Abstract
Purpose
To report increased corneal bioavailability of allogenic serum when used in combinations with Therapeutic Hyper-CL™ soft contact lens in a patient with severe Sjögren's syndrome-associated dry eye.
Observations
A 57-year-old woman with a medical history of bilateral severe Sjögren's syndrome-associated dry eye and previous amniotic membrane patch for autoimmune corneal perforation in her left eye was referred for left eye recurrence of progressive melting and pending perforation. After manual corneal trephination, full thickness transplant and sutured amniotic membrane patch, a Therapeutic Hyper-CL™ soft contact lens (EyeYon Medical, Ness Tziona, Israel) was fit. The patient was commenced in the left eye with topical corticosteroid, antibiotic, and allogenic serum eye drops. In the right eye the patient had silicone hydrogel bandage contact lens and was under same treatment of the left eye for previous endothelial keratoplasty. In order to evaluate the efficacy and increased corneal availability of drugs provided by Therapeutic Hyper-CL™ compared with silicone hydrogel soft contact lens, anterior segment OCT was performed.
Conclusions and importance
The anterior segment OCT showed a thicker meniscus of fluid and possibly subsequent increase of trophic factors bioavailability in left eye compared with right eye. Therefore, in case of severe and refractory dry-eye disease the combination of Therapeutic Hyper-CL™ and serum eye drops may be representing a valid therapeutic approach.
Keywords: dry eye, Cornea, Hyper-cl, Sjogren, Serum eye
Abbreviations: SS, Sjögren's syndrome; allo-SED, allogenic serum eye drops; auto-SED, autologous serum eye drops; AM, amniotic membrane
1. Introduction
Sjögren's syndrome (SS) is defined as chronic systemic progressive autoimmune disease, characterized by lymphocytes B and T activation and infiltration of the exocrine glands and epithelia, with subsequent onset of related sicca symptoms, most commonly dry eyes and dry mouth.1
A correct management of patients with SS-associated dry eye is essential. If left untreated, it may lead to wide and serous ocular manifestation as chronic conjunctivitis, persistent epithelia defects, recurrent infective keratitis, neovascularization, keratolysis, corneal melting and perforation.2, 3, 4 Currently, the topical management of SS-associated dry eye is based on the severity of ocular findings and implies a four-step level approach, ranging from topical lubricants (level 1) to more drastic approach as eyelid surgery (level 4).5 At level 3 are reported the use of punctum plugs, serum eye drops (SED), and contact lenses.
The efficacy of SED, both allogenic (allo-SED) and autologous (auto-SED), has been well proved in case of dry eye associated with SS, while the literature regarding the efficacy of contact lens is manly based on no-SS-associated dry eye, with only few studies reporting the results of standard bandage contact lenses and scleral contact lens in patient with SS.6, 7, 8, 9, 10, 11
Therapeutic Hyper-CL™ (EyeYon Medical, Ness Tziona, Israel) is a novel soft contact lens designed to increase the contact time of eye drops on the corneal surface, enabling increased bioavailability of the active drug. This is possible due to its design, forming a cavity between the lens and the cornea. The eye drops, instilled in the cavity, become trapped, extending their contact time with the cornea. Up to date, the literature reporting the use of this type of contact lens is limited to two articles and both in case of chronic corneal edema.12,13
Here we report the first case of using allogenic serum eye drops in combination with Hyper-CL™ versus standard bandage contact lens in a patient with severe SS-associated dry eye.
2. Case report
A 57-year-old woman was referred to our hospital for recurrence of corneal melting and pending perforation in her left eye (LE). Her past medical history was positive for primary Sjögren's Syndrome with associated cryoglobulinemia, diagnosed at the age of 20 for which she was under treatment with systemic daily prednisolone 5mg, hydroxychloroquine 200mg and pilocarpine 5mg three times per day. Moreover, she was on weekly methotrexate 15mg and folic acid 10mg. Systemically, the SS was under control, with no major complain, no arthritis nor arthralgia and no active skin lesions. The systemic compensation of SS was also proved by laboratory tests order at time of the referral. Indeed, cryoglobulins test and rheumatoid factor were negative, and serum concentration of complement factors C3 and C4, complement hemolytic activity measured with CH50, and C-reactive protein were within normal values. Uranalysis result was unremarkable as well.
Unfortunately, same grade of compensation was not observed for her SS-associated dry eye disease. She had past ocular history in her right eye (RE) of corneal endothelial decompensation, possible due to her underlying systemic disease,14 for which she underwent a Descemet stripping automated endothelial keratoplasty (DSAEK), followed by persistent epithelial defect, managed by bandage contact lens.15,16 Her LE had instead history of multiple corneal ulcers and melting with perforation for which she had a human amniotic membrane (AM) patch.
At time of the referral, she was in both eyes under treatment with allo-SED 50%, 8–10 times per day, started in the previous 5 years and never discontinued, plus, in her RE, a topical combination of levofloxacin and dexamethasone four time per day.
On examinations, the best corrected visual acuity (BCVA) was count finger at 30 cm in the RE and hand movement in the LE.
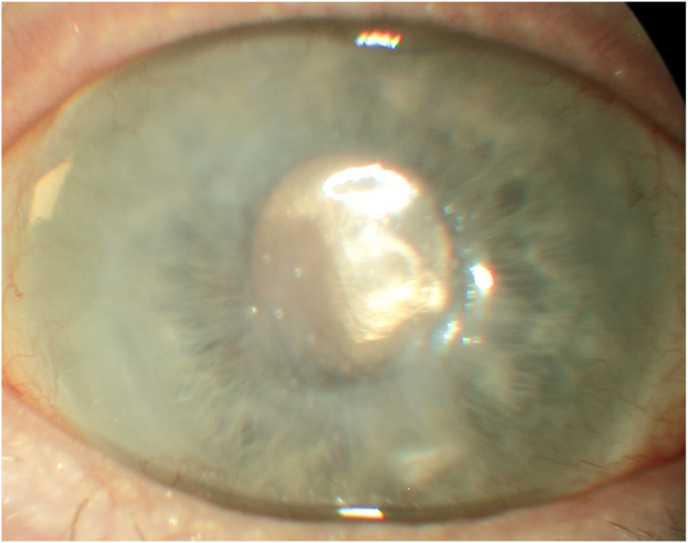
At slit lamp examination, the RE showed diffuse conjunctiva inflammation and central corneal scarring in correspondence of an area of stromal thinning, possible due to inflammation related to SS-associated dry eye. Corneal epithelium was instead positive of superficial punctate keratopathy. The DSAEK graft was attached, and the soft contact lens (Bausch and Lomb PureVision™) was in situ (Fig. 1).
Fig. 1.
Anterior segment photo of the right eye at time of the referral.
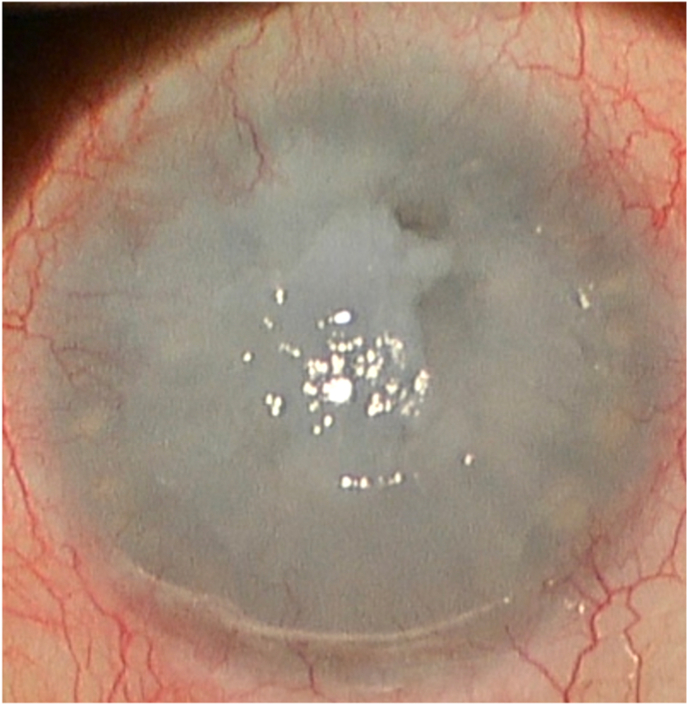
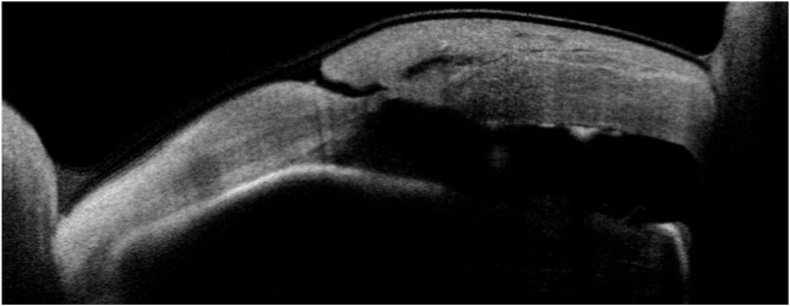
The LE was instead positive for diffuse conjunctiva inflammation, corneal melting, and pending perforation, sealed anteriorly by the previous applied amniotic patch membrane and posteriorly by iris. (Fig. 2). To better estimate the cornea thinning in both eyes, an anterior segment optical coherence tomography (AS-OCT, CASIA SS-2000, Tomey Corporation, Nagoya, Aichi, Japan) was performed. In the RE, the central corneal thickness was 209 μm while in LE a pending perforation was noticeable (Fig. 3).
Fig. 2.
Anterior segment photo of the left eye at time of the referral.
Fig. 3.
Anterior segment OCT of the left eye at time of the referral, positive for pending perforation.
In view of the evaluation and risk of corneal perforation in her LE, the patient was listed for triple surgery of penetrating keratoplasty, phacoemulsification of cataract, and intraocular lens implantation. After manual corneal trephination of 8.00 mm, a graft of 8.25 mm was transplanted and sutured with interrupted suture. At the end of surgery, an AM patch was placed on the ocular surface and sutured to the sclera. The Therapeutic Hyper-CL™ was positioned when the AM was reabsorbed, at day 10 after the transplant. The decision of using this novel contact lens was based on the ocular history of the patient, positive for severe and refractory SS-associated dry eye and clinical finding of her RE, which showed signs of persistent corneal inflammation with stromal thinning, despite she was already under treatment with allo-SED. As post-operation treatment, in the LE she was prescribed to continue the allo-SED six times per day and add the same topical combination of levofloxacin and dexamethasone four time per day which she was already using in the RE.
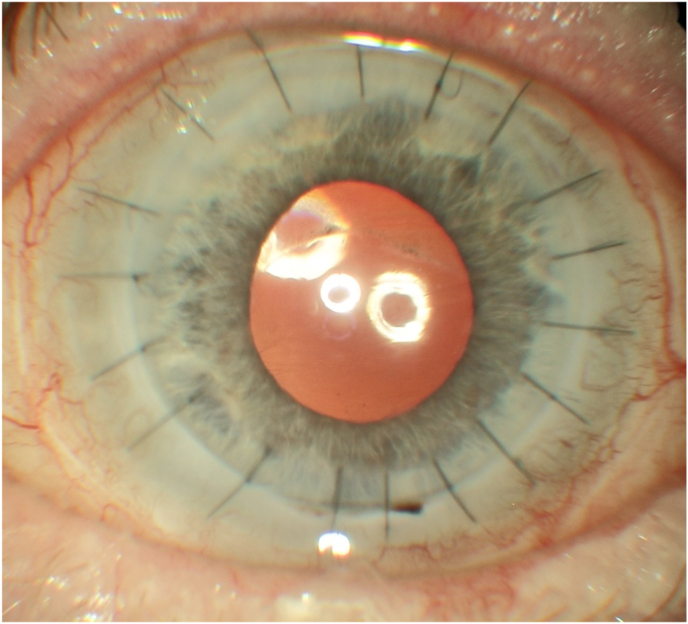
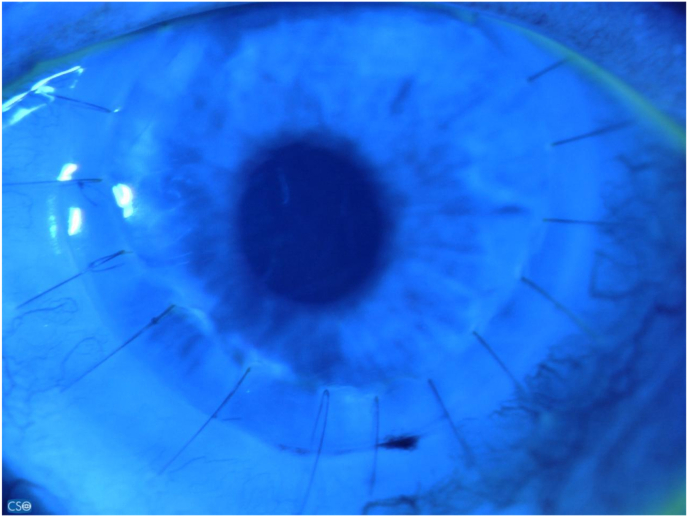
At review, after 1 months, her BCVA in the RE was stable while in the LE was 20/200. At slit lamp the RE ocular findings were unchanged while in the LE the cornea was clear, the interrupted sutures were well buried, the corneal epithelium was intact, the Hyper-CL™ was in place (Fig. 4A and B), and the Schirmer test result of 3 mm (4 mm in the RE). The patients had no major complaint in the LE, with no longer gritty sensation.
Fig. 4.
A and B: Anterior segment photos of the left eye at follow-up (A) with intact corneal epithelium at fluo-test (B).
In order to better evaluate the therapeutic effect of the Hyper-CL™, the thickness of corneal meniscus of allo-SED and topical corticosteroids and antibiotic was compared with the one provided by the standard soft bandage lens. The comparison was performed using a high resolution AS-OCT (Spectralis SD-OCT, Heidelberg Engineering, Heidelberg, Germany). The thickness was measured using the caliper function and was measured from the posterior surface of the contact lens to the corneal epithelium.
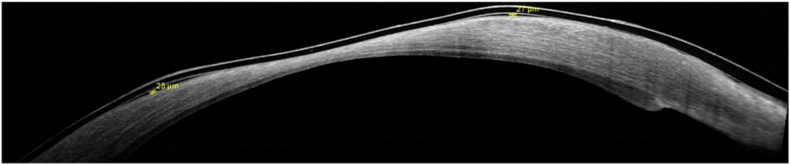
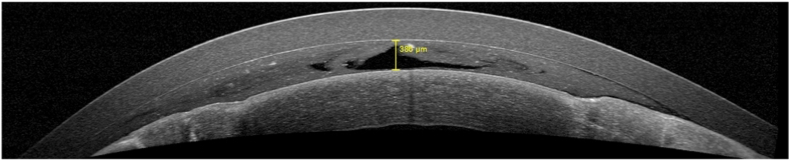
The AS-OCT showed a fluid thickness of 25 and 27 μm in the RE (Fig. 5) and 386 μm in the LE (Fig. 6), with subsequently higher persistence contact time of trophic and therapeutic factor in the LE, compared with RE.
Fig. 5.
Anterior segment OCT of the right eye with the fluid meniscus thickness provided by standard bandage contact lens.
Fig. 6.
Anterior segment OCT of the left eye with the fluid meniscus thickness provided by Therapeutic Hyper-CLTM
3. Discussion
The efficacy of allogenic and autologous serum eye drops for the treatment of ocular surface damage (OSD) caused by SS-associated dry eye has been widely recognized in literature and it is based on its anti-inflammatory, epithelio- and neuro-trophic functions.5,10,17,18
However, as reported by Jones et al., the treatment with serum eye drops, despite significantly improving signs and symptoms within a few weeks and carrying low risk of complications, is resource intensive and the OSD can relapse after cessation of treatment,17 so that the therapy with serum eye drops may be continued for an indefinite period.
Therefore, finding a way to extent the effect of single eye drops may be beneficial for a time and cost consuming treatment as SED.
Also, another question arises about which should be a valid therapeutic approach in case of patients, as the one of this case report, who present with OSD refractory to the SED, as proved by progressive sterile corneal melting and perforation in her LE and stromal thinning of her RE, despite the SS was systemically under control.
Our aim was to use the novel Therapeutic Hyper-CL™ in combination with allo-SED 50% to better manage refractory cases of SS-associated dry eye and possibly in the future to use them in well managed cases to reduce the number of instillations per day. This idea comes from the literature which suggest that in case of dry eye a longer a contact time on the ocular surface of topical lubricants is associated with better results.19 Subsequently, also lengthen the time on ocular surface of trophic factors, increasing the biovailability, could be a valid approach.
Allo-SED was preferred to auto-SED in view of similar efficacy and better logistic organization, as it is made using previously stored blood, with subsequent quicker and more convenient production.8,17
About the concentration of SED, we chose the 50% and not the 100%. Cho et al.20 report that auto-SED at 100% is associated with higher reduction of symptoms and corneal epitheliopathy compared with serum at 50%, but we preferred the dilution at 50% for the following reasons.
First, the human serum concentration of TGF-β, which has an antiproliferative effect, is approximately 50 ng/mL, which is five times higher than the amount of TGF-β in normal tears.21 Subsequently, high concentrations of this molecule may suppress corneal epithelium wound healing and is not possible to evaluate the long-term effect considering also that the follow-up time of Cho et al. was of 12 weeks.20, 21, 22 Second, serum at 100% has higher concentration of proteins which may alter the osmolarity of the tear film, which is an important pathogenic factor in the dry-eye disease.23 Lastly, SED at 100% requires the double of amount of blood for its preparation, compared to 50%. In view of the above, we believed that a concentration at 50% was safer and more manageable.
Lengthen the contact time using the Therapeutic Hyper-CL™ is more efficient compared with using standard bandage contact lens, as can be observed by our case. The thickness of fluid meniscus between the lens and the anterior surface of the cornea was more than 14 times thicker in case of Therapeutic Hyper-CL™ and also the distribution of fluid was homogenous, whereas with standard contact lens was limited at few areas with no area of meniscus in mostly of the corneal surface.
Therapeutic Hyper-CL™ have a water content of 59% and its oxygen permeability (Dk) (35 °C) of 26 x 10−11 (cm2/sec) (ml O2/ml x mm Hg) does not require oxygen support.13,24 Their ability to create a reservoir of eye drops in the corneal surface, increasing the contact length of time on the cornea at least of 10 minutes and up to 20 minutes, versus 20–30 seconds without the lens, is possible thanks to their design.13
Indeed, in the Therapeutic Hyper-CL™ the central base curve of the lens is steeper than the peripheral curve, creating in this way an elevation of the lens at the center of the cornea. The formed elevation creates a gap, a reservoir bag of fluids. The lens fit on the corneal limbus and has multiple holes in the mid-periphery which represents the way in of eye drops and improve the oxygen supply to the cornea (Fig. 7). Their tolerability and safety are similar to standard contact lens.13
Fig. 7.
Therapeutic Hyper-CL™ lens.
It can be used continuously, day and night, for 7 days plus additional 7 days, in case it is removed, cleaned and re-inserted. After 14 days the lens needs to be disposed.
So, compared with soft contact lenses and scleral lenses, Therapeutic Hyper-CL™ provide greater liquid meniscus and prolonged contact time on the cornea, whereas disadvantages are that they need to be changed every 14 days and the price, higher that standard contact lenses and scleral lenses.
In our case, the patients did not report any discomfort in the LE where Hyper-CL™ was fit compared to the RE, where she had a standard contact lens. No epithelial defects were present at last follow up (8 weeks) in the LE and the patient was very satisfied.
In conclusion, although limited to a single case report, we consider that the use of novel Therapeutic Hyper-CL™ is promising also in case of SS-associated dry eyes. Further studies with larger sample size, and prolonged follow-up time are necessary to prove the efficacy.
Funding sources/financial disclosure
None.
Patient consent
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient.
Funding support
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Kassan S.S., Moutsopoulos H.M. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164(12):1275. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 2.Akpek E.K., Bunya V.Y., Saldanha I.J. Sjögren’s syndrome: more than just dry eye. Cornea. 2019;38(5):658–661. doi: 10.1097/ICO.0000000000001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner M., Romano V., Steger B., et al. Imaging of corneal neovascularization: optical coherence tomography angiography and fluorescence angiography. Investig Ophthalmol Vis Sci. 2018;59(3):1263–1269. doi: 10.1167/iovs.17-22035. [DOI] [PubMed] [Google Scholar]
- 4.Liu S., Romano V., Steger B., et al. Gene-based antiangiogenic applications for corneal neovascularization. Surv Ophthalmol. 2018;63(2):193–213. doi: 10.1016/j.survophthal.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Foulks G.N., Forstot S.L., Donshik P.C., et al. Clinical guidelines for management of dry eye associated with sjögren disease. Ocul Surf. 2015;13(2):118–132. doi: 10.1016/j.jtos.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Zhang X., Zheng Q., et al. Comparative evaluation of silicone hydrogel contact lenses and autologous serum for management of Sjögren syndrome-associated dry eye. Cornea. 2015;34(9):1072–1078. doi: 10.1097/ICO.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 7.la Porta Weber S., Becco de Souza R., Gomes J.Á.P., Hofling-Lima A.L. The use of the esclera scleral contact lens in the treatment of moderate to severe dry eye disease. Am J Ophthalmol. 2016;163:167–173.e1. doi: 10.1016/j.ajo.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez Calvo‐de‐Mora M., Domínguez‐Ruiz C., Barrero‐Sojo F., et al. Autologous versus allogeneic versus umbilical cord sera for the treatment of severe dry eye disease: a double‐blind randomized clinical trial. Acta Ophthalmol. 2022;100(2) doi: 10.1111/aos.14953. [DOI] [PubMed] [Google Scholar]
- 9.Semeraro F., Forbice E., Braga O., et al. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. BioMed Res Int. 2014;2014:1–11. doi: 10.1155/2014/826970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semeraro F., Forbice E., Nascimbeni G., et al. vol. 30. 2016. Effect of autologous serum eye drops in patients with Sjögren syndrome-related dry eye: clinical and in vivo confocal microscopy evaluation of the ocular surface; pp. 931–938. (Vivo). 6. [DOI] [PubMed] [Google Scholar]
- 11.van der Meer P.F., Verbakel S.K., Á Honohan, et al. Allogeneic and autologous serum eye drops: a pilot double‐blind randomized crossover trial. Acta Ophthalmol. 2021;99(8):837–842. doi: 10.1111/aos.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdinest N., London N., Levinger N., lavy I. Therapeutic contact lens for Fuchs endothelial corneal dystrophy: monitoring with Scheimpflug tomography. Am J Ophthalmol Case Rep. 2022;25 doi: 10.1016/j.ajoc.2021.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daphna O., Mimouni M., Keshet Y., et al. Therapeutic HL-contact lens versus standard bandage contact lens for corneal edema: a prospective, multicenter, randomized, crossover study. J Ophthalmol. 2020;2020:1–5. doi: 10.1155/2020/8410920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekhon A.S., He B., Iovieno A., Yeung S.N. Pathophysiology of corneal endothelial cell loss in dry eye disease and other inflammatory ocular disorders. Ocul Immunol Inflamm Published online October. 2021;22:1–11. doi: 10.1080/09273948.2021.1980808. [DOI] [PubMed] [Google Scholar]
- 15.Romano V., Steger B., Myneni J., et al. Preparation of ultrathin grafts for Descemet-stripping endothelial keratoplasty with a single microkeratome pass. J Cataract Refract Surg. 2017;43(1):12–15. doi: 10.1016/j.jcrs.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Romano V., Tey A., Hill N.M.E., et al. Influence of graft size on graft survival following Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol. 2015;99(6):784–788. doi: 10.1136/bjophthalmol-2014-305648. [DOI] [PubMed] [Google Scholar]
- 17.Jones L., Downie L.E., Korb D., et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Cao K., Wei Z., et al. Autologous serum eye drops versus artificial tear drops for dry eye disease: a systematic review and meta-analysis of randomized controlled trials. Ophthalmic Res. 2020;63(5):443–451. doi: 10.1159/000505630. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y.J., Lee W.Y., Kim Y., jin, Hong Y., pyo A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Publ Health. 2021;18(5):2383. doi: 10.3390/ijerph18052383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho Y.K., Huang W., Kim G.Y., Lim B.S. Comparison of autologous serum eye drops with different diluents. Curr Eye Res. 2013;38(1):9–17. doi: 10.3109/02713683.2012.720340. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A., Monroy D., Ji Z., et al. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996;15(6):605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 22.Terai K., Call M.K., Liu H., et al. Crosstalk between TGF-β and MAPK signaling during corneal wound healing. Investig Opthalmol Vis Sci. 2011;52(11):8208. doi: 10.1167/iovs.11-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemp M.A., Bron A.J., Baudouin C., et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798.e1. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.H., Lin M.C., Radke C.J. Limbal metabolic support reduces peripheral corneal edema with contact-lens wear. Transl Vis SciTechnol. 2020;9(7):44. doi: 10.1167/tvst.9.7.44. [DOI] [PMC free article] [PubMed] [Google Scholar]