Abstract
The pathway of cysteine biosynthesis in archaea is still unexplored. Complementation of a cysteine auxotrophic Escherichia coli strain NK3 led to the isolation of the Methanosarcina barkeri cysK gene [encoding O-acetylserine (thiol)-lyase-A], which displays great similarity to bacterial cysK genes. Adjacent to cysK is an open reading frame orthologous to bacterial cysE (serine transacetylase) genes. These two genes could account for cysteine biosynthesis in this archaeon. Analysis of recent genome data revealed the presence of bacteria-like cysM genes [encoding O-acetylserine (thiol)-lyase-B] in Pyrococcus spp., Sulfolobus solfataricus, and Thermoplasma acidophilum. However, no orthologs for these genes can be found in Methanococcus jannaschii, Methanobacterium thermoautotrophicum, and Archaeoglobus fulgidus, implying the existence of unrecognizable genes for the same function or a different cysteine biosynthesis pathway.
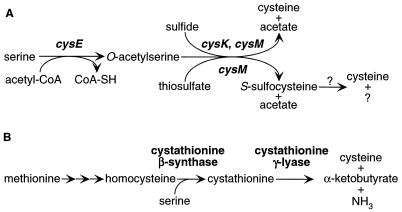
Cysteine is an essential amino acid, unique in its ability to form disulfide linkages and also critical in the catalytic centers of many proteins. In bacteria, cysteine is synthesized from serine by incorporation of sulfide or thiosulfate (Fig. 1A). In the first step, O-acetylserine is formed by serine transacetylase, the cysE gene product. Cysteine is then produced in a reaction catalyzed by the enzyme O-acetylserine (thiol)-lyase-A or O-acetylserine (thiol)-lyase-B, encoded by the cysK and cysM genes, respectively (11). Cysteine biosynthesis in plants is quite similar, although the respective genes have only recently been cloned and only one isozyme of O-acetylserine (thiol)-lyase has so far been identified (7). In animals, the transsulfuration pathway derives the sulfur group of cysteine from methionine and the carbon skeleton and amino group from serine (Fig. 1B). Methionine is first converted to homocysteine through the intermediate S-adenosylmethionine. Cystathionine β-synthase then combines homocysteine and serine to form cystathionine, which yields cysteine upon the action of cystathionine γ-lyase (6).
FIG. 1.
Known biosynthetic pathways to cysteine. (A) Pathway in enteric bacteria. The question marks indicate a step that is incompletely characterized but in which cysteine may be formed by hydrolysis or by reduction with glutathione, also generating sulfate or sulfite, respectively (19). CoA, coenzyme A. (B) The transsulfuration pathway in animals. The first three reactions involve methyl group transfer via S-adenosylmethionine.
The pathway of cysteine biosynthesis in the archaeal domain is at present unknown. Identifiable homologs of the bacterial cysE, cysK, and cysM genes have not been identified in the archaeal genomes of Methanococcus jannaschii, Methanobacterium thermoautotrophicum, Archaeoglobus fulgidus, and Pyrococcus horikoshii (3, 9, 10, 15). Similarly, homologs of cystathionine β-synthase and cystathionine γ-lyase are currently found only in the genomic sequence of Aeropyrum pernix (8). A single isotopic study in Halobacterium marismortui and Sulfolobus acidocaldarius showed that the sulfur of protein-bound cysteine may be derived from exogenously supplied methionine (20). Still unresolved, however, is whether the conversion occurs directly as in the animal transsulfuration pathway or by a more indirect route. In any case, a different biosynthetic pathway may also be present. It is known that in some bacteria (e.g., Deinococcus radiodurans) asparagine is made from aspartate by a tRNA-dependent amidation reaction (5). This route suggested the possibility that cysteine in archaea may be formed from serine in a reaction that involves thiolation of serine misacylated to tRNACys, a reaction formally analogous to the synthesis of selenocysteine (4).
To address the question of cysteine biosynthesis in archaea, we attempted to complement a cysteine auxotroph of Escherichia coli with a genomic library of Methanosarcina barkeri, a mesophilic, autotrophic member of the methylotrophic group of methanogens and the type species of its genus (1). Here we report that bacteria-like cysE and cysK genes are present in M. barkeri.
MATERIALS AND METHODS
Bacterial strains, growth conditions, plasmids, and libraries.
E. coli NK3 (cysK cysM) is a cysteine auxotroph (13). Cells were plated on M9 solid medium (14) supplemented with 20 mg of each l-amino acid except cysteine per liter. Where necessary, plates were supplemented with 22 mg of cysteine and 20 mg of kanamycin per liter. pCBS1 is a pBR322-derived plasmid in which the cloned gene is placed under control of the E. coli trpS promoter. A genomic M. barkeri (Fusaro) library (18) was kindly provided by Dieter Jahn (Freiburg University, Freiburg, Germany). It had been prepared with Sau3A1-digested genomic fragments ligated into the BamHI site of the pBK-CMV vector (Stratagene).
Cloning and sequencing techniques.
Strain NK3 was transformed by electroporation in a Bio-Rad gene pulser. The cysK sequence in M. barkeri was PCR amplified from the complementing clone with PfuTurbo DNA polymerase (Stratagene) and primers containing NdeI and BglII restriction sites. To add 3′-deoxy-adenosine residues, the reaction was incubated at 72°C for 15 min with Taq polymerase (Boehringer Mannheim). The PCR product was gel isolated by using the QIAEX II gel extraction kit (Qiagen) and directly cloned into the pCRII-TOPO vector (Invitrogen). The DNA sequence of the cloned cysK PCR product was confirmed prior to subcloning into pCBS1 at the NdeI and BglII restriction sites. Sequencing was performed by the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University. The GenBank accession number of the M. barkeri sequence is AF174138.
Phylogenetic methods.
The phylogenetic tree was assembled with the maximum likelihood method (20,000 puzzling steps) implemented in the program PUZZLE 4.0 (16). Sequences were aligned with the CLUSTAL X (1.8) program (17). The unpublished Thermoplasma acidophilum sequence was determined by A. Ruepp (Max-Planck-Institut für Biochemie, Martinsried, Germany).
RESULTS AND DISCUSSION
A cysK-like M. barkeri gene complements a cysteine auxotrophic mutant of E. coli.
We obtained a plasmid by complementing the cysteine auxotrophic E. coli strain NK3 (13) with a genomic library of M. barkeri and selecting for growth on minimal medium lacking cysteine. The ability of the isolated plasmid to confer cysteine prototrophy was confirmed upon retransformation of NK3. Sequencing of this DNA revealed open reading frames (ORFs) with high similarity to bacterial cysK and cysE genes, in addition to a nifS-like gene. As shown in Fig. 1, the bacterial cysE and cysK genes encode the enzymes for biosynthesis of cysteine from serine and sulfide. The M. barkeri cysK sequence was PCR amplified and cloned into pCBS1, allowing constitutive expression of the gene from the E. coli trpS promoter. The pCBS1-cysK construct conferred cysteine prototrophy on the NK3 strain as judged by growth on minimal medium lacking cysteine. Thus, the M. barkeri cysK gene was active in vivo in complementing the E. coli cysteine-deficient strain.
Characterization of the cysE and cysK genes of M. barkeri.
The cysE and cysK genes of M. barkeri are adjacent, separated by 260 nucleotides. This is reminiscent of some of the bacteria, such as Mycobacterium tuberculosis and Thermotoga maritima, where these genes are in a similar arrangement. The cysE and cysK sequences of M. barkeri have 48.2 and 49.7 mol% G+C contents, respectively, slightly above the 39 to 44 mol% G+C content of the M. barkeri species as determined by buoyant density (2).
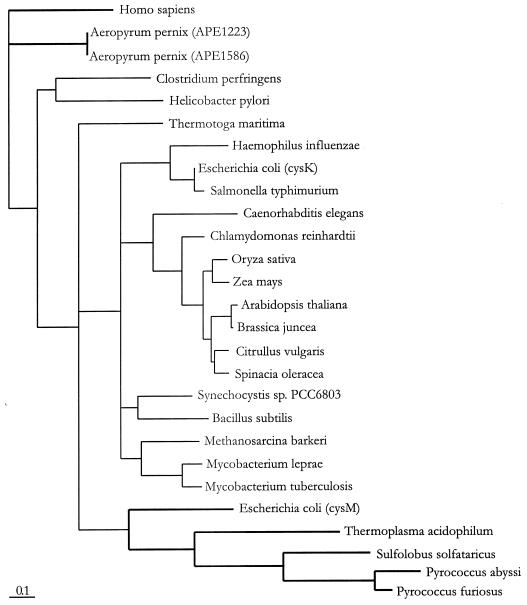
The M. barkeri cysK gene product was highly similar to O-acetylserine (thiol)-lyase-A sequences from bacteria and plants (62% identity with M. tuberculosis, 60% identity with Synechocystis sp., 58% identity with E. coli, 57% identity with Bacillus subtilis, and 56% identity with Chlamydomonas reinhardtii). The sequence of the two O-acetylserine (thiol)-lyases (A and B) are clearly closely related (43% identity for the E. coli enzymes), and there is also similarity between these enzymes and cystathionine β-synthase from the animal pathway (Fig. 1). A database search also revealed cysK-related genes in other archaea. The A. pernix genome (8) contains two ORFs (APE1223 and APE1586) with some similarity (in TblastN searches) to bacterial cysK. However, they also show similarity to cystathionine β-synthase. In the phylogenetic analysis (Fig. 2), these ORFs do not group with the bacterial genes. Rather, they cluster with the human cystathionine β-synthase gene and therefore may have a related activity. Furthermore, the genomes of T. acidophilum (A. Ruepp and W. Baumeister, unpublished results), Pyrococcus abyssi, Pyrococcus furiosus, and Sulfolobus solfataricus contain orthologs to the bacterial cysM-encoded O-acetylserine (thiol)-lyase-B. Thus, these organisms may use the bacterial cysteine biosynthesis pathway, if cysE orthologs were also present. A search of these genomes did not reveal an obvious ORF; therefore, biochemical analysis will be required. Likewise, the methanogens M. jannaschii and M. thermoautotrophicum do not contain recognizable cysE- and cysK-related ORFs, which still leaves open the question of whether a different pathway of cysteine formation exists.
FIG. 2.
Unrooted phylogeny of cysK-like sequences.
We generated a phylogenetic tree of cysK- and cysM-related sequences to gain some insight into the relationship of these genes (Fig. 2). The M. barkeri gene is related to bacterial cysK orthologs and may have been transferred from bacteria. A wide-host-range plasmid from Synechococcus sp. containing cysE- and cysK-like genes was also capable of complementing the respective mutations in E. coli (12), as was the gene for O-acetylserine (thiol)-lyases from watermelon (Citrullus vulgaris [13]). Ignoring thermophilic differences, it is conceivable that complementation of NK3 could also be achieved with the archaeal sequences which group with cysM from E. coli (Fig. 2). However, this has yet to be demonstrated. Likewise, the possible functions of the cysK-like sequences from A. pernix (Fig. 2) are intriguing. Finally, while M. barkeri may contain a typical bacterial cysteine-biosynthetic pathway, cysteine formation remains unexplained in archaea lacking homologous sequences of the well-described cysteine biosynthetic pathways.
ACKNOWLEDGMENTS
Makoto Kitabatake and Man Wah So contributed equally to this work.
We are indebted to A. Ruepp and W. Baumeister for sharing their unpublished results. We also thank Dieter Jahn for providing the genomic library, M. Noji for the NK3 strain, and Kamilah Ali for pCBS1 DNA.
D.L.T. is a postdoctoral fellow of the National Institute of General Medical Sciences. M.K. was a postdoctoral fellow of the Japan Society for the Promotion of Science.
This work was supported by a grant from the Department of Energy (DE-FG02-98ER20311).
REFERENCES
- 1.Boone D R, Whitman W B, Rouvière P. Diversity and taxonomy of methanogens. In: Ferry J G, editor. Methanogenesis: ecology, physiology, biochemistry and genetics. New York, N.Y: Chapman and Hall; 1993. pp. 35–80. [Google Scholar]
- 2.Bryant M P, Boone D R. Emended description of strain MST (DSM 800T), the type strain of Methanosarcina barkeri. Int J Syst Bacteriol. 1987;37:169–170. [Google Scholar]
- 3.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J-F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 4.Commans S, Böck A. Selenocysteine inserting tRNAs: an overview. FEMS Microbiol Rev. 1999;23:335–351. doi: 10.1111/j.1574-6976.1999.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 5.Curnow A W, Tumbula D L, Pelaschier J T, Min B, Söll D. Glutamyl-tRNAGln amidotransferase in Deinococcus radiodurans may be confined to asparagine biosynthesis. Proc Natl Acad Sci USA. 1998;95:12838–12843. doi: 10.1073/pnas.95.22.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith O W. Mammalian sulfur amino acid metabolism: an overview. Methods Enzymol. 1987;143:366–376. doi: 10.1016/0076-6879(87)43065-6. [DOI] [PubMed] [Google Scholar]
- 7.Hell R. Molecular physiology of plant sulfur metabolism. Planta. 1997;202:138–148. doi: 10.1007/s004250050112. [DOI] [PubMed] [Google Scholar]
- 8.Kawarabayasi Y, Hino Y, Horikawa H, Yamazaki S, Haikawa Y, Jin-no K, Takahashi M, Sekine M, Baba S, Ankai A, Kosugi H, Hosoyama A, Fukui S, Nagai Y, Nishijima K, Nakazawa H, Takamiya M, Masuda S, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Kubota K, Nakamura Y, Nomura N, Sako Y, Kikuchi H. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 1999;6:83–101. doi: 10.1093/dnares/6.2.83. [DOI] [PubMed] [Google Scholar]
- 9.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 10.Klenk H-P, Clayton R A, Tomb J-F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Peterson S, Reich C I, McNeil L K, Badger J H, Glodek A, Zhou L, Overbeek R, Gocayne J D, Weidman J F, McDonald L, Utterback T, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Sadow P W, D'Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olsen G J, Fraser C M, Smith H O, Woese C R, Venter J G. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 11.Kredich N M. Biosynthesis of cysteine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 514–527. [Google Scholar]
- 12.Nicholson M L, Gaasenbeek M, Laudenbach D E. Two enzymes together capable of cysteine biosynthesis are encoded on a cyanobacterial plasmid. Mol Gen Genet. 1995;247:623–632. doi: 10.1007/BF00290354. [DOI] [PubMed] [Google Scholar]
- 13.Noji M, Murakoshi I, Saito K. Molecular cloning of a cysteine synthase cDNA from Citrullus vulgaris (watermelon) by genetic complementation in an Escherichia coli Cys− auxotroph. Mol Gen Genet. 1994;244:57–66. doi: 10.1007/BF00280187. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. p. A.3. [Google Scholar]
- 15.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Mao J-I, Rice P, Nölling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strimmer K, von Haeseler A. Quarter puzzling: a quarter maximum-likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 17.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorholt J A, Vaupel M, Thauer R K. A polyferredoxin with eight [4Fe-4S] clusters as a subunit of molybdenum formylmethanofuran dehydrogenase from Methanosarcina barkeri. Eur J Biochem. 1996;236:309–317. doi: 10.1111/j.1432-1033.1996.t01-1-00309.x. [DOI] [PubMed] [Google Scholar]
- 19.Woodin T S, Segel I H. Glutathione reductase-dependent metabolism of cysteine-S-sulfate by Penicillium chrysogenum. Biochim Biophys Acta. 1968;167:78–88. doi: 10.1016/0005-2744(68)90278-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, White R H. Transsulfuration in archaebacteria. J Bacteriol. 1991;173:3250–3251. doi: 10.1128/jb.173.10.3250-3251.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]