as it became available from ongoing studies – accelerating the authorisation process significantly. 9 However, due to transparency concerns and the circulating misinformation, dissenting voices regarding the vaccines’ safety got louder, causing insecurity and vaccine-hesitancy in the general public and even among health care professionals.10,11
The large pre-authorisation clinical trials were conducted appropriately, and no regulatory processes were bypassed. However, it must be acknowledged that any large phase III trial has limitations, such as a short follow-up period prior to authorisation as well as the inability to detect rare (⩾ 1 in 10,000 to < 1 in 1,000 vaccines) and very rare (⩽ 1 in 10,000 vaccinees) adverse events that become apparent only when the vaccines start to be used widely.12,13 This makes well-functioning post-marketing safety surveillance systems and international communication and collaboration necessary.
Furthermore, it must be kept in mind that many countries followed prioritised vaccine rollout resulting in immunisation of populations that were excluded or underrepresented in the clinical trials, resulting in increased uncertainty about the safety in these groups. 13 Other important milestones, such as the amendment of the vaccination scheme to include a third dose (September 6th, 2021) as well as the vaccines’ use in adolescents (May 28th, 2021) and younger children (November 25th, 2021) (see Figure 1) were achieved based on real-world data.
Figure 1.
Timeline of vaccine development.
Anticipated challenges and limitations
Although the COVID-19 pandemic has in many ways been unparalleled, efforts have been made to learn from experiences during previous pandemics and epidemics. The lessons for safety surveillance centred around public concerns of vaccine safety with the suggestion to tackle these issues with suitable vaccine safety surveillance that can provide timely and effective communication. 14 The requirement for demonstrating effect and safety is typically greater than compared to other medicinal products used for therapeutic reasons in patients due to the differences in the target population. 15 It is possible that the severity and risk of the COVID-19 pandemic along with the potential benefit of vaccination may have affected individual perceptions. The key challenges were to strengthen surveillance systems, dependent on resources, to be able to accommodate large numbers of reports and to facilitate early detection, investigation and analysis of adverse events with subsequent appropriate and rapid response including public communication to maintain vaccine confidence. 14
Specific considerations for the COVID-19 vaccines safety surveillance systems include preparing for simultaneous and sequential use of different vaccines (e.g. mixed schedule vaccination), and detection of local clusters of immunisation-error related adverse events. 14 Both challenges require complete information regarding the vaccine used, other medications administered concomitantly, and the experienced adverse events. Reporting of adverse events can be particularly challenging given the non-uniformity of coding adverse events, as well as the possibility of certain conditions to present themselves with a variety of symptoms, both of which may lead to a significant number of reports containing different terms but describing the same phenomenon. 16 This particularly impedes statistical signal detection processes. Further considerations included increasing the awareness of adverse event reporting, particularly in those who work with adult healthcare and may not be familiar with procedures since the majority of national vaccination programmes involve childhood vaccinations. 14 However, due to the number of people to be vaccinated, safety surveillance systems were expected to receive a large volume of reports. 14
The safety surveillance of vaccines and collection of reports on suspected adverse events following immunisation is the responsibility of national vaccine regulatory systems, including national regulatory authorities (NRAs) and national immunisation programmes (NIPs). Especially in countries where NRAs do not have extensive capacities, the NIPs may take responsibility for certain NRA tasks. 17 Even though the WHO global manual on the surveillance of adverse events following immunisation (AEFIs) 18 foresees an active and close collaboration between NIPs and NRAs for the safety surveillance of vaccines, data exchange in many countries can still be improved. 19 The extent of the COVID-19 vaccination campaign necessitated a closer collaboration between NIPs and NRAs as well as between international NRAs.
Passive surveillance, defined as collection and analysis of unsolicited reports of suspected adverse events in the form of individual case safety reports (ICSRs) that are sent to a central database or health authority, is the basis of safety surveillance for immunisation programmes, with the ability to identify rare events, to assess clusters of reports and to detect safety signals for further evaluation. 14 More recently, rapid cycle analysis of active surveillance data has been used to assess pre-specified adverse events in vaccine surveillance. 20 In contrast to passive surveillance, active surveillance aims to determine event rates in defined populations following pre-established processes. However, in the context of the COVID-19 vaccination campaign, passive surveillance required additional active surveillance and post-authorisation safety studies that can estimate event rates and facilitate risk evaluation.14,21 The improvement of safety surveillance systems particularly in low- and middle-income countries (LMICs) is strongly recommended. 21 An effective and quick system of sharing data between vaccines manufacturers, vaccine sponsors and regulatory authorities is important to interpret analysis from passive surveillance data which can supplement clinical trial safety information by detecting adverse events that are rare, occur in certain populations or have a longer latency period than follow up. 14 Furthermore, timely reporting, reviewing, and sharing of data–at a national, and global level–strengthens surveillance systems. 14
Spontaneous reporting systems are usually limited to varying degrees by under-reporting of adverse events. 22 In the context of mass vaccination campaigns with increased public attention and widely spread (mis-)information on safety issues, a bias towards the opposite phenomenon – a relative increase in reporting also of coincidental events in association with vaccination – can be observed and complicate signal detection. 23 This phenomenon can be observed under ‘normal’ conditions as well. However, the extent to which it was experienced for the COVID-19 vaccines was unprecedented. Furthermore, follow-up processes differ from regular PV activities for other medicinal products (e.g. active follow-up after vaccination and a defined follow-up period following the administration of a vaccine dose). In addition, a generally lower tolerance regarding adverse events following immunisation (AEFIs) of a mostly healthy population compared to adverse events following pharmacotherapy of conditions affecting ill patients can be observed, additionally intensifying the issue. COVID-19 and the safety of the vaccines, remain in the public spotlight and the enhanced PV efforts in that context are to be considered very positive, but at the same time bear the risk for several types of bias (e.g. notoriety bias, surveillance bias, and recall bias) potentially negatively impacting signal detection efforts.24,25
Causality assessment (CA) of AEFIs is mainly based on four criteria: consistency (evidence in the published, peer-reviewed literature), biological plausibility, temporality, and specificity. 26 CA of AEFIs differs from that of medicines due to their immunological mechanism of action compared to the mechanism of action of therapeutically, or diagnostically used medicinal products. 27 Although many COVID-19 vaccines are administered as two or more doses, there is limited opportunity to assess rechallenge in these cases, as reports often occur after the second dose, or between the doses but with limited information on follow up and the effect of the second dose, if administered. In the context of the new vaccines, CA is complicated by the absence of available evidence, as well as the lack of available data impeding the assessment of biological plausibility. 24
Another possible effect of the level of public interest in COVID-19 vaccinations is the spread of information from various sources. Vaccine hesitancy was listed as one of the 10 top global health threats by the WHO in 2019 28 and the relationship between social media and doubts of vaccine safety have been widely discussed.29,30 As stated by Black et al.: 31 ‘In the modern era of rapid dissemination of rumours on social media, a rapid response to a vaccine safety signal is needed to maintain public confidence’. This sentiment is widely published and further reinforced by recommendations from the WHO to provide rapid communication regarding safety concerns of the COVID-19 vaccines to maintain public confidence and to prevent alarm or uncertainty.14,32
Preparations for the safety surveillance of the novel vaccines
The first steps in the preparation for developing and deploying new vaccines against the novel SARS-CoV-2 included the increase of resources. Expertise needed to be acquired and infrastructure for development and production of vaccines in large scale needed to be provided. As preparation for efficient deployment, several logistical and administrative challenges had to be faced: defining prioritised patient groups (invitations, vaccine centres, transport, recalls for 2nd dose), vaccine storage (cold chain supply), and more. However, these areas are outside the scope of the current review and are therefore not being addressed in-depth.
The development of several vaccines using different platforms as well as the extent of the COVID-19 vaccination campaign was foreseeable. Therefore, the role and requirements for COVID-19 vaccine safety surveillance systems were established with advance. A safety surveillance manual was published by the WHO to give guidance on the establishment of appropriate safety surveillance systems on the national level ensuring an appropriate and rapid response to emerging AEFIs and Adverse Events of Special Interest (AESIs). 14
A list of AESIs for the COVID-19 vaccines was created by the Brighton Collaboration’s Task Force for Global Health via the Coalition for Epidemic Preparedness Innovations (CEPI)-funded Safety Platform for Emergency vACcines (SPEAC) project. 33 COVID-19 vaccine AESI definitions were preliminarily based on existing Brighton Collaboration definitions of adverse events considered most likely to be of relevance for the vaccine candidates, and a review of the literature using PubMed. 34
Based on the Brighton Collaboration’s AESI definitions, national and international projects to investigate AESI background rates were started and published.35–37 While it is not possible to estimate incidence from passive surveillance data, nor to directly compare reporting rates to background incidence rates, knowledge of these background rates provided key information in the assessment of potential safety signals. In particular, active surveillance programmes benefitted from the definition of AESI background rates, allowing a more direct comparison of observed to expected rates. However, background rates play a crucial role in causality assessment of AEFIs. 12 Furthermore, AESI background rates proved useful for prioritisation (e.g. as a parameter for clinical importance) and for granting a rapid response to vaccine safety concerns. 31
Part of the post-marketing safety surveillance is the identification and anticipation of important (potential) risks of a medicinal product. Those are summarised in a product’s risk management plan (RMP). Guidance documents for industrial stakeholders included the advice to consider the vaccines’ construct and formulation, the antigen’s characteristics, possible adjuvants, as well as to include important identified risks with other vaccines using similar technologies [e.g. vaccine-associated enhanced respiratory disease (VAERD) as observed with SARS-CoV-1 and MERS vaccine candidates].38,39 The inclusion of important potential risks aims to cover risks with possible impact on the benefit/risk balance, for which a causal relationship is presumed but could not be inferred yet, due to lack of evidence. RMPs are supposed to also cover missing information. For the COVID-19 vaccines the regulators advised to focus especially on the vaccines’ use in risk groups (e.g. pregnant and breastfeeding women, patients with severe co-morbidities, and children), and on the long-term safety, and interaction with other vaccines.38,39
Periodic safety update reports (PSURs) are another important part of post-marketing safety surveillance. In the context of the COVID-19 vaccines, their adapted approval process, and the subsequent limited follow up period of pre-marketing clinical studies, the timeline for the PSURs (usually annually to twice per year) has been shortened and the submission of monthly condensed reports was agreed on. Issues addressed in the monthly PSURs are suggested to include the risks of vaccination errors (especially in the context of mass vaccination campaigns), safety of mixed schedules, estimated antibody waning, and the anticipated need for administration of booster doses or revaccinations.38,39
The published guidance documents contain also specific advice for signal detection and management. Stakeholders were strongly advised to include international databases of ICSRs (namely EudraVigilance and VigiBase, the WHO global database of ICSRs) as data sources for their signal detection activities.38,39
Action taken
Many national and international regulatory bodies used their established PV monitoring systems as the basis for the safety surveillance of the COVID-19 vaccines40,41 as well as information campaigns. Improvement of pre-existing systems were undertaken, for example, by introducing mobile reporting apps, to facilitate, stimulate and monitor spontaneous reporting. Furthermore, in many places active surveillance measures were introduced or increased. Methods used included Cohort Event Monitoring (CEM) (e.g. within the European ACCESS study), 42 Sentinel Surveillance (SS), Data Linkage (e.g. in the Rapid Cycle Analysis in the UK), 43 and e- and m-Health. 14 Transparency measures were set out by all stakeholders. Regular publication of news and updates were made available to a wider audience to increase the public’s confidence in the vaccines and to fight the circulating misinformation.41,44–53 The exact scope of adaptations made by the regulatory bodies falls outside the scope of this paper and will not be discussed in-depth.
What did UMC do?
AEFI reporting by the member states was facilitated by the implementation of a digital reporting form designed for mobile devices and accessible via a QR code allowing reporting directly into VigiFlow in countries using it as national PV database.
With guidance from WHO headquarters, entry of incoming reports was facilitated by the expansion of VigiFlow for the WHO AEFI form. 54 The scope of this amendment was the facilitated efficient and effective AEFI data transfer using the 25 AEFI reporting core variables from the periphery of a health care system into a central database for processing and conversion into information that can guide decision making and actions in a timely manner. Furthermore, the international standardisation of data collection enables data analysis on a global scale in VigiBase.
The possibility to extract AEFI line listings from VigiFlow was introduced as requested by WHO to allow timely analysis on the national level. The line listings facilitate an overview of the data, supporting initial identification of clusters or unusual/significant reporting of events requiring further analysis. Using AEFI line listings, it will be possible to identify vaccine batch-related problems as well as locally confined immunisation-related errors.
The described changes were conceptualised by the WHO and involved close collaboration with WHO headquarters and other stakeholders, such as national PV centres. Changes were implemented on local levels according to WHO guidelines.
A monthly descriptive report regarding COVID-19 vaccine reporting in VigiBase was made available for member countries of the WHO PIDM via VigiLyze. The intention of this report is to give a qualitative overview of ICSR characteristics such as demographics, reported events, and the different vaccines. This facilitates data interpretation for national pharmacovigilance stakeholders during their signal detection activities.
For safety signal analysis, further adaptations to VigiLyze have been undertaken. More frequent updates of VigiBase data were started to allow timely data analyses. The creation of a COVID-19 vaccine-specific standardised drug grouping (SDG) enables the data analysis on a vaccine platform level. In addition, the option to switch between ‘active ingredients’ and ‘active ingredient variants’ facilitates data analysis on individual vaccine level.
Uppsala Monitoring Centre
WHO PIDM.
The WHO Programme for International Drug Monitoring (PIDM) was established in 1968 as a response to the thalidomide catastrophe. It was established with the scope to ensure the collection of evidence about patient harm from as many sources as possible. The programme started with 10 member states from three continents and has grown to 148 full members and 23 associated members by 12 July 2021. UMC has been responsible for the technical and operational aspects of the programme since 1978.
VigiFlow.
VigiFlow is a web-based individual case safety report (ICSR) management system that is available for use by national PV centres of the WHO PIDM. It supports the collection, processing and sharing of ICSR data to facilitate effective data analysis. Features include manual data entry, and data sharing and exchange between stakeholders.
VigiBase
VigiBase is the WHO global database for suspected adverse reactions to medicinal products. VigiBase collects reports of suspected adverse effects of medicines and vaccines from all member countries of the WHO PIDM.
CAVEAT:
Reports sent to VigiBase describe suspected adverse reactions. Causality is not established at the time of reporting but must be assessed individually. Reporting practices vary between regional PV centres. The volume of reports depends highly on the extent of a product’s use, publicity, the nature of the suspected adverse event, and other factors. As the nature of included reports is spontaneous and unsolicited, reporting rates must not be confused with incidence rates.
VigiLyze
VigiLyze is a signal detection and management system available free of charge for national PV centres in all member countries of the WHO PIDM. Its main feature is the application of quantitative signal detection methods on national, regional, or global data. It provides tools facilitating the entire signal management process (from signal detection, over signal prioritisation, to qualitative signal assessment).
Signal detection
Signal detection activities at UMC include regular screenings of VigiBase for previously unknown medicine-ADR combinations. Due to the size of the database, statistical methods–described elsewhere55,56–are used in signal detection, followed by manual in-depth assessment by PV experts after a combination is prioritised and evaluated to represent a new risk or a new aspect of a known risk (validation). Signals are shared with countries participating in the WHO Programme for International Drug Monitoring (PIDM). 57
The characteristics, rate, and quantity of incoming ICSRs for the COVID-19 vaccines required the adaptation of existing processes and the development of optimization strategies. Statistical disproportionality analysis is the base for signal detection at UMC. It has been used to great effect during the pandemic. However, disproportionality analysis only highlights single drug-event combinations. When there are inconsistencies in reported terms, or when an adverse event can be characterised by multiple terms, standard disproportionality analysis can sometimes fail to highlight safety issues. vigiGroup is a novel statistical method developed at the UMC to address this issue, by considering the co-reporting patterns for different terms on an ICSR, in order to assign reports to clinically meaningful groups representing the same underlying condition. This technique has been used successfully for retrospective analyses of safety profiles of medicines at UMC. 58 During the pandemic, the use of vigiGroup has been applied to prospectively sort through the COVID-19 vaccine ICSRs to highlight potential safety issues.
In the past, signal detection at UMC was performed during periodical focussed signal detection workshops. Due to the extent of incoming reports as well as the fast-moving reporting landscape, another approach was adapted. Changes included direct monitoring in VigiLyze, as well as performing more frequent signal detection workshops using UMC’s internal tools, such as vigiMine and vigiRank, 55 and performing statistical disproportionality analysis against different data backgrounds. Furthermore, international regulatory authorities’ communications and the rapidly growing medical literature were screened, as well as communication and collaboration with external sources (e.g. clinical experts and WHO headquarters), in order to detect and assess any potential emerging safety issues as early as possible to trigger in-depth analysis using VigiBase data.
Signal prioritisation
The unprecedented rate of incoming reports caused a rethinking of UMC’s internal signal prioritisation strategy. In weekly meetings, a multidisciplinary team discussed and prioritised potential safety signals with the aim to warrant timely assessment and communication. Criteria taken into consideration during signal prioritisation were the available evidence, the geographic spread of received reports, the seriousness of the observed reaction, the clinical importance, the impact of the observed reaction on public health, and the type of message a potential signal communication could have. Case definitions together with Standardised MedDRA Queries (SMQs) for AESIs have been used in internal monitoring of these events at the Uppsala Monitoring Centre (UMC). Furthermore, the monitoring of international regulatory activities was supposed to avoid performing work already conducted elsewhere maintain a global perspective to potentially detect rare safety issues earlier.
The options to add tags and deadlines to investigations in VigiLyze, as well as advanced filtering and searching options of the investigations list allow for signal prioritisation directly in VigiLyze without the need for a separate platform.
Signal assessment
UMC usually limits signal detection activities and assessments to emerging suspected adverse drug reactions. CA of COVID-19 vaccine AEFIs was complicated by very large case series. This problem has led to the development and application of tools, like a vaccine report-specific de-duplication tool, strategies to prioritise the analysis of ICSRs, and the use of vigiPoint, 59 a tool for the data driven exploration and comparison of key features of two or more case series.
An important step in CA consists of the validation of an adverse event diagnosis. For that purpose, case definitions, such as those prepared by the Brighton Collaboration, are used for guidance, although in case series where clinical case information is constrained both validation and the utility of diagnostic guidelines can be limited. However, in case of new or emergent events, such definitions might not exist at the time of assessment. 24 Therefore, collaborations with other stakeholders as well as attentive reviews of the published medical literature are key to case validation.
An approach to assess causality, as proposed by Butler and colleagues could be using a combined methodology considering temporal relationship, individual risk factors and the likelihood of alternative causes and integrate results from different epidemiological methods in order to compensate for various types of bias. 24 Another approach was to address several related reactions in a comprehensive assessment, discussing potential causes and underlying conditions and highlighting reporting patterns and any potential need for further appropriate studies or considerations. Reviews of emerging safety issues as well as analyses of reporting patterns of specific areas were performed and shared with other stakeholders.
Reflections
The global vaccine rollout is far from complete, and there are still lessons to be learnt by reflecting on the progress so far. While concerns over the expedited development of the COVID-19 vaccines have been raised and despite widespread misinformation that can dampen the confidence in the vaccines, 60 the rollout of vaccines has overall been successful. As of September 1st, 2021, 5.34 billion doses have been administered. 61 While a large number of ICSRs have been reported to VigiBase (1.8 million reports), the figure remains small in comparison to the total of administered doses. However, case series associated to the COVID-19 vaccines are often larger than series studied for signal detection associated to other medicinal products, challenging clinical assessors and PV experts. Due to the characteristics of the emerging case series (e.g. lack of reliable denominators, high numbers of reports, and a high variety of completeness of documentation) and the relatively short follow up period, in most cases during safety signal assessment it is only possible to formulate hypotheses and the recommendation to pursue further epidemiological studies where appropriate can be found in the assessment texts. Differences compared to safety surveillance activities for other medicines further challenged signal management for the novel vaccines. The rate of incoming reports reflected the real-world situation. Due to the increased public focus, a timely as possible assessment of emerging safety issues was required. The information shared with other stakeholders has contributed to the global safety surveillance of the COVID-19 vaccines.
UMC’s unique position allows for research and method development apart from signal detection and assessment. As outlined above, different methodologies of signal detection and data analysis were implemented during the COVID-19 vaccine safety monitoring with the efficacy of these techniques often being evaluated simultaneously and therefore being fit for future use not only in the context of COVID-19 vaccine monitoring. However, the strengthening of PV systems and tools, such as the adaptations to VigiFlow and more frequent updates of VigiBase, allowed assessors to cope with the increased demands, and timely assessment and updating of potential adverse events for analysis has taken place. Future safety monitoring efforts will benefit from the experience made during the monitoring of the pandemic vaccines. Furthermore, although the use of mobile apps in PV has previously been integrated into PV systems with good effect, 62 the COVID-19 pandemic has prompted their more extensive use. The effects of this, with regards to the vaccine rollout, have not fully been evaluated yet. Another aspect UMC’s position allows for, is the focussing on the early detection of emerging potential safety signals (e.g. by restricting signal detection to small case series and applying statistical methods). In this way, safety monitoring efforts taken by international PV stakeholders can be complemented by work performed at UMC. Collaborations with other international PV stakeholders can benefit from UMC’s global perspective. Reviews of subjects of interest from this perspective can support risk evaluation on national and international level.
In general, safety information from the vaccine manufacturers and regulatory authorities has been transparent, with ongoing investigations, decisions and clinical trial data made widely available.32,41,44–51,63–65
While passive surveillance is the traditional cornerstone of PV and it has played a crucial role in identifying safety signals related to the COVID-19 vaccines, active surveillance and the ability to differentiate from background rates has been crucial in the vaccine safety surveillance. Although not possible in all cases, attempts have been made to calculate background rates of adverse reactions of interest.36,66 In combination with estimates of incidence rates from active surveillance, this has allowed for confirmation of safety signals and highlighted subgroups at higher risk with updated safety information made publicly available67,68 as well as comparison of adverse events related to COVID-19 disease and the COVID-19 vaccines. 69 Furthermore, there have been investigations into hypothesised adverse effects that have gained notable media traction, such as concerns regarding fertility,70,71 with clarifying statements of no evidence of effect.65,72
Outlook
Looking back, adaptations of PV processes can be observed everywhere, and their impacts are impressive. However, the vaccine rollout is not complete, and the COVID-19 pandemic has not ended yet. The number of vaccines authorised for use is increasing, and with help from the WHO’s COVAX programme, 73 vaccines are reaching more countries, with sometimes less well-established pharmacovigilance systems. Identified problems for the vaccine deployment in LMICs were vaccine safety concerns impacting vaccine hesitancy, vaccination service-related, social/religious- and culture-related, vaccine production and cost-related, vaccine distribution and storage-related, as well as vaccination programme monitoring and evaluation barriers. 74 Despite extensive experience from the Expanded Programme on Immunisation, the importance of strengthening and adaptation of vaccine safety monitoring programmes in LMICs represents an important but challenging task for the scientific community in the future.
The extension of the vaccines’ authorisations for the use in additional patient groups, the anticipated approval of new vaccines, as well as the dynamic change of vaccination schedules (including heterologous vaccine schedules) and the anticipation of required booster doses the need for further process adaptations and shifting of focus areas can be anticipated. The introduction of extensive transparency measures regarding the vaccines and related issues by regulatory authorities worldwide are very positive and it is likely that they will continue for the COVID-19 vaccines. However, it is important to make sure that transparency does not stop once the pandemic ends and that the dialogue between stakeholders of drug safety will continue.
Acknowledgments
The authors sincerely thank the PV products specialists Jenny Jansson Liikamaa and Anna Baumgarten for patiently lining out what adaptations to VigiFlow and VigiLyze were made in order to facilitate data collection and analysis for the WHO PIDM members but also enable the signal detection team’s work at UMC. In that context we would like to acknowledge also Dr. Madhava Balakrishna for conceptualising expansions of VigiFlow to accommodate AEFI reports, as well as Ayako Fukushima for changes to mobile devices. Special thanks go to UMC’s Global Communications Team, especially to Mårten Leo for graphic design support.
We, furthermore, want to thank Dr. Ruth Savage in her function as consultant, who gave important insights into New Zealand’s national pharmacovigilance system. Another special thank you goes to pharmacovigilance scientist Cecilia Wikner who gave important input during the final round of internal revision of the manuscript. We would like to acknowledge also Noah Iessa for external feedback from WHO’s side. And last but not least we warmly thank the head of UMC’s WHO Collaboration Centre department, Pinelopi Lundquist, who helped us to get the work for this review article going, supervised the process, managed resources, and gave critical input during internal revision
The authors are indebted to the national centres which make up the WHO Programme for International Drug Monitoring and contribute reports to VigiBase. The opinions and conclusions of this review are not necessarily those of the national centres which make up the WHO Programme for International Drug Monitoring nor of the WHO
Footnotes
ORCID iD: Annette Rudolph  https://orcid.org/0000-0003-2360-7174
https://orcid.org/0000-0003-2360-7174
Contributor Information
Annette Rudolph, WHO CC, Signal Management, Uppsala Monitoring Centre, Bredgränd 7B, Uppsala 753 20, Sweden.
Joseph Mitchell, WHO CC, Signal Management, Uppsala Monitoring Centre, Uppsala, Sweden.
Jim Barrett, Research, Data Science, Uppsala Monitoring Centre, Uppsala, Sweden.
Helena Sköld, Operations, PV Portfolio, Uppsala Monitoring Centre, Uppsala, Sweden.
Henric Taavola, Research, Data Science, Uppsala Monitoring Centre, Uppsala, Sweden.
Nils Erlanson, Research, Data Science, Uppsala Monitoring Centre, Uppsala, Sweden.
Carlos Melgarejo-González, WHO CC, Signal Management, Uppsala Monitoring Centre, Uppsala, Sweden.
Qun-Ying Yue, WHO CC, Signal Management, Uppsala Monitoring Centre, Uppsala, Sweden.
Declarations
Ethics approval and consent to participate: Not applicable
Consent for publication: Not applicable
Author contributions: Annette Rudolph: Conceptualisation; Methodology; Writing – original draft; Writing – review & editing.
Joseph Mitchell: Conceptualisation; Methodology; Writing – original draft; Writing – review & editing.
Jim Barrett: Conceptualisation; Methodology; Writing – original draft; Writing – review & editing.
Helena Sköld: Conceptualisation; Writing – review & editing.
Henric Taavola: Conceptualisation; Writing – review & editing.
Nils Erlanson: Conceptualisation; Methodology; Writing – review & editing.
Carlos Melgarejo-González: Conceptualisation; Writing – review & editing.
Qun-Ying Yue: Conceptualisation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Uppsala Monitoring Centre (UMC) is a non-profit organisation and the WHO Collaborating Centre for International Drug Monitoring. All authors declare being employed by UMC and not having any conflicts of interest.
Availability of data and materials: Not applicable.
References
- 1. Pertwee E, Simas C, Larson HJ. An epidemic of uncertainty: rumors, conspiracy theories and vaccine hesitancy. Nat Med 2022; 28: 456–459. [DOI] [PubMed] [Google Scholar]
- 2. Li Y-D, Chi W-Y, Su J-H, et al. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci 2020; 27: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z, Yuan Z, Matsumoto M, et al. Immune responses with DNA vaccines encoded different gene fragments of severe acute respiratory syndrome coronavirus in BALB/c mice. Biochem Biophys Res Commun 2005; 327: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munoz FM, Cramer JP, Dekker CL, et al. Vaccine-associated enhanced disease: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021; 39: 3053–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bok K, Sitar S, Graham BS, et al. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity 2021; 54: 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. London School of Hygiene & Tropical Medicine. Vaccine landscape, https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/# (2021, accessed 8 May 2021).
- 7. Regulatory Affairs Professionals Society. COVID_19 vaccine tracker. J Craven, https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (18 February 2021, accessed 23 February 2021).
- 8. World Health Organization. COVID-19 – landscape of novel coronavirus candidate vaccine development worldwide, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (2021, accessed 8 October 2021).
- 9. European Medicines Agency. EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines, https://www.ema.europa.eu/en/documents/other/ema-initiatives-acceleration-development-support-evaluation-procedures-covid-19-treatments-vaccines_en.pdf (2021, accessed 8 March 2021).
- 10. Maraqa B, Nazzal Z, Rabi R, et al. COVID-19 vaccine hesitancy among health care workers in Palestine: a call for action. Prev Med 2021; 149: 106618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med 2021; 27: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black S, Eskola J, Siegrist C-A, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet 2009; 374: 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma O, Sultan AA, Ding H, et al. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol 2020; 11: 585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. COVID-19 vaccines: safety surveillance mannual, https://apps.who.int/iris/bitstream/handle/10665/338400/9789240018280-eng.pdf?sequence=1&isAllowed=y (accessed 22 July 2021).
- 15. Atouf F, Chakrabarti R, Uppal A. Building trust in the quality of vaccines. Hum Vaccines Immunother 2022; 18: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai C, Peng Y, Shen E, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther 2021; 29: 2794–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Vaccine safety basics – e-learning course. Module 5: vaccine safety institutions and mechanisms, https://www.who.int/vaccine_safety/initiative/tech_support/Part-5.pdf?ua=1 (accessed 8 April 2021).
- 18. World Health Organization. Global manual on surveillance of adverse events following immunization. 2nd ed. Geneva: World Health Organization, https://www.who.int/vaccine_safety/publications/Global_Manual_on_Surveillance_of_AEFI.pdf (2014, accessed 8 May 2021). [Google Scholar]
- 19. Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol 2015; 8: 449–460. [DOI] [PubMed] [Google Scholar]
- 20. Leite A, Andrews NJ, Thomas SL. Near real-time vaccine safety surveillance using electronic health records-a systematic review of the application of statistical methods: Near real-time vaccine safety surveillance. Pharmacoepidemiol Drug Saf 2016; 25: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kochhar S, Salmon DA. Planning for COVID-19 vaccines safety surveillance. Vaccine 2020; 38: 6194–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29: 385–396. [DOI] [PubMed] [Google Scholar]
- 23. Ellenberg SS, Chen RT. The complicated task of monitoring vaccine safety. Public Health Rep 1997; 112: 10–20; discussion 21. [PMC free article] [PubMed] [Google Scholar]
- 24. Butler M, Tamborska A, Wood GK, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry 2021; 92: 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pariente A, Gregoire F, Fourrier-Reglat A, et al. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf 2007; 30: 891–898. [DOI] [PubMed] [Google Scholar]
- 26. WHO Team Pharmacovigilance. Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. 2nd ed. Geneva: World Health Organization, https://www.who.int/publications/i/item/causality-assessment-aefi-user-manual-2019 (2019, accessed 17 August 2021). [Google Scholar]
- 27. Le Louët H, Loupi E, Haramburu F, et al. Which pharmacovigilance for vaccines? Therapies 2007; 62: 245–247. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Ten threats to global health in 2019, https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (2019, accessed 17 September 2021).
- 29. Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Glob Health 2020; 5: e004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puri N, Coomes EA, Haghbayan H, et al. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum Vaccines Immunother 2020; 16: 2586–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Black SB, Law B, Chen RT, et al. The critical role of background rates of possible adverse events in the assessment of COVID-19 vaccine safety. Vaccine 2021; 39: 2712–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. COVID vaccine confidence requires radical transparency. Nature 2020; 586: 8. [DOI] [PubMed] [Google Scholar]
- 33. Brighton Collaboration. Priority list of adverse events of special interest: COVID-19, https://brightoncollaboration.us/priority-list-aesi-covid/ (2020, accessed 7 June 2022).
- 34. Law B, Sturkenboom M. Priority list of adverse events of special interest: COVID-19. SPEAC Work Package: WP2 Standards and tools. V2.0, May 25, 2020; p. 53. [Google Scholar]
- 35. US Food and Drug Administration. Draft Protocol: Background rates of adverse events of special interest for COVID-19 vaccine safety monitoring. 2020;p. 32. [Google Scholar]
- 36. Li X, Ostropolets A, Makadia R, et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. Epidemiology. Epub ahead of print 26 March 2021. DOI: 10.1101/2021.03.25.21254315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. VAC4EU. COVID-19 vaccine monitoring. VAC4EU, https://vac4eu.org/covid-19-vaccine-monitoring/ (accessed 7 June 2022).
- 38. European Medicines Agency. Consideration on core requirements for RMPs of COVID-19 vaccines. coreRMP19 guidance v2.0, https://www.ema.europa.eu/en/documents/other/consideration-core-requirements-rmps-covid-19-vaccines_en.pdf (2021, accessed 17 September 2021).
- 39. Pan American Health Organization. Risk management plans and periodic safety reports for COVID-19 vaccines. Recommendations for their request, preparation, management, and assessment, https://iris.paho.org/bitstream/handle/10665.2/53631/PAHOHSSMTCOVID-19210003_eng.pdf?sequence=1&isAllowed=y (2021, accessed 17 September 2021).
- 40. Centers for Disease Control and Prevention. Ensuring the safety of COVID-19 vaccines in the United States. Centers for Disease Control and Prevention, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html (2021, accessed 7 June 2022). [Google Scholar]
- 41. U.S. Food and Drug Administration. COVID-19 vaccine safety surveillance, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/covid-19-vaccine-safety-surveillance (2021, accessed 17 September 2021).
- 42. Sturkenboom M. Version 1.0: final report ACCESS – feasibility analysis of an EU infrastructure for COVID-19 vaccine monitoring, https://vac4eu.org/wp-content/uploads/2021/02/4.FinalreportACCESS.pdf (2021, accessed 9 June 2022).
- 43. Report of the Commission on Human Medicines Expert Working Group on COVID-19 vaccine safety surveillance. GOV.UK, https://www.gov.uk/government/publications/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance (accessed 7 June 2022).
- 44. Australian Government. COVID-19 vaccine safety monitoring and reporting. Department of Health – Therapeutic Goods Administration, https://www.tga.gov.au/covid-19-vaccine-safety-monitoring-and-reporting (2021, accessed 17 September 2021). [Google Scholar]
- 45. US Centers for Disease Control and Prevention. Safety of COVID-19 vaccines, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/safety-of-vaccines.html (2021, accessed 17 September 2021).
- 46. European Medicines Agency Pharmacovigilance Risk Assessment Committee. PRAC: agendas, minutes and highlights, https://www.ema.europa.eu/en/committees/prac/prac-agendas-minutes-highlights (accessed 17 September 2021).
- 47. European Medicines Agency. Human regulatory – COVID-19 vaccines, https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed 17 September 2021).
- 48. Government of Canada. Vaccine safety and possible side effects, https://www.canada.ca/en/public-health/services/vaccination-children/safety-concerns-side-effects.html (accessed 17 September 2021).
- 49. Medicines & Healthcare Products Regulatory Authority. Coronavirus (COVID-19) vaccine adverse reactions, https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions (2021, accessed 17 September 2021).
- 50. New Zealand Medicines and Medical Devices Safety Authority – Medsafe. COVID-19: vaccine safety monitoring process, https://www.medsafe.govt.nz/COVID-19/monitoring-process.asp#adrs (2020, accessed 17 September 2021).
- 51. World Health Organization. COVID-19 vaccines, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (2021, accessed 17 September 2021).
- 52. US Centers for Disease Control and Prevention. ACIP meetings information, https://www.cdc.gov/vaccines/acip/meetings/index.html (2022, accessed 7 June 2022).
- 53. European Centre for Disease Prevention and Control. COVID-19. European Centre for Disease Prevention and Control, https://www.ecdc.europa.eu/en/covid-19 (accessed 7 June 2022).
- 54. World Health Organization. Reporting form for AEFI, https://www.who.int/publications/m/item/reporting-form-for-aefi (2021, accessed 17 September 2021).
- 55. Caster O, Juhlin K, Watson S, et al. Improved statistical signal detection in pharmacovigilance by combining multiple strength-of-evidence aspects in vigiRank. Drug Saf 2014; 37: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf 2014; 37: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Uppsala Monitoring Centre. Signal detection – signal detection at UMC, https://www.who-umc.org/research-scientific-development/signal-detection/signal-detection-at-umc (2020, accessed 17 August 2021).
- 58. Ekhart C, van Hunsel F, van Puijenbroek E, et al. Post-marketing safety profile of vortioxetine using a cluster analysis and a disproportionality analysis of global adverse event reports. Drug Saf 2022; 45: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Juhlin K, Star K, Norén GN. A method for data-driven exploration to pinpoint key features in medical data and facilitate expert review. Pharmacoepidemiol Drug Saf 2017; 26: 1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines 2020; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav 2021; 5: 947–953. [DOI] [PubMed] [Google Scholar]
- 62. Bahk CY, Goshgarian M, Donahue K, et al. Increasing patient engagement in pharmacovigilance through online community outreach and mobile reporting applications: an analysis of adverse event reporting for the essure device in the US. Pharmaceut Med 2015; 29: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. New Zealand Medicines and Medical Devices Safety Authority – Medsafe. COVID-19: adverse events following immunization with COVID-19 vaccines. Safety report #18, https://www.medsafe.govt.nz/COVID-19/safety-report-18.asp (2021, accessed 17 September 2021).
- 64. World Health Organization. WHO pharmaceuticals newsletter, https://www.who.int/publications/i/item/who-pharmaceuticals-newsletter—n-4-2021 (2021, accessed 17 September 2021).
- 65. Medicines & Healthcare products Regulatory Authority. Coronavirus vaccine – weekly summary of Yellow Card reporting, https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (2021, accessed 24 August 2021).
- 66. Willame C, Dodd C, Gini R, et al. Background rates of Adverse Events of Special Interest for monitoring COVID-19 vaccines, https://vac4eu.org/covid-19-tool/
- 67. European Medicines Agency. Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis, https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis (2021, accessed 24 August 2021).
- 68. Food Drug Administration. Coronavirus (COVID-19) update: June 25 2021, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-june-25-2021 (2021, accessed 14 July 2021).
- 69. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med 2021; 385: 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Orvieto R, Noach-Hirsh M, Segev-Zahav A, et al. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod Biol Endocrinol 2021; 19: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Male V. Are COVID-19 vaccines safe in pregnancy? Nat Rev Immunol 2021; 21: 200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. US Centers for Disease Control and Prevention. COVID-19 vaccines for people who would like to have a baby, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/planning-for-pregnancy.html (2021, accessed 24 August 2021).
- 73. World Health Organization. COVAX: working for global equitable access to COVID-19 vaccines, https://www.who.int/initiatives/act-accelerator/covax (accessed 17 September 2021).
- 74. Tagoe ET, Sheikh N, Morton A, et al. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Pub Heal 2021; 9: 709127. [DOI] [PMC free article] [PubMed] [Google Scholar]