Abstract
Calcium and zinc carboxylates were prepared from palm fatty acid distillate, a byproduct of palm oil refining, via metathesis in aqueous ethanol. The formations of both metal carboxylates have been confirmed by infrared spectroscopy and x-ray diffraction analysis. Thermogravimetric analysis has shown that the prepared calcium and zinc carboxylates are practically stable while their weight losses are 14% at 393 °C and 19% at 311 °C, respectively. The efficacy of the metal carboxylates in their mixtures in stabilizing polyvinyl chloride against heat has also been studied by using static and dynamic tests. The calcium to zinc ratio of 4:1 has been found to give the longest stability time under the studied condition. The mixed calcium/zinc carboxylates demonstrate a synergism effect with pentaerythritol. The results reveal that mixed calcium/zinc carboxylates from palm fatty acid distillate are effective in stabilizing polyvinyl chloride against heat.
Keywords: Metal carboxylate, Metathesis, Palm Fatty Acid Distillate, Polyvinyl chloride, Thermal stabilizer
Highlights
-
•
Ca/Zn carboxylate from PFAD is stable at the processing temperature of PVC.
-
•
Mixed Ca/Zn carboxylate from PFAD is an effective PVC thermal stabilizer.
-
•
PFAD offers a potential raw material for a mixed metal base thermal stabilizer.
Metal carboxylate; Metathesis; Palm fatty acid distillate; Polyvinyl chloride; Thermal stabilizer.
1. Introduction
Polyvinylchloride (PVC) is the third-largest thermoplastic polymer in demand volume, right behind polyethylene and polypropylene (PlasticsEurope, 2019). The PVC market was valued at more than USD 40,000 million in 2021, and it is expected to register a CAGR of over 4% during the 2022–2027 period (Mordor Intelligence, 2022). Pipes and fittings accounted for a major application share by volume and value, followed by profiles, hoses, and tubing (Mordor Intelligence, 2022). However, PVC is thermally unstable at processing temperatures (Jubsilp et al., 2021). Its poor thermal stability is the biggest weakness of PVC (Li et al., 2019). In consequence, thermal stabilizers must be added to PVC resin to prevent thermal degradation when processed into the final products (Han et al., 2019; Li et al., 2019). The compounds used as thermal stabilizers can be divided into two groups: metal-free organic and metal-based groups (Folarin and Sadiku, 2011; Schiller, 2015). The first group is relatively new technology (Jubsilp et al., 2021). The disadvantage of organic thermal stabilizers are their poor stability (Guangbao et al., 2020). The second group, which has been industrialized and well applied in PVC production, includes salts or carboxylates of lead, tin, or mixed metals (Ye et al., 2019). Lead salts are known as the oldest thermal stabilizer (Schiller, 2015). It is very effective but not environmentally friendly (Asawakosinchai et al., 2017). Lead salts have been banned in many countries due to their high toxicity (Zhang et al., 2020). Tin-based organic compounds and mixed metal carboxylates are alternatives to lead salts. Organic tin groups are known to be effective, but they are expensive (Song et al., 2022). Mixed metal thermal stabilizers are popularly used due to their advantages of being non-toxic and low price (Ye et al., 2019). The commonly used metal combinations are alkaline earth and transition IIB metals, especially the Ca/Zn pair (El-Ghaffar et al., 2019). Mixed Ca/Zn thermal stabilizers are metal carboxylate mixtures of calcium and zinc which are generally stearic acid-based.
Numerous works on the synthesis and thermal stabilizing effects of mixed Ca/Zn thermal stabilizers on PVC have been published. Stearate is the most commonly studied carboxylate of mixed Ca/Zn as found in the literature (Atakul et al., 2005; Benaniba et al., 2003; Borukaev et al., 2021; El-Ghaffar et al., 2019; Gökçel et al., 1999; Zhang et al., 2014). Mixed Ca/Zn thermal stabilizers derived from Ximinea americana seed oil (Folarin et al., 2012), Balanites aegyptiac seed oil (Folarin et al., 2013), Castor (Ricinuscommunis) seed oil (Folarin and Ayinde, 2016), rubber seed oil (Okieimen et al., 2009), tung-maleic anhydride (Wang et al., 2016a), N-(3-amino-benzoic acid) terpene-maleamic acid (Wang et al., 2016b), and tung oil (Li et al., 2017) have also been studied. However, stabilization of PVC by mixed metal carboxylate made from industrial byproducts has not received much attention. One of the byproducts that can be utilized is palm fatty acid distillate (PFAD).
PFAD is a byproduct of Crude Palm Oil (CPO) refining processes and is composed mainly of free fatty acids (FFA) in which palmitic and oleic acids are the major FFA (Abdul Kapor et al., 2017; Ng et al., 2022; Top, 2010). The presence of FFA in CPO is a result of lipase enzyme activity during harvest (Ahmad Nizam et al., 2020; Gibon et al., 2007). The global production of CPO in 2019/2020 reached 73 million tons (Foreign Agricultural Service, 2021). Approximately, 90% of CPO is processed for food (Mba et al., 2015). There is an average of 4% FFA in CPO (Top, 2010; Xu et al., 2020). To meet the standard for refined, bleached, and deodorized palm oil (food), the FFA in CPO must be removed to a maximum level of 0.1% (Xu et al., 2020). The removed FFA leaves the purification process as PFAD. Based on these data, PFAD production can be estimated at 2.6 million tons in the marketing year 2019/2020. Currently, PFAD is used as raw materials for the manufacture of animal feeds, laundry soaps, and biodiesel (Xu et al., 2020; Yeong et al., 2022). Due to its composition which is dominated by FFA and its abundant availability as a by-product, PFAD can be considered a potential raw material for producing mixed Ca/Zn thermal stabilizers. In addition, as a by-product, PFAD is available at a lower price than stearic acid which is commonly used as a raw material for mixed Ca/Zn thermal stabilizers. PFAD is priced at 1395 USD/ton (Agropost, 2022), while stearic acid is priced at 2159 USD/ton (ECHEMI, 2022). The utilization of PFAD as raw materials for mixed Ca/Zn thermal stabilizer will increase the added value of PFAD as a by-product and provide an alternative raw material for producing PVC thermal stabilizers. One of the important questions is whether the mixed Ca/Zn thermal stabilizer produced from PFAD provides a good stabilizing effect.
This research is aimed to evaluate the efficacy of mixed calcium and zinc carboxylates from PFAD in stabilizing PVC against heat. At first, calcium and zinc carboxylates were prepared from PFAD by a metathesis method. The calcium and zinc carboxylates, henceforth, are called calcium palmate (Ca-Palmate) and zinc palmate (Zn-Palmate), respectively, as they are made from palm-based fatty acids. This is analogous to the use of potassium tallate for potassium carboxylate made from tall oil fatty acid (Burnett, 2018) and sodium tallawate for tallow-based sodium carboxylate (Coiffard and Couteau, 2020; Cristiano and Guagni, 2022). The Ca- and Zn-Palmates were then characterized and their stabilizing effects on the thermal degradation of PVC were tested. The results of this research are expected to provide an answer to whether the mixed Ca/Zn stabilizer from PFAD has an effective thermal stabilizing effect on PVC.
2. Materials and methods
2.1. Material
PFAD was supplied by Tunas Baru Lampung Company, a palm cooking oil company in Indonesia. Sodium hydroxide (NaOH, 99.0%, Merck), calcium chloride (CaCl2⋅2H2O, 99.0%, Merck), and zinc sulfate (ZnSO4⋅7H2O, 99.5%, Merck) were used for synthesizing sodium, calcium, and zinc carboxylates, respectively. Pentaerythritol (C5H12O4, 98%, Merck) was used as a costabilizer. The PVC resin used for the thermal stability test was supplied by Asahimas Chemical Company, Cilegon, Banten Province, Indonesia. The PVC resin has a k value of 65 with a white appearance, degree of polymerization of 1030 ± 40, bulk density of 0.5 g/ml, and 100% passing particle size of 80 mesh.
2.2. Synthesis of Ca-Palmate and Zn-Palmate
Ca-Palmate and Zn-Palmate were prepared by a metathesis technique. The preparation consists of two synthesis steps, i.e., synthesis of sodium carboxylate from PFAD and sodium hydroxide solution and synthesis of Ca-Palmate and Zn-Palmate from sodium carboxylate and metal salt solutions. A total of 15 g of PFAD, 2.2088 g of sodium hydroxide, 100 ml of ethanol, and 50 ml of deionized water were put into a flat bottom flask and refluxed for two hours. After cooling the reaction mixture, the excess sodium hydroxide was neutralized by hydrochloric acid solution. The mixture was then transferred into a jacketed beaker glass which was equipped with a condenser. The mixture was kept at 60 °C by controlling the temperature of water circulated through the jacket of the beaker glass. While agitating, a 100 ml of calcium or zinc salt aqueous solution was then gradually added. The calcium and zinc salts were added in stoichiometric amounts with respect to the PFAD. After the salt solution was completely added, the mixture was continuously agitated for two hours. The reaction mixture was then filtered by using a Büchner funnel. The cake was washed using deionized water and dried at 60 °C under vacuum for 6 hours.
2.3. Analyses
The PFAD analyses done included acid value, saponification value, iodine value, titer, and moisture content. The analyses on Ca (or Zn)-Palmate included total fatty matter, calcium (or zinc), and moisture contents. Analyses of PFAD were done three times while those of Ca and Zn-Palmates were done twice. The analysis results were expressed as 95% confidence intervals which were calculated according to the literature (Montgomery, 2012).
Acid value, saponification value, iodine value, titer, and moisture content of the PFAD were measured according to ISO-660-2020, ISO-3657-2020, ISO-3961-2018, ISO-935-1988, and ISO-662-2016, respectively. The total fatty matters of metal carboxylates were quantified according to ISO-685-2020. Calcium contents in Ca-Palmate were determined by the titrimetric method according to ISO-6490-1-1985. Zinc contents in Zn-Palmate were determined by digesting the Zn-Palmate according to ISO-685-2020 and analyzing the aqueous phase obtained by Atomic Absorption Spectrophotometry. Samples for analysis were weighed to the nearest 0.0001 g. The analysis methods are detailed below.
2.3.1. Determination of acid value
0.5 g of sample was weighed in a 250 ml conical flask. Ethanol (50 ml) was added and shaken well. After adding a few drops of phenolphthalein indicator, the solution was titrated against a standard potassium hydroxide solution until the solution developed a light red color which persisted for at least 15 s. The acid value (AV), expressed in mg KOH/g of sample, was calculated using Eq. (1):
| (1) |
where NK is the normality of the potassium hydroxide solution, VK,A is the volume in ml of the potassium hydroxide solution consumed in the sample test, VK,B is the volume in ml of the potassium hydroxide solution consumed in the blank test, and wS is weight in g of sample.
2.3.2. Determination of saponification value
2 g of sample was weighed in a 250 ml conical flask and mixed with 0.5 N ethanolic potassium hydroxide (25 ml). The mixture was refluxed for 2 hours. After cooling the mixture, 1 ml of phenolphthalein was added. The solution was then titrated against a standard hydrochloric solution. The saponification value (SV), expressed in mg KOH/g, was calculated using Eq. (2):
| (2) |
where NH is the normality of the hydrochloric solution, VH,A is the volume in ml of the hydrochloric solution consumed in the sample test, VH,B is the volume in ml of the hydrochloric solution consumed in the blank test, and wS is weight in g of sample.
2.3.3. Determination of iodine value
0.4 g of sample was weighed in a 250 ml conical flask and mixed with 20 ml of solvent (equal volume of cyclohexane and acetic acid glacial mixture). After adding a Wijs reagent (25 ml), the flask was closed using a stopper. The content of the flask was swirled and placed in the dark for 1 hour. A potassium iodide solution (20 ml) was added, and the solution was titrated against a standard sodium thiosulfate solution with a starch solution as indicator. The iodine value (IV), expressed in g I2/100 g, was calculated using Eq. (3):
| (3) |
where NT is the normality of the sodium thiosulfate solution, VT,A is the volume in ml of the sodium thiosulfate solution consumed in the sample test, VT,B is the volume in ml of the sodium thiosulfate solution consumed in the blank test, and wS is weight in g of sample.
2.3.4. Determination of titer
50 g of sample was heated to about 10 °C above the expected titer and poured into a glass tube (25 mm × 100 mm) to a height of 55 mm. The tube was placed in a water bath which was maintained at a temperature of 20–25 °C below the expected titer and then stirred using a vertical stirrer. Titer was observed as the temperature which, after a rapid initial decrease, fell slowly and then became constant within 30 s.
2.3.5. Determination of moisture content
20 g of sample was weighed in a dish. The dish was placed in an oven which was maintained at 103 °C for 1 hour, cooled in a desiccator to room temperature, and then weighed. The heating and cooling were repeated until the difference between the results of two successive weightings did not exceed 2 mg. Moisture content was calculated as the loss in weight of the sample.
2.3.6. Determination of calcium content
5 g of sample was weighed into the incineration dish. The sample was combusted in a furnace at 550 °C for 4 hours. The ash was then treated with hydrochloric acid solution and ammonium oxalate solution to precipitate the calcium as calcium oxalate. The precipitate was dissolved in sulfuric acid solution to form oxalic acid. The oxalic acid was then titrated against a standard potassium permanganate solution.
2.3.7. Determination of zinc content
5 g of sample was weighed in a conical glass. The sample was treated with sulfuric acid solution (10%-excess) to hydrolyze the sample. The aqueous phase was separated from the oil phase and diluted with distilled water. The zinc in the aqueous phase was then analyzed by AAS.
2.3.8. Determination of total fatty matter
5 g of sample was weighed in a conical glass. The sample was treated with nitric acid solution in excess to hydrolyze the sample. The oil phase was extracted by n-hexane. After washing the extract using distilled water, the n-hexane was removed by vacuum distillation. The distillation residue was dissolved in ethanol for acid value measurement. The total fatty matter content was calculated from the measured acid value and sample weight.
2.4. Characterization
Infrared spectra were measured with a Shimadzu IR-Prestige-21 Spectrophotometer. The spectra were acquired in the range of 4,000–400 cm−1. The crystalline structure of metal carboxylate was characterized by XRD (Bruker's D8 advance diffractometer) with Cu Kα radiation (1.54 Å) at 40 kV and 35 mA. The diffraction pattern was collected at a step size of 0.02° and a step time of 0.4 s in 2θ ranging from 5° to 70°. Thermogravimetric analysis (TGA) was carried out using a thermogravimetric analyzer Linseis STA PT 1600 (Linseis Messgeraete GmbH) under a nitrogen atmosphere (50 ml min−1) at a heating rate of 10 °C min−1. The test was conducted from room temperature to 600 °C.
2.5. Stability test of PVC
Static thermal stability was observed through Congo red and dehydrochlorination tests. The Congo red test was carried out at 180 °C in a glass test tube (7 mm ID, 1 mm thickness) using a 5 mm × 25 mm Congo red paper of which the bottom edge was placed 25 mm above the sample surface. The Congo red tests were conducted four times for each variation. Dehydrochlorination tests were carried out in a glass test tube (7 mm ID, 1 mm thickness) at 180 °C with a nitrogen gas rate of 60 ml min−1. The nitrogen was passed through a 75 cm long glass tube coil which was soaked at the same temperature to ensure the required temperature before passing through the sample bed. The nitrogen gas leaving the sample was dispersed in 200 ml distilled water in which the conductivity was measured using a Martini Instrument conductivity meter (model Mi170) to monitor the liberation of hydrogen chloride. The dehydrochlorination tests were done twice for each sample. The temperatures for Congo red and dehydrochlorination tests were achieved by immersing the test tubes in a silicone oil batch that was equipped with a temperature controller. Each test used 1 g of PVC sample. Each sample was prepared by mixing 5 g of PVC powder with a predetermined amount of thermal stabilizer. All samples were ground using a porcelain mortar before being tested.
Table 1 presents the variations of Congo red and dehydrochlorination tests. Stabilizers #1, #2, #3, and #4 have Ca/Zn ratios of 0.25, 1.00, 4.00, and 7.00, respectively, aimed to study the effects of Ca-Palmate-to-Zinc-Palmate weight ratio (Ca/Zn ratio) on the stability time at the same total dose, i.e., 3 phr. Stabilizers #3 and #5 are used to observe the effect of doses at the same Ca/Zn ratio. It has been reported that, in the case of stearate, the efficacy of mixed Ca/Zn stabilizers can be enhanced by using a co-stabilizer (El-Ghaffar et al., 2019; Gao et al., 2012; Ikeda et al., 2003). It is known also that pentaerythritol is a good co-stabilizer and has synergistic effects with mixed Ca/Zn stearates (Liao et al., 2011; Liu et al., 2007; Schiller, 2015). To check whether the same synergy exhibited between mixed Ca/Zn-Palmate and pentaerythritol, two samples with mixed Ca/Zn-Palmate/pentaerythritol have been evaluated. They are represented by stabilizers #6 and #7. A mixture of commercial calcium and zinc stearates (stabilizer #8) is added as a comparison.
Table 1.
Doses of stabilizers for Congo red and dehydrochlorination tests.
| Stabilizer | Ca-Palmate (phr) | Zn-Palmate (phr) | Total Ca/Zn-Palmate (phr) | Pentaerythritol (phr) |
|---|---|---|---|---|
| None | - | - | - | - |
| #1 | 0.600 | 2.400 | 3 | - |
| #2 | 1.500 | 1.500 | 3 | - |
| #3 | 2.400 | 0.600 | 3 | - |
| #4 | 2.625 | 0.375 | 3 | - |
| #5 | 4.000 | 1.000 | 5 | - |
| #6 | 2.400 | 0.600 | 3 | 0.600 |
| #7 | 4.000 | 1.000 | 5 | 1.000 |
| Ca-Stearate (phr) | Zn-Stearate (phr) | Total Ca/Zn-Stearate (phr) | ||
| #8 | 2.400 | 0.600 | 3 | - |
Dynamic discoloration test was conducted on a two-roll mill (Zongli model ZL-3018). The additives included in the formulations were thermal stabilizer, calcium stearate (0.4 phr) and stearic acid (0.6 phr) as internal lubricants, paraffin wax (0.6 phr) as an external lubricant, PA20 (1 phr) as a processing aid, CPE (4 phr) as an impact modifier, and CaCO3 (15 phr) as a filler. Before being rolled, the formulated samples were homogenized using a Bamix-M133 hand blender.
3. Results and discussion
3.1. Raw material properties
Quantitative analyses of the raw PFAD give the acid value, saponification value, iodine value, moisture content, and titer as presented in Table 2. Table 3 compares the properties obtained in this work to the ranges of PFAD properties found in the literature. The observed properties in this paper are in the ranges reported in the literature. The FFA content as palmitic acid is calculated from the measured acid value which is 193 ± 1 mg KOH/g. Since the acid value is lower than the saponification value, it can be ascertained that the PFAD used in this paper still contains neutral oil. Because of that, when the PFAD was refluxed in the presence of NaOH solution, two reactions occurred. The first reaction is the neutralization of fatty acids through the reaction in Eq. (4) (Protasova et al., 2021):
| R-COOH(l) + NaOH(aq) → R-COONa(aq) + H2O(l) | (4) |
Table 2.
Results of quantitative analyses of PFAD.
| Sample | Property | CI∗ |
|---|---|---|
| Acid Value (mg KOH/g) | ||
| 1 | 193.1 | |
| 2 | 193.9 | 193.4 ± 1.0 |
| 3 | 193.3 | |
| Saponification value (mg KOH/g) | ||
| 1 | 205.4 | |
| 2 | 206.3 | 205.5 ± 2.0 |
| 3 | 204.7 | |
| Iodine value (g I2/100 g) | ||
| 1 | 56.1 | |
| 2 | 56.8 | 56.3 ± 1.1 |
| 3 | 56.0 | |
| Titer (oC) | ||
| 1 | 47.3 | |
| 2 | 47.6 | 47.4 ± 0.4 |
| 3 | 47.4 | |
| Water content (%) | ||
| 1 | 0.24 | |
| 2 | 0.25 | 0.23 ± 0.04 |
| 3 | 0.21 |
CI = Confidence interval at 95%-confidence level.
Table 3.
Properties of PFAD.
| # | Property | This work | Tay et al. (2009) | Chang et al. (2016) |
|---|---|---|---|---|
| 1 | FFA (%-as Palmitic acid) | 88 ± 1 | 73–93 | 66–95 |
| 2 | Saponification value (mg KOH/g) | 205 ± 2 | 200–215 | 195–220 |
| 3 | Iodine value (g I2/100 g) | 56 ± 1 | 46–58 | 38–63 |
| 4 | Moisture (%) | 0.23 ± 0.04 | 0.03–0.24 | 0.06–7.50 |
| 5 | Titer (°C) | 47.4 ± 0.4 | 46.0–48.3 | - |
The second reaction is the saponification of neutral oil through the reaction in Eq. (5) (Protasova et al., 2021):
| (5) |
Since the saponification reaction in Eq. (5) is an equilibrium reaction, an excess in NaOH is necessary to shift the reaction to the right. The weight ratio of NaOH to PFAD used in this paper has taken into account a 10% excess with respect to the neutral oil present in the PFAD.
3.2. Characterization of Ca/Zn-Palmate
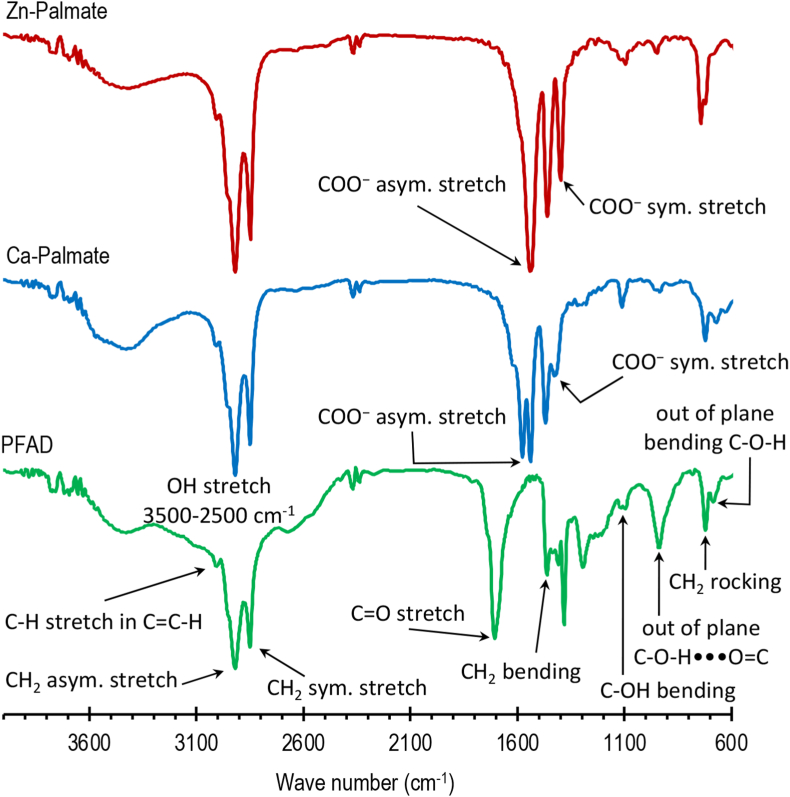
Figure 1 presents the IR spectra of PFAD, Ca-Palmate, and Zn-Palmate. The IR spectra of fatty acids and their metal salts have been reviewed by Filopoulou et al. (2021) which is referred to in this paper in interpreting the spectra in Figure 1. Since PFAD is mainly composed of FFA, its spectra are characterized by the existence of acidic hydroxyl stretching region, acidic carbonyl stretching region, hydroxyl C–O stretch, and C–O–H bending region (Filopoulou et al., 2021). The O–H stretching of carboxylic acid groups is shown by the broad peak in the 3500-2500 cm−1 region which includes the band region assigned to hydroxyl-carbonyl interactions due to acid dimers at 2750–2500 cm−1. The unsaturated double bound (=CH-cis) and acidic carbonyl stretching vibration are observed at 3007 and 1705 cm−1, respectively. The out-of-plane bending vibration C–O–H, the in-phase CH2 rocking, the out-of-plane dimer vibration C–O–H•••O=C, the stretching vibration of the carbon-oxygen C–O of the acidic hydroxyl, and the symmetric in-plane bending (or ‘scissoring’) CH2 are observed at 686 cm−1, 721 cm−1, 937 cm−1, 1097 cm−1, and 1462 cm−1, respectively. The spectra of Ca-Palmate are characterized by the peaks observed at 1542 cm−1 and 1578 cm−1 indicating the asymmetric stretching vibration of COO− and at 1429 cm−1 indicating the symmetric stretching vibration of COO−. The spectra of Zn-Palmate are characterized by the peaks observed at 1541 cm−1 and at 1401 cm−1 indicating the asymmetric and symmetric stretching vibrations of COO−, respectively. It is clearly shown that the bands of hydroxyl O–H, dimer vibration, and C–O–H do not appear in both Ca-Palmate and Zn-Palmate spectra. The peak observed at 3400 cm−1 indicates the presence of water, as moisture or hydrate. Both calcium and zinc ions can produce hydrated salts with fatty acids. According to Filopoulou et al. (2021), the formation of hydrate is indicated by the intense, broad, crystalline water peaks at ∼3440 cm−1 and ∼1626 cm−1 for the calcium carboxylate and at 3565–3580 cm−1 and 1620–1600 cm−1 for the zinc carboxylate. The Ca/Zn-Palmate IR spectra obtained in this study are practically similar to the Ca/Zn stearate patterns presented in the literature (El-Ghaffar et al., 2019; Filopoulou et al., 2021). The difference is only in the presence of the double bond peak which indicates the presence of oleic acid in PFAD.
Figure 1.
IR spectra of PFAD, Ca-Palmate, and Zn-Palmate.
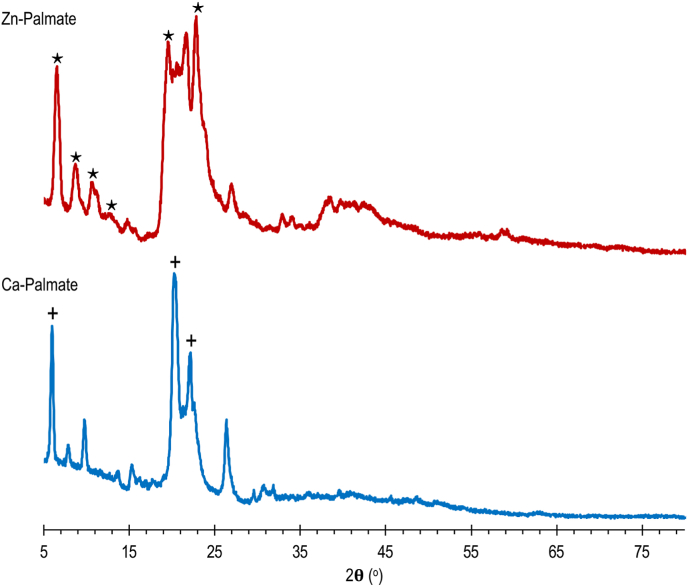
As reported by Corbeil and Robinet (2002), the XRD patterns between zinc palmitate and zinc stearate are not different, but the XRD peaks shift toward a lower angle when going from palmitate to stearate. Corbeil and Robinet (2002) also added that the presence of double bonds does not affect the pattern of spectra in the region below 20°. Therefore, the XRD spectra of Ca-Palmate and Zn-Palmate are interpreted by referring to the spectra of calcium stearate and zinc stearate, respectively. Figure 2 presents the diffraction patterns for the Ca-Palmate and Zn-Palmate. The XRD pattern of Ca-Palmate has distribution peaks at 5.97°, 7.88°, 9.79°, 13.64°, and 15.29°. The XRD pattern of Zn-Palmate has distribution peaks at 5.54°, 8.72°, 10.72°, 12.67°, and 14.77° below 20° and a cluster of weak peaks between 20–30°. The “∗” and “+” signs in Figure 2 indicate the characteristic peaks at diffraction angles (2θ) between 5° to 20° for zinc stearate and calcium stearate, respectively, which are obtained from El-Ghaffar et al. (2019). Table 4 compares the diffraction data of Ca-Palmate and the diffraction data of calcium stearate from a previous study (El-Ghaffar et al., 2019). Table 5 compares the diffraction data of Zn-Palmate and the diffraction data of zinc stearate from the previous study (El-Ghaffar et al., 2019). Some agreement in position and intensity were found for several phases which support the results of IR spectra that Ca-Palmate and Zn-Palmate have been successfully prepared from PFAD. Some variations occurred due to the variety of carboxylate parts in both Zn-Palmate and Ca-Palmate. Since there are made from PFAD, the carboxylate parts of both Zn-Palmate and Ca-Palmate consist of palmitate and oleate as the major carboxylates and stearate as well as linoleate.
Figure 2.
XRD spectra of Ca-Palmate and Zn-Palmate.
Table 4.
Diffraction data of Ca-Palmate.
| Ca-Stearate (El-Ghaffar et al., 2019) |
Prepared Ca-Palmate (This work) |
||
|---|---|---|---|
| Relative intensity (%) | Interplanar spacing (Å) | Relative intensity (%) | Interplanar spacing (Å) |
| 50.29 | 24.12 | - | - |
| 100 | 15.84 | 65 | 14.85 |
| 44.73 | 4.37 | 100 | 4.37 |
| 22.11 | 4.1 | 63.7 | 4.02 |
| 20.78 | 9.47 | 22.9 | 9.05 |
| 26.77 | 5.82 | 11.2 | 5.77 |
| 12.35 | 3.87 | 37 | 3.95 |
Table 5.
Diffraction data of Zn-Palmate.
| Zn-Stearate (El-Ghaffar et al., 2019) |
Prepared Zn-Palmate (This work) |
||
|---|---|---|---|
| Relative intensity (%) | Interplanar spacing (Å) | Relative intensity (%) | Interplanar spacing (Å) |
| 100 | 13.8 | 69.9 | 13.51 |
| 19.8 | 10 | 22.9 | 10.13 |
| 33 | 8.05 | 15.2 | 8.26 |
| 1.6 | 6.44 | 5 | 6.97 |
| 36.4 | 4.51 | 96.2 | 4.53 |
| 46.9 | 3.88 | 100 | 3.9 |
| 7.1 | 2.37 | - | - |
3.3. Analyses of Ca/Zn-Palmate
The Ca/Zn-Palmates obtained are creamy white and easy to flow after grinding. The results of quantitative analyzes of Ca/Zn-Palmates are presented in Table 6. The calcium and fatty mater contents of the Ca-Palmate are found to be 6.6 ± 0.9 wt% and 80.4 ± 5.3 wt%, respectively. On the other hand, the zinc and fatty mater contents of the Zn-Palmate are found to be 9.5 ± 1.0 wt% and 76.5 ± 2.7 wt%, respectively. From the acid and saponification values, the fatty acid in the PFAD can be predicted to have an average molecular weight of 267 g/mol. Theoretically, therefore, the anhydrous Ca-Palmate contains 7.0 wt% calcium and 93.4 wt% fatty matter. The anhydrous Zn-Palmate, on the other hand, contains 10.9 wt% zinc and 89.4 wt% fatty matter. The measured metal and fatty matter contents which are lower than the levels in the anhydrous state indicate the existence of water, bound water or hydrate, in the prepared Ca-Palmate and Zn-Palmate. This is supported by water content data. Heating in an oven at 103 °C showed that the prepared Ca-Palmate and Zn-Palmate have water contents of 9.0 ± 1.1 wt% and 17.2 ± 1.0 wt%, respectively.
Table 6.
Results of quantitative analyses of Ca-Palmate and Zn-Palmate.
| Sample | Property | CI∗ |
|---|---|---|
| Calcium content of Ca-Palmate (%) | ||
| 1 | 6.24 | |
| 2 | 6.48 | 6.6 ± 0.9 |
| 3 | 6.98 | |
| Zinc content of Zn-Palmate (%) | ||
| 1 | 9.84 | |
| 2 | 9.51 | 9.5 ± 1.0 |
| 3 | 9.03 | |
| Total fatty matter of Ca-Palmate (%) | ||
| 1 | 79.98 | 80.4 ± 5.3 |
| 2 | 80.82 | |
| Total fatty matter of Zn-Palmate (%) | ||
| 1 | 76.33 | 76.5 ± 2.7 |
| 2 | 76.76 | |
| Water content of Ca-Palmate (%) | ||
| 1 | 9.1 | 9.0 ± 1.1 |
| 2 | 8.9 | |
| Water content of Zn-Palmate (%) | ||
| 1 | 17.1 | 17.2 ± 1.0 |
| 2 | 17.3 |
CI = Confidence interval at 95%-confidence level.
3.4. Thermal stability of Ca-Palmate and Zn-Palmate
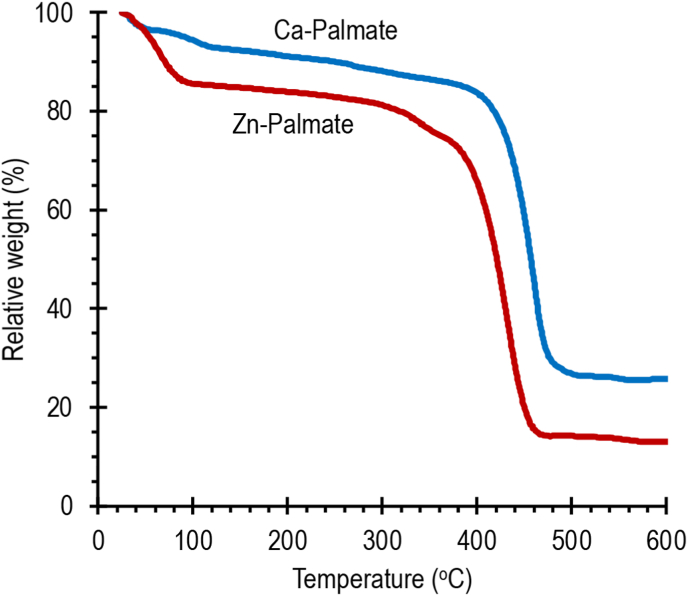
The thermal stability of Ca-Palmate and Zn-Palmate need to be tested to ensure that both palmates do not deteriorate under the applied conditions. Figure 3 presents the TGA curves of the prepared Ca-Palmate and Zn-Palmate. Both metal palmates exhibit two stages of weight loss. The same is also found for calcium and zinc stearates (El-Ghaffar et al., 2019). In the first stage, the prepared Ca-Palmate and Zn-Palmate are practically stable while the weight loss was 14% at 393 °C and 19% at 311 °C for Ca-Palmate and Zn-Palmate, respectively. These two weight losses represent the weight reduction due to the release of moisture and water hydrates. For Zn-Palmate, a weight loss of 15% was observed at 100 °C. This weight loss indicated that the Zn-Palmate obtained is still moist. As stated in the previous section, moisture analysis showed that the obtained Zn-Palmate has a moisture content of about 17%. The weights of the Ca-Palmate and Zn-Palmate decrease drastically in the temperature ranges of 311–359 °C and 420-379 °C, respectively. Within these temperature ranges, the Ca-Palmate and Zn-Palmate lost 55% and 65% by weight, respectively. It is due to the thermal decomposition of both metal carboxylates. Above 500 °C, the Ca-Palmate and Zn-Palmate lost all of their organic parts leaving residues of 26 wt% and 13 wt%, respectively. The decomposition products were not analyzed in this research. It has been reported that the thermal decomposition products of metal carboxylates contain metal oxide, ketone, and carbon dioxide as main products, whilst the solid residues take forms as metal carbonates (Akanni et al., 1992).
Figure 3.
TGA curves of Ca-Palmate and Zn-Palmate.
3.5. Stabilization mechanism
The stabilization mechanism of the mixed Ca/Zn metal thermal stabilizer is characterized by the synergy between the two metal carboxylates. Zinc carboxylates impart a good early color to PVC by substituting labile chlorine atoms according to the reaction in Eq. (6) (Schiller, 2015):
| PVC-Cl + Zn(OOCR)2 → PVC-OOR + ZnCl2 | (6) |
On the other hand, calcium carboxylate reacts with ZnCl2 through the reaction in Eq. (7) (Schiller, 2015):
| ZnCl2 + Ca-(OOCR)2 → Zn(OOCR)2 + CaCl2 | (7) |
Calcium carboxylate also binds hydrogen chloride through the reaction in Eq. (8) (Schiller, 2015):
| 2 Ca-(OOCR)2 + HCl → 2 RCOOH + CaCl2 | (8) |
Polyol enhances PVC stability when used in combination with calcium/zinc stearate system by acting as an acceptor of chelating agent for metal chloride to form inert complex (Wang et al., 2006).
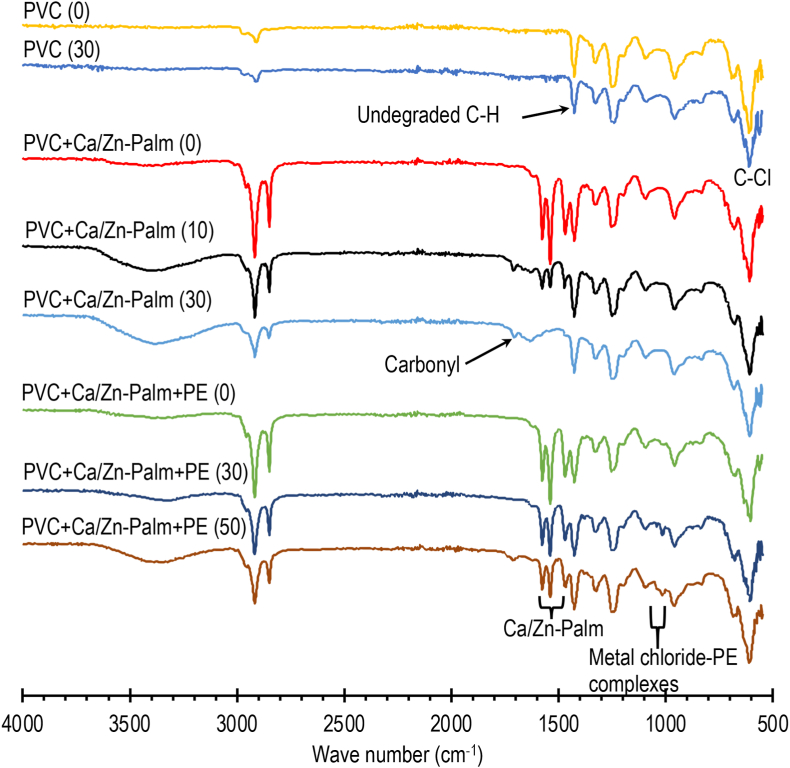
Since Ca/Zn-Palmate are basically Ca/Zn carboxylates, it can be expected that their stabilization mechanism follows the reactions in Eqs. (6), (7), and (8). To confirm the mechanism, two mixtures, PVC+Ca/Zn-Palmate and PVC+Ca/Zn-Palmate+Pentaerythritol, were prepared. The doses used were 4 phr for Ca-Palmate, 1 phr for Zn-Palmate, and 1 phr for Pentaerythritol. Samples of the mixtures, about 0.5 g, were placed in glass tubes and heated at 180 °C in a thermal oil batch at various times. The IR spectra of the heated mixtures were then measured. Figure 4 presents the IR spectra of the mixtures at various heating times. The spectra of PVC are characterized by the presence of the C–Cl band (609 m−1) and the C–H bending vibration band at –CH2– in undegraded PVC segments (1427 m−1). After being heated, each mixture showed a decrease in intensity at 1427 m−1 indicating a degradation phenomenon. The IR spectra of the PVC+Ca/Zn-Palmate mixture after heating showed the presence of a carbonyl bending vibration band at 1705 m−1 indicating the presence of carboxylic acid formed by the reaction in Eq. (8). The increasing intensity of the band near 3400 cm−1 was also caused by the formation of carboxylic acids. The presence of Ca/Zn-Palmate in the mixtures is shown by the presence of COO− vibration bands in the range of 1460–1575 cm−1. The longer the PVC+Ca/Zn-Palmate mixture is heated, the lower the intensity of the bands in this region indicating the consumption of Ca/Zn-Palmate. The role of pentaerythritol can be observed by the presence of metal chloride-PE complexes. For calcium/zinc stearate mixtures, their characteristic bands were reported in the range of 1001–1033 cm−1 (Wang et al., 2006). The presence of the characteristic bands of metal chloride-PE complexes in this region was also observed in the spectra of heated PVC+Ca/Zn-Palmate+PE mixtures. After being heated for 30 minutes, the bands indicating the presence of Ca/Zn-Palmate in the PVC+Ca/Zn-Palmate mixture practically disappeared. However, with the presence of pentaerythritol, after heating for the same time or even after heating for 50 minutes, the COO− vibration bands of Ca/Zn-Palmate can still be observed. This indicates the addition of pentaerythritol saves the consumption of Ca-Palmate and, therefore, longer stabilization can be expected. The presence of an ester bond, near 1735 cm−1, indicating the substitution of allylic chloride by zinc stearate (Mackenzie et al., 1983), however, could not be observed. It may be due to the small portion of Zn-Palmate or the decomposition of the ester by hydrogen chloride formed.
Figure 4.
IR spectra of heated PVC, numbers in parenthesis indicate heating times in minutes, Palm is Palmate, PE is pentaerythritol.
3.6. PVC thermal stability
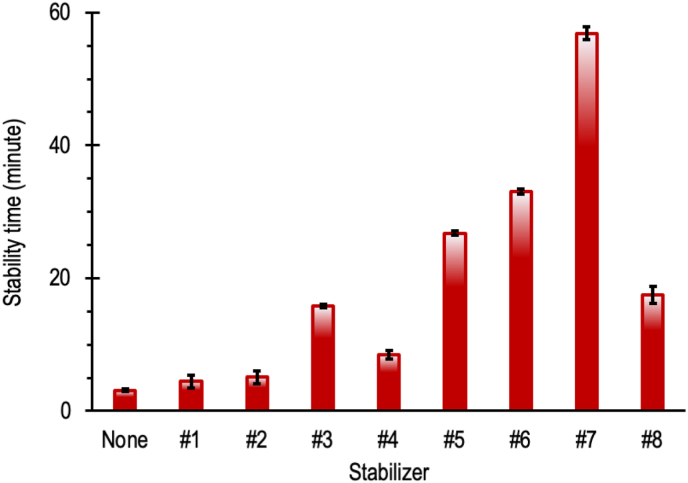
Figure 5 illustrates the Congo red stability time of PVC with various mixed Ca/Zn-Palmate where the doses of the stabilizers are specified in Table 1. On comparing the results at various Ca/Zn-Palmate ratios (stabilizers #1, #2, #3, and #4), the Ca/Zn-Palmate ratio of 4.00 gives the greatest stability time. At lower Ca/Zn-Palmate ratios, the mixed Ca/Zn-Palmates do not exhibit stabilizing effects. It is due to catalytical effect of ZnCl2 as the stabilization product of Zn-Palmate which promotes dehydrochlorination process. When the Ca/Zn ratio is increased further to a value of 7.00, the stability time decreases. A very high Ca/Zn-Palmate ratio means the presence of Zn-Palmate is very low. As a result, the allylic chloride substitution is limited thereby accelerating the liberation of hydrogen chloride. On comparing stabilizers #3 and #5 to the unstabilized PVC, it is clearly shown that the addition of mixed Ca/Zn-Palmate at 3–5 phr and a Ca/Zn ratio of 4.00 increases the stability of PVC. It indicates the synergic effects of Ca/Zn-Palmate following the reactions in Eqs. (6), (7), and (8). The synergic effects of pentaerythritol with Ca/Zn-Palmate are demonstrated by stabilizers #6 and #7. The stability time is greatly increased by combining Ca/Zn-Palmate with pentaerythritol. Adding pentaerythritol with the same dose of Zn-Palmate to the mixed Ca/Zn-Palmate with Ca/Zn ratio of 4.00 doubles the stability time compared to that without pentaerythritol. As can be expected, the synergies between pentaerythritol and mixed calcium/zinc stearate as stated in the literature (Liao et al., 2011; Liu et al., 2007; Schiller, 2015) also occur between pentaerythritol and mixed Ca/Zn-Palmate. Comparing the stability provided by stabilizers #3 and #8, it can be observed that the mixed calcium/zinc stearate is slightly better than the mixed Ca/Zn-Palmate.
Figure 5.
Effects of stabilizers on stability time of PVC by Congo red test at 180 °C, the error bars represent the confidence interval of measurements for four samples at 95% confidence level.
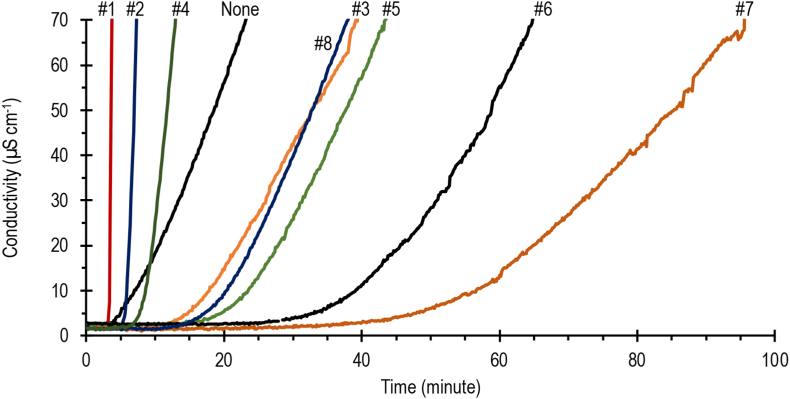
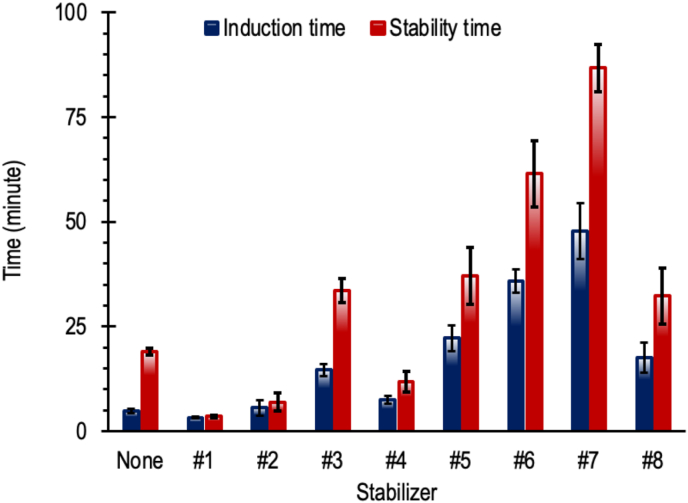
Figure 6 illustrates the time course of conductivity of water through with the nitrogen gas was dispersed after passing the PVC sample stabilized by various stabilizers in Table 1. In contrast to the Congo red tests which provide only one time value, the dehydrochlorination test gives two-time values, namely the induction and the stability times. The induction time indicates the time when the hydrogen chloride begins to evolve and the stability time is taken as the period during which the conductivity increases by 50 μS cm−1 which is the maximum acceptable level of degradation (Han et al., 2019; Li et al., 2019). In this study, the induction time is taken as the period during which the conductivity increases by 3 μS cm−1 Figure 7 summarizes the results of the dehydrochlorination tests. The trend shown by the induction time is like the trend of the Congo red test results. The impact of 'zinc burning' as mentioned in the literature (Dong et al., 2019; Li et al., 2017; Wang et al., 2006, 2016b; Ye et al., 2018) became clearer with the dehydrochlorination test. As can be seen from Figure 6, the curves of stabilizers #1 and #2 after passing the induction times are very steep, steeper than that of PVC without stabilizer. Consistent with the results of the Congo red test, the dehydrochlorination test also showed that Ca/Zn-Palmates at a ratio of 4 and a dose of 3 or 5 phr, with or without pentaerythritol, increase the stability of PVC significantly. Adding pentaerythritol into the mixed Ca/Zn-Palmate not only delayed the release of hydrogen chloride, as indicated by an increase in the induction time, but also reduced the rate of hydrogen chloride release. This is evidence that the hydrogen chloride scavenging by pentaerythritol works well and may even be faster than that by Ca-Palmate. This is understandable because the reactivity of pentaerythritol is increased in the presence of ZnCl2 and CaCl2 (Liao et al., 2011), which are produced through the stabilization of PVC by the mixed Ca/Zn-Palmate system. As can be seen from Figure 4, with the addition of pentaerythritol into the formula, even though it has been heated for 50 minutes, the presence of Ca/Zn-Palmate can still be observed. In contrast to using only Ca/Zn-Palmate, the characteristic peaks of Ca/Zn-Palmate disappeared when the mixture was heated for 30 minutes.
Figure 6.
Change in conductivity of aqueous solution with respect to time at 180 °C for PVC with different thermal stabilizers.
Figure 7.
Summary of dehydrochlorination tests, the error bars represent the confidence interval of measurements for two samples at 95% confidence level.
At a Ca/Zn ratio of 4 and a dose of 3 phr, stabilizers #4 versus #8, calcium/zinc stearate gives an induction time of 3 minutes longer than Ca/Zn-Palmate. However, the final stability times given are almost the same. Overall, as also observed from the results of the Congo red test, the mixed calcium/zinc stearate gives a slightly better stabilizing effect than the mixed Ca/Zn-Palmate. The calcium stearate and zinc stearate used in this paper are of commercial grade. The manufacture of calcium/zinc stearate on a commercial scale uses commercial stearic acid which is a mixture of stearic acid and palmitic acid with stearic acid as the main component. On the other hand, PFAD is composed of palmitic acid and oleic acid as the main components. It has been reported that the thermal stability of metal oleate is lower than that of metal stearate and palmitate (Ushikusa, 1990). This may be the reason why the mixed calcium/zinc stearate is slightly better than Ca/Zn-Palmate.
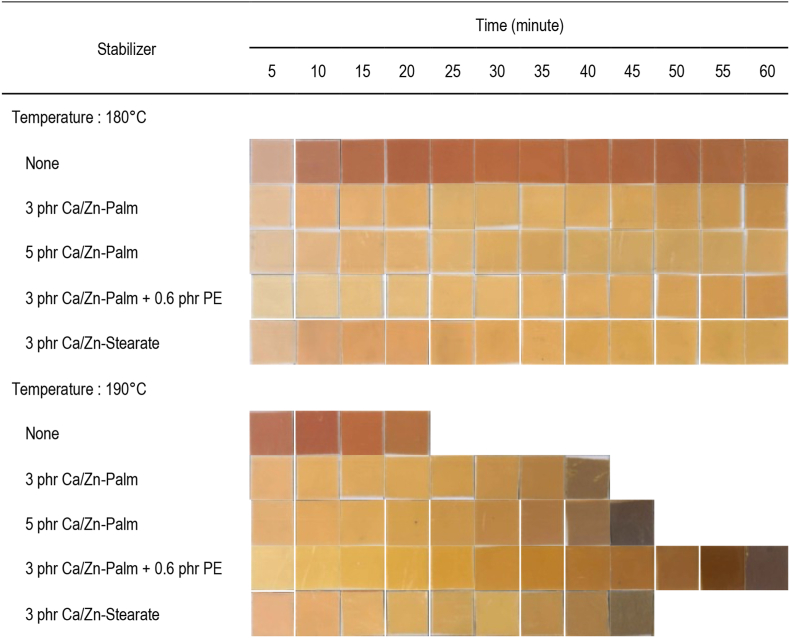
To comprehensively evaluate the efficacy of mixed Ca/Zn-Palmate, the color stability of PVC was tested on a two-roll mill. A 100 gram of PVC was blended with thermal stabilizers and other additives with doses as given in the experimental section. The blends were rolled at 180–190 °C and a roll speed of 11 rpm with a film thickness of 0.25 mm. Four stabilizers were tested including a mixed commercial calcium and zinc stearates. Figure 8 illustrates the color of PVC samples taken every 5 minutes from the roll mill. The PVC without stabilizer was found to become dark in a short time. At 180 °C, all stabilized PVC could be rolled in one hour with light color. At 190 °C, PVC stabilized with Ca/Zn-Palmates at 3 and 5 phr become dark in 40 and 45 minutes, respectively. Initial color improvement and longer stability by the addition of pentaerythritol as a co-stabilizer are evident from the results of the discoloration test at 190 °C. At the same dose, the roll mill tests at 190 °C showed that the efficacy of mixed Ca/Zn-Palmate is slightly lower than that of the mixed calcium/zinc stearate. This is consistent with the results of the Congo red test and the dehydrochlorination test which is thought to occur due to differences in the composition of the carboxylate of both mixed metal thermal stabilizers. Overall, none of the tested formulas gave a good initial whiteness. This is because the tested formula uses high Ca/Zn ratios. As reported in the literature (Balköse et al., 2001; Ye et al., 2019), a high Ca/Zn ratio has advantages in long stability times but is lacking in initial color. The recommended Ca/Zn ratio in a mixed metal formulation is 0.25–4.00 with the lower and higher ratios being prioritized for initial color and long-term stability, respectively (Balköse et al., 2001).
Figure 8.
Discoloration of PVC on roll-mill at various mixed Ca/Zn Palmate.
As a product made from a new material, it is interesting to compare the performance of mixed Ca/Zn-Palmate with the mixed metal thermal stabilizers of other previously reported materials. However, comparative studies are not easy to do because of the different PVC resins and the test conditions used. Literature with the same testing conditions as the tests in this work is Folarin and Ayinde (2016). The authors synthesized metal carboxylates including calcium and zinc carboxylates from castor seed oil (CSO). Although the specification for the PVC resin used is not provided, the authors reported that their PVC resin gives a Congo red stability time of 3 minutes, almost the same as that obtained from this work, i.e., 3.13 minutes. The PVC resin used, therefore, can be considered to have the same specification as that of this work. Furthermore, Folarin and Ayinde (2016) reported that PVC with mixed Ca/Zn–CSO at 3 phr with a Ca/Zn ratio of 4, resulted in a Congo red stability time of 17 minutes. On the other hand, at the same dose and ratio, Ca/Zn-Palmate gave a quite similar stability time of 15.8 minutes. Thus, besides competing with mixed calcium/zinc stearate, the thermal stabilizing effect provided by Ca/Zn-Palmate also competes with mixed Ca/Zn thermal stabilizer made from CSO.
Both static and dynamic tests have proven that Ca/Zn-Palmate can stabilize PVC against heat well, thus confirming the hypothesis that PFAD can be used to produce an effective mixed metal PVC thermal stabilizer. It is worthy to note that, PFAD was used in this work as received, without any pretreatment. The fact that no pretreatment is required and the availability as a byproduct will increase the opportunities for PFAD as an economical raw material for mixed metal-based PVC thermal stabilizers. In addition, the use of PFAD as a raw material for mixed Ca/Zn thermal stabilizers will support sustainable development by utilizing industrial by-products to enhance resource efficiency.
4. Conclusions
Calcium and zinc carboxylates have been prepared from palm fatty acid distillate via the metathesis method. The formations of both metal carboxylates were confirmed by FTIR and XRD spectrophotometry. TGA analyses have shown that the prepared calcium and zinc carboxylates are practically stable while their weight losses are 14% at 393 °C and 19% at 311 °C, respectively. The efficacy of both metal carboxylates in their mixtures in stabilizing PVC against heat has also been studied by using Congo red, dehydrochlorination, and two roll mill tests. The calcium to zinc carboxylate weight ratio of 4:1 has been found to give the longest stability time under the studied condition. The mixed calcium/zinc carboxylates demonstrate a synergism effect with pentaerythritol. The results reveal that mixed calcium/zinc carboxylates from palm fatty acid distillate are effective thermal stabilizers for polyvinyl chloride.
Declarations
Author contribution statement
I Dewa Gede Arsa Putrawan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Antonius Indarto: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yona Octavia: Performed the experiments.
Funding statement
This work was supported by Institut Teknologi Bandung (ITB) research grant under PPMI 2021 Program.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The PFAD was provided by Tunas Baru Lampung Company and the PVC resin was supplied by Asahimas Chemical Company to whom the authors gratefully acknowledge.
References
- Abdul Kapor N.Z., Maniam G.P., Rahim M.H.Ab., Yusoff M.M. Palm fatty acid distillate as a potential source for biodiesel production-a review. J. Clean. Prod. 2017;143:1–9. [Google Scholar]
- Agropost Palm oil market report. 2022. https://agropost.wordpress.com/2022/05/ URL.
- Ahmad Nizam A.F., Muthiyah K., Mahmud M.S. Free fatty acid formation in oil palm fruits during storage. IOP Conf. Ser. Mater. Sci. Eng. 2020;991(1) [Google Scholar]
- Akanni M.S., Okoh E.K., Burrows H.D., Ellis H.A. The thermal behaviour of divalent and higher valent metal soaps: a review. Thermochim. Acta. 1992;208:1–41. [Google Scholar]
- Asawakosinchai A., Jubsilp C., Mora P., Rimdusit S. Organic heat stabilizers for polyvinyl chloride (PVC): a synergistic behavior of eugenol and uracil derivative. J. Mater. Eng. Perform. 2017;26(10):4781–4788. [Google Scholar]
- Atakul S., Balköse D., Ülkü S. Synergistic effect of metal soaps and natural zeolite on poly(vinyl chloride) thermal stability. J. Vinyl Addit. Technol. 2005;11(2):47–56. [Google Scholar]
- Balköse D., Gökçel H.İ., Göktepe S.E. Synergism of Ca/Zn soaps in poly(vinyl chloride) thermal stability. Eur. Polym. J. 2001;37(6):1191–1197. [Google Scholar]
- Benaniba M.T., Belhaneche-Bensemra N., Gelbard G. Stabilization of PVC by epoxidized sunflower oil in the presence of zinc and calcium stearates. Polym. Degrad. Stabil. 2003;82(2):245–249. [Google Scholar]
- Borukaev T.A., Kharaev A.M., Kh Shaov A., Kh Salamov A., Borodulin A.S. Influence of a mixture of calcium and zinc stearates on the thermal and mechanical properties of PVC plastic. J. Phys. Conf. Ser. 2021;1990(1) [Google Scholar]
- Burnett C.L. Cosmetic Ingredient Review; Washington: 2018. Safety Assessment of Fatty Acids & Soaps as Used in Cosmetics, Cosmetic Ingredient Review. [Google Scholar]
- Chang A.S., Sherazi S.T.H., Kandhro A.A., Mahesar S.A., Chang F., Shah S.N., Laghari Z.H., Panhwar T. Characterization of palm fatty acid distillate of different oil processing industries of Pakistan. J. Oleo Sci. 2016;65(11):897–901. doi: 10.5650/jos.ess16073. [DOI] [PubMed] [Google Scholar]
- Coiffard L., Couteau C. Soap and syndets: differences and analogies, sources of great confusion. Eur. Rev. Med. Pharmacol. Sci. 2020;24(21):11432–11439. doi: 10.26355/eurrev_202011_23637. [DOI] [PubMed] [Google Scholar]
- Corbeil M.-C., Robinet L. X-ray powder diffraction data for selected metal soaps. Powder Diffr. 2002;17(1):52–60. [Google Scholar]
- Cristiano L., Guagni M. Zooceuticals and cosmetic ingredients derived from animals. Cosmetics. 2022;9(1):13. [Google Scholar]
- Dong T., Li D., Li Y., Han W., Zhang L., Xie G., Sunarso J., Liu S. Design and synthesis of polyol ester-based zinc metal alkoxides as a bi-functional thermal stabilizer for poly(vinyl chloride) Polym. Degrad. Stabil. 2019;159:125–132. [Google Scholar]
- ECHEMI Stearic acid China domestic price. 2022. https://www.echemi.com/productsInformation/pid_Seven41144-stearicacid.html URL.
- El-Ghaffar A.M.A., Youssef E.A.M., Afify M.F. High performance metal stearates thermal stabilizers for poly vinyl chloride. Int. J. Petrochem. Sci. Eng. 2019;4(4):162–168. [Google Scholar]
- Filopoulou A., Vlachou S., Boyatzis S.C. Fatty acids and their metal salts: a review of their infrared spectra in light of their presence in cultural heritage. Molecules. 2021;26(6005):1–26. doi: 10.3390/molecules26196005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folarin O.M., Ayinde A.A. Thermal stability study of some metal carboxylates of Castor (ricinuscommunis) seed oil on poly(vinyl chloride) Res. J. Chem. Sci. 2016;6(2):23–28. [Google Scholar]
- Folarin O.M., Eromosele I.C., Eromosele C.O. Stabilizing effect of metal carboxylates of Balanites aegyptiaca seed oil (BSO) on poly(vinyl chloride) Environ. Nat. Resour. Res. 2013;3(2) [Google Scholar]
- Folarin O.M., Eromosele I.C., Eromosele C.O. Thermal stabilization of poly(vinyl chloride) by metal carboxylates of ximenia americana seed oil under inert condition. J. Mater. Environ. Sci. 2012;3(3):507–514. [Google Scholar]
- Folarin O.M., Sadiku E.R. Thermal stabilizers for poly(vinyl chloride): a review. Int. J. Phys. Sci. 2011;6(18):4323–4330. [Google Scholar]
- Foreign Agricultural Service . United States Department of Agriculture; 2021. Oilseeds: World Markets and Trade. [Google Scholar]
- Gao J.G., Liu X.Q., Yang J.B., Zhu F.L. Influence of polyols as a Co-stabilizer on stabilization efficiency of calcium/zinc stabilizers to PVC. Adv. Mater. Res. 2012;549:251–254. [Google Scholar]
- Gibon V., De Greyt W., Kellens M. Palm oil refining. Eur. J. Lipid Sci. Technol. 2007;109(4):315–335. [Google Scholar]
- Gökçel H.I., Balköse D., Köktürk U. Effects of mixed metal stearates on thermal stability of rigid PVC. Eur. Polym. J. 1999;35(8):1501–1508. [Google Scholar]
- Guangbao W., Shangsuo Y., Jijun X. Thermal degradation kinetics of calcium stearate/PVC composite. Results Mater. 2020;8 [Google Scholar]
- Han W., Zhang M., Li D., Dong T., Ai B., Dou J., Sun H. Design and synthesis of a new mannitol stearate ester-based aluminum alkoxide as a novel tri-functional additive for poly(vinyl chloride) and its synergistic effect with zinc stearate. Polymers. 2019;11(6):1031. doi: 10.3390/polym11061031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Ishikawa M., Nakamura Y., Iida T. Dispersibility of macromolecular polyols as Co-stabilizer in poly(vinyl chloride) and their stabilization effect combined with synergetic metal soap. Polym. Polym. Compos. 2003;11(8):649–662. [Google Scholar]
- Jubsilp C., Asawakosinchai A., Mora P., Saramas D., Rimdusit S. Effects of organic based heat stabilizer on properties of polyvinyl chloride for pipe applications: a comparative study with Pb and CaZn systems. Polymers. 2021;14(1):133. doi: 10.3390/polym14010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang M., Li S., Huang K., Mao W., Xia J. Effects of preparation methods of mixed calcium and zinc thermal stabilizers derived from dimer fatty acid and tung-oil based C22 triacid on properties of PVC. Pol. J. Chem. Technol. 2017;19(2):78–87. [Google Scholar]
- Li Y., Li D., Han W., Zhang M., Ai B., Zhang L., Sun H., Cui Z. 2019. Facile Synthesis of Di-mannitol Adipate Ester-Based Zinc Metal Alkoxide as Bi-functional Additives for PVC 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., He B., Chen X. Chlorinated poly(vinyl chloride) stabilization by pentaerythritol/calcium-zinc stearate mixtures: the fate of pentaerythritol. J. Vinyl Addit. Technol. 2011;17(1):1–8. [Google Scholar]
- Liu Y.-B., Liu W.-Q., Hou M.-H. Metal dicarboxylates as thermal stabilizers for PVC. Polym. Degrad. Stabil. 2007;92(8):1565–1571. [Google Scholar]
- Mackenzie M.W., Willis H.A., Owen R.C., Michel A. An infra-red spectroscopic study of the stabilization of poly(vinyl chloride) by zinc and calcium stearates. Eur. Polym. J. 1983;19(6):511–517. [Google Scholar]
- Mba O.I., Dumont M.-J., Ngadi M. Palm oil: processing, characterization and utilization in the food industry – a review. Food Biosci. 2015;10:26–41. [Google Scholar]
- Montgomery D.C. eighth ed. John Wiley & Sons; Hoboken, New Jersey: 2012. Design and Analysis of Experiments. [Google Scholar]
- Mordor Intelligence Polyvinyl chloride (PVC) market - growth, trends, COVID-19 impact, and forecast (2022 - 2027) 2022. https://www.mordorintelligence.com/industry-reports/polyvinyl-chloride-pvc-market URL.
- Ng B.Y.S., Ong H.C., Lau H.L.N., Ishak N.S., Elfasakhany A., Lee H.V. Production of sustainable two-stroke engine biolubricant ester base oil from palm fatty acid distillate. Ind. Crop. Prod. 2022;175 [Google Scholar]
- Okieimen F.E., Egbuchunam T.O., Balköse D.B. Studies in the utilization of biobased additives as thermal stabilizer for plasticized PVC. Adv. Mater. Res. 2009;62–64:335–344. [Google Scholar]
- PlasticsEurope . PlasticsEurope AISBL; Brussel: 2019. Plastics - the Facts 2019. [Google Scholar]
- Protasova N.N., Korchagin M.V., Protasov A.V., Korchagin V.I. Analysis and synthesis of kinetic parameters of soapstocksaponification stage in sunflower oil production. IOP Conf. Ser. Earth Environ. Sci. 2021;640(4) [Google Scholar]
- Schiller M. Carl Hanser Verlag; Munich: 2015. PVC Additives: Performance, Chemistry, Development, and Sustainability. [Google Scholar]
- Song L., Huo H., Zhang W., Xia H., Niu Y. The facile strategy of improving the long-term stability of highly transparent polyvinyl chloride by introducing unsaturated Zn oleate and uracil derivatives. Materials. 2022;15(7):2672. doi: 10.3390/ma15072672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay B., Ping Y., Yusof M. Characteristics and properties of fatty acid distillates from palm oil. Oil Palm Bull. 2009;59:5–11. [Google Scholar]
- Top A.G.M. Production and utilization of palm fatty acid distillate (PFAD) Lipid Technol. 2010;22(1):11–13. [Google Scholar]
- Ushikusa T. Decomposition temperature of fatty acid metal salts used as the constituent molecules of molecular cognizance thin solid films. Jpn. J. Appl. Phys. 1990;29(11):2460–2464. [Google Scholar]
- Wang M., Xia J., Jiang J., Li S., Huang K., Mao W., Li M. A novel liquid Ca/Zn thermal stabilizer synthesized from tung-maleic anhydride and its effects on thermal stability and mechanical properties of PVC. Polym. Degrad. Stabil. 2016;133:136–143. [Google Scholar]
- Wang M., Xia J., Jiang J., Li S., Li M. Mixed calcium and zinc salts of N-(3-amino-benzoic acid)terpene-maleamic acid: preparation and its application as novel thermal stabilizer for poly(vinyl chloride) RSC Adv. 2016;6(99):97036–97047. [Google Scholar]
- Wang M., Xu J., Wu H., Guo S. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride) Polym. Degrad. Stabil. 2006;91(9):2101–2109. [Google Scholar]
- Xu H., Lee U., Wang M. Life-cycle energy use and greenhouse gas emissions of palm fatty acid distillate derived renewable diesel. Renew. Sustain. Energy Rev. 2020;134 [Google Scholar]
- Ye F., Guo X., Zhan H., Lin J., Lou W., Ma X., Wang X. The synergistic effect of zinc urate with calcium stearate and commercial assistant stabilizers for stabilizing poly(vinyl chloride) Polym. Degrad. Stabil. 2018;156:193–201. [Google Scholar]
- Ye Q., Ma X., Li B., Jin Z., Xu Y., Fang C., Zhou X., Ge Y., Ye F. Development and investigation of lanthanum sulfadiazine with calcium stearate and epoxidised soyabean oil as complex thermal stabilizers for stabilizing poly(vinyl chloride) Polymers. 2019;11(3):531. doi: 10.3390/polym11030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong S.P., Chan Y.S., Law M.C., Ling J.K.U. Improving cold flow properties of palm fatty acid distillate biodiesel through vacuum distillation. J. Bioresour. Bioprod. 2022;7(1):43–51. [Google Scholar]
- Zhang M., Han W., Hu X., Li D., Ma X., Liu H., Liu L., Lu W., Liu S. Pentaerythritol p-hydroxybenzoate ester-based zinc metal alkoxides as multifunctional antimicrobial thermal stabilizer for PVC. Polym. Degrad. Stabil. 2020;181 [Google Scholar]
- Zhang X., Zhou L., Pi H., Guo S., Fu J. Performance of layered double hydroxides intercalated by a UV stabilizer in accelerated weathering and thermal stabilization of PVC. Polym. Degrad. Stabil. 2014;102:204–211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.