Abstract-
Purpose
This systematic review was executed to determine the influence of proton pump inhibitors on biomechanical efficiency of dental implants.
Materials and methods
The comprehensive online literature search was conducted on digital database of Pubmed, Cochrane database and EBSCO host, Web of Science and Scopus from 2010 to 2021(Dec).The studies included in our research comprised of randomized controlled trials and animal studies. Literature review, Letter to the editor, short communication and studies not related to the dental implants were excluded. A total of 6 studies were finalized and included in the systemic review.
Result
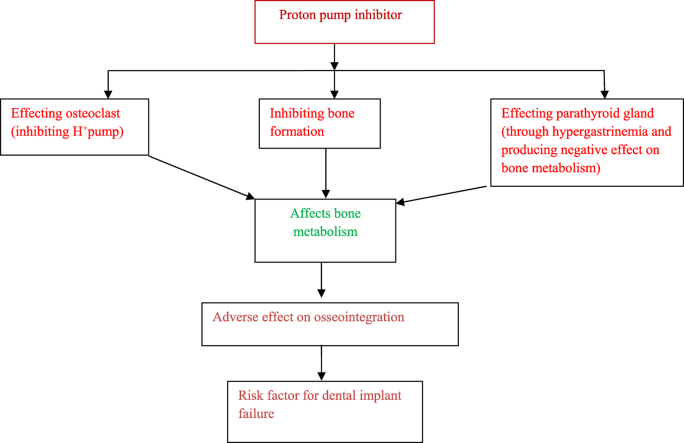
The proton pump inhibitors have a negative influence on the bone metabolism and adversely affect the Osseointegration of the dental implants. Further they reduce the biomechanical efficiency of dental implant which ultimately results in their failure.
Conclusion
Proton pump inhibitors are a risk factor for dental implant survival. This conclusion has been drawn from the limited research available. Hence well designed prospective randomized controlled trials should be carried out on a large population including the users and non-users, to more thoroughly elucidate the effect of proton pump inhibitor on osseointegration process of dental implants.
Keywords: Bone metabolism, Dental implant, Calcium absorption, Osseointegration, Proton pump inhibitor
Graphical abstract
1. Introduction-
Dental implants provide the most effective and predictable solution for the partial or completely edentulous spaces with elevated survival rates, but failures are still encountered.1 One of the main goal of dental implant treatment is the preservation of ridge and long term osseointegration which forms the direct functional and structural association between the loading surface of implant and bone.2,3 Implant failures are classified as early failures associated with impaired bone-implant contact and late implant failures related to functional and parafunctional overloading and pathogenic oral microbiota.2,4 The frequency of early failure ranges from 0.7% to 2%.5 Numerous factors affecting the metabolism of bone such as age, gender, use of tobacco, systemic diseases, radiotherapy, and some systemic medication have been suggested to play a vital role in the early failure of dental implants.6,7 The systemic medications taken by the patient affects the treatment outcome of the implants by directly or indirectly affecting the bone metabolism.6,7
Proton pump inhibitors are the most commonly used drug for the treatment of disorders related to acid such as a gastric ulcer or gastrointestinal disorders for instance eosinophilic gastritis, gastroesophageal reflux, dyspepsia, and helicobacter infection. Nowadays, there is an increase in the usage of Proton pump inhibitors (PPIs) as long-term or continuous therapy. A significant relation has been observed between the usage of PPIs and high risk of bone fracture due to osteoporotic changes.8, 9, 10, 11, 12, 13, 14Further they have been recently recognized to impair the dental implant osseointegration because of their adverse effect on bone metabolism.
Therefore the present systematic review was executed to determine whether there is negative influence of systemic PPIs on the dental implant biomechanical efficiency.
2. Materials and method-
The present review is based on the guidelines of Preferred Reporting Items for Systematic reviews and Meta-analysis(PRISMA).
3. Searched question-
The PICO (population, intervention, comparison and outcome) framework was used for question “Does the proton pump inhibitors(intervention) usage in patients undergoing dental implant treatment modality(population) influences the biomechanical efficiency(outcome) of dental implant as compared to controls(non-users)?
4. Search strategy and selection criteria-
The comprehensive literature search was conducted in Pubmed, Cochrane database, EBSCO host, Web of Science and Scopus from 2010 to 2021(Dec). Hand search of different journals related to proton pump inhibitors and dental implants was preformed to find studies related to topic of review. The hand searched journals included were Clinical Implant Dentistry and Related Research, Clinical Oral Implant Research, Journal of Oral Implantology and Journal of Implant Dentistry. The following keywords were used in search strategy such as proton pump inhibitors, bone metabolism, osseointegration, dental implant failure and risk factors for dental implants.
Kappa statistics was used to calculate level of agreement among the reviewers (AB,BC). A discussion with other authors (GV,DM) was done and consent on final decision was taken. Inclusion criteria comprised of randomerized controlled trails and animal studies in between the period from 2010 to 2021(Dec). Articles in English were included. Case reports and case series, Literature reviews, Letter to the editor, short communications and studies not related to the dental implants were excluded. Articles in Language other than English were excluded. Table 1 shows the inclusion and exclusion criteria.
Table 1.
showed the inclusion and exclusion criteria for systematic review.
| Inclusion Criteria | Exclusion criteria |
|---|---|
| Studies in between 2010 and 2021(Dec) were included. | Studies outside the proposed time slot were excluded. |
| Randomized controlled trials were included. | Letter to editor, literature reviews, case reports, and studies not related to dental implants were excluded. |
| Studies in English were included. | Studies in language other than English were excluded. |
5. Data extraction-
Data was extracted from the included studies for the final investigation. The study data is presented as-author, year, study type, number of patients(PPIs users and non users), mean age, total number of implants(PPIs users and non users), implant dimensions (length × diameter), implant location, implant survival, implant failure and follow-up.
6. Quality and risk of bias assessment for included studies-
Cochrane collaboration tools were used to assess the risk of bias of randomerized controlled trials. SYRCLE'S tool was used to assess the risk of bias of animal studies.
7. Result-
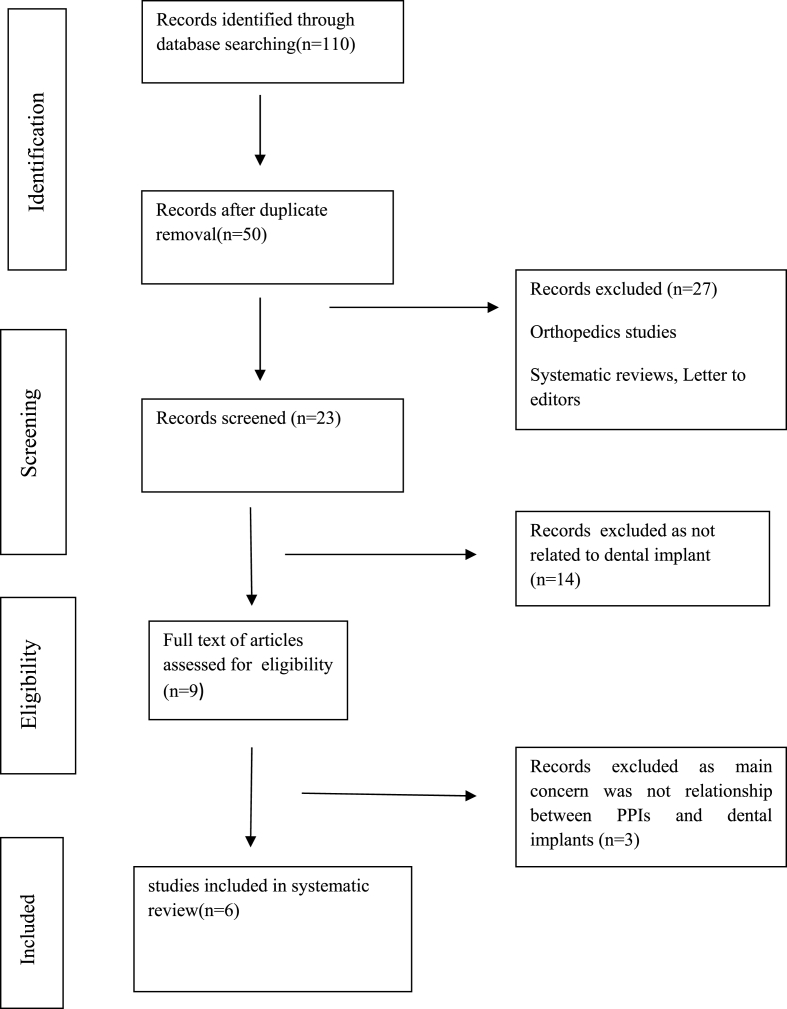
Figure-1 shows the flowchart diagram of the details of the selected study. Total 110 articles related to question raised were identified. Each reviewer further screened the articles, duplicated articles and other articles not related to the topic of interest were excluded. A total of 6 studies including randomerized controlled trials15, 16, 17, 18 and animal studies7,19 were reviewed. Kappa statistics showed the high level of agreement among the reviewers.(ᵏ>0.80). Table 2, Table 3 showed the detailed analysis of the included studies i,e 4 randomerized controlled trials and 2 animal studies. In 4 randomized controlled trials total number of patients were 3025 and total implants placed were 8750 (653 in PPIs users and 8097 in non users). The total number of Implants that survived were 8435 (579 in PPIs users and 7856 in non users) and those failure were 310 (74 in PPIs and 236 in non users). In animal studies, Sprague Dawley rat(48) received 72 dental implants and both the studies showed contradictory result on osseointegration. The Cochrane collaboration tool was used to assess the risk of bias in randomized controlled trials (Table 4) and SYRCLE'S tool for assessment of risk of bias in animal study (Table 5).All the studies included in the systematic review had a low bias risk and were unlikely to altered the result of systematic review. ARRIVE guidelines 2.0(July 2020) were used to analyzed the quality of the animal studies (Table 6) and had a score of 30 out of total score 31.
Fig. 1.
Flowchart shows the Screening of articles for their eligibility to be included in the systematic review.
Table 2.
showed the detailed analysis of the 4 human studies(randomerized controlled trials).
| S NO. | Author/Year/Study type | Number of PPI Users Non Users |
Mean age | Total number of implant | Location of implants | Dimension of implant (length × Diameter) |
Follow up | Implant survival | Implant failed | Study outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Wu et al., 2017 Retrosceptive cohort study |
799 Pts 58 PPIs users 741 non users |
18–93 years | 1773 implants 133 in PPIs users 1640 in non-users |
Maxilla & Mandible Anterior and posterior region |
12.1±.1 mm non users 12.1±.3 mm PPIs × 4.2±.5 mm non users( Mean ± SD) 4.1±.4 mm(Mean ± SD) |
Mean ± SD <12 months935 implants in non users 69 in PPIs users ≥12 months- 705 non users 64 implants in PPIs users |
124 in PPIs users 1587 in non users |
9 in PPIs users 53 in non users |
The study observed the increased risk of osseointegrated dental implant failure in PPIs users. |
| 2 | Chrcanovic et al., 2017 | 999 Pt 67 PPIs users 932 Non users |
≤30 years 31 ≤ 60 Years >60 years |
3559 implants 250 implants in PPIs users 3309 implants in non users |
Mandible anterior and posterior region | 6–10 mm, 10.5–14 mm, 15–20mm × 3-3.5omm, 3.70–4.10 mm, 4.20–5 mm |
94.8± 78.7 months |
220 in PPIs users 3161 in non users |
30 in PPIs users 148 in non users |
The study reported the association between PPIs administration and increased implant failure. |
| 3 | Altay et al., 2018 Retrospective study |
592 patients (316 female pt 276 male pt) 24(18 female and 6 males) PPIs Users 568 Non users |
18–84years | 1918 Implants 69 implants in PPIs Pt 1849 Implants in 568 non users |
Anterior region-506 implants Premolar region-603Implants Molar region 809 Implants (Mandible-957& Maxilla-961 Implants) |
Not mention | 29.02 ± 17.90 months for PPIs users 28.97 ± 17.59 months for non users | 45 implants in PPIs pt 1838 Implants in Non users |
24 in PPIs Pt 11 in Non users |
The study suggested association of PPIs with early dental implant failure. |
| 4 | Brendon et al., 2019 | 635 patients | 21 year or above | 201 implants in PPIs pt | Not mention | Not mention | 2–3 years | 190 implants in PPIs pt | 11 implants in PPIs pt | The study observed more crestal bone loss in dental implant patient having PPIs prescription history. |
| 1299 in Non users | 1270 implants in non users. | 24 in non users |
Table 3.
showed the detailed analysis of the 2 animal studies.
| S no | Author/Year | Total number of animals | Total number of implants placed | Age of animal | Study summary | Location of implant | Implant dimension | Inference |
|---|---|---|---|---|---|---|---|---|
| 1. | Subaie et al., 2016 | 24 Sprague Dawley Rat | 24 implants | 10 weeks | In 24 rats implants were placed in left tibia and defect was created in the right tibia. After surgery rat were on omeprazole (5 mg/kg) and others on saline. After euthanasia rat, percentage of new bone formation in the defect was assessed using microcomputed tomography and peri-implant bone mass or tissue volume and percentage of bone implant contact was observed by histomorphometry. | Tibia left | 1.5mm × 2.0 mm | Rats on PPIs shoe larger cortical bone defects. The systematic administration of these drugs impaired the bone healing. |
| 12 treated with Omeprazole | ||||||||
| 12 with saline | ||||||||
| 2. | Tekin et al., 2021 | 24 Sprague Dawley Rat | 48 implants | 2.5–3 months | In 24 sprague Dawley rats after surgical placement of Ti implant into the metaphyseal part of left and right tibia and randomly divided into three groups n = 8 omeprazole 5 mg/kg and n = 8 10 mg/kg and n = 8 control group. Total 48 implants were placed. After experiment animals from each group was scarified and study completed with 7 rats in each group. Blood samples were collected for biochecimal analysis and implant and surrounding tissue were used for biomechanical reserve torque analysis. | Left and right Tibia | 4mm × 2.5 mm | PPIs have no biomechanical or biomchecimal effect on dental implant osseointegration. |
| N = 8 controls | 32 in PPIs treated | |||||||
| N = 8 on 5 mg/kg of omeprazole | 16 in non treared | |||||||
| N = 8 on 10 mg/kg of omprazole |
Table 4.
Cochrane collaboration tool was used to assess the risk of bias in randomized controlled trials.
|
S no |
Study | Selection Bias |
Blinding of outcome assessment(detection bias) | Incomplete outcome data(Attrition bias) | Selective reporting(reporting bias) | Anything else ideally pre-specified | |
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | ||||||
| 1. | Wu et al., 2017 | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias |
| 2. | Chrcanovic et al., 2017 | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias |
| 3. | Altay et al., 2018 | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias |
| 4. | Brendon et al., 2019 | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias |
Table 5.
SYRCLE'S tool for assessment of risk of bias in animal study.
| S no | Study | Selection Bias |
Preformed bias |
Detection Bias |
Incomplete outcome of data (attrition bias) | Selective outcome reporting(reporting Bias) | Other sources of bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Blinding | |||||
| 1. | Subaie et al., 2016 | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias | unclear | Unclear |
| 2 | Tekin et al., 2021 | Low bias | Low bias | Low bias | Low bias | unclear | Low bias | unclear | unclear | unclear | Unclear |
Table 6.
ARRIVE guidelines 2.0 for assessing the risk bias of animal studies-.
| S.No | Items (Essential & Recommended) | Score range | Tekin et al., 2021 | Subaie et ql 2016 |
|---|---|---|---|---|
| 1. | Study Design | 0–2 | 2 | 2 |
| 2. | Sample size | 0–2 | 2 | 2 |
| 3. | Inclusion & Exclusion criteria | 0–3 | 3 | 3 |
| 4. | Blinding | 0–1 | 1 | 1 |
| 5. | Outcome measures | 0–2 | 2 | 2 |
| 6. | Statistical method | 0–2 | 2 | 2 |
| 7. | Experimental animals | 0–2 | 2 | 2 |
| 8. | Result | 0–2 | 2 | 2 |
| 9. | Abstract | 0–1 | 1 | 1 |
| 10. | Background | 0–1 | 1 | 1 |
| 11. | Objectives | 0–2 | 1 | 1 |
| 12. | Ethical state | 0–1 | 1 | 1 |
| 13. | Housing and Husbandry | 0–1 | 1 | 1 |
| 14. | Animal care and Monitoring | 0–3 | 2 | 2 |
| 15. | Interpretation or Scientific implication | 0–2 | 2 | 2 |
| 16. | Generalization | 0–1 | 1 | 1 |
| 17. | Data Access | 0–1 | 1 | 1 |
| 18. | Declaration of interests | 0–2 | 2 | 2 |
| Total Score | 31 | 30 | 30 |
8. Discussion-
Now a days, the association between the PPIs administration and bone metabolism disorders has become a topic of interest for research scholars. The exact mechanism is still unknown, but it has been assumed that bone loss around dental implant in PPIs patient might be associated with alteration in homeostasis of bone, microbial flora or inflammatory response.20, 21, 22Further few studies have observed the negative influence of PPIs administration on intestinal calcium absorption leading to negative calcium balance in body.23Certain studies have reported significant rise in hip, spine and other site fracture in both genders in PPI users.24 The administration of PPIs employed for gastro esophageal reflux disorders symptoms have been positively associated with deficiency of vitamin B12, and increased homeocysteine level.25This raised homeocysteine level leads to increased rate of bone formation and resorption ultimately resulting in a reduction in broadband ultrasound attenuation(BUA) which measures the mass of bone as ultrasound waves penetrate through the mineralized tissue.26, 27, 28Hence it can be concluded that PPI use has a negative influence on mineral density and volume of bone trabeculae.28, 29, 30 PPIs also hamper the absorption and excretion of magnesium which offers a negative effect on bone metabolism either directly or indirectly, thus affecting survival of biointegration of dental implant.31
PPIs inhibited the proton pump of osteoclast responsible for bone resorption thus impeding the osteoclastic activity and leading to inhibition of bone remodeling.32In vitro studies have shown that administration of PPIs inhibits the vacuolar H+K+ ATPase of osteoclasts.33PPIs usage inhibits the phosphoethanolamine/Phosphocholine phosphatase and tissue non-specific alkaline phosphatase in bone matrix vesicles.34
In vivo study it was observed that PPIs might inhibit the release of calcium from calvaria of the neonatal mouse and reduces the bone resorption by osteoclast.33 PPIs decreased the transverse growth of endosteum and reduces the ratio of mineral substance mass to bone mass resulting in inhibition of formation of bone and impaired mineralization of bone tissue.35This is assumed to be due to reduction of expression markers responsible for bone accrual and formation such as Bone Morphogenic protein 2,4 and Cysteine rich protein-61.35
The chronic gastric acid suppression was observed with PPIs intake, leading to hypergastrinemia[40].This can negatively influence the bone metabolism through parathyroid gland hyperplasia and hypertrophy leading to increased level of PTH. The consistant raised level of PTH in relation to calcium concentration of serum may result to loss of quality and strength of the bone.35
Studies by Tuukkanen et al.33 in 1986 and Mizunashi et al.36 in 1993 reported decrease resorption of bone and its turnover in patients on PPIs. Cottrell et al.37 in 2010 and Bodele et al.38 in 2008 in their studies observed no significant influence on formation of bone. Hasanin et al.39 in 2014 observed that omeprazole had both type of positive and negative influence on bone remodeling. Histing et al. [40] in the murine fracture model observed that there was significantly a lower bony tissue amount within callus and an elevated amount of fibrous and cartilaginous tissue. Pantoprazole delays the healing of fracture by affecting both bone remodeling and formation.
However, till date few studies investigated the influence of PPIs on dental implant biointegration with bone. In vivo study conducted on Sprague Dawley rat tibia by Al Subaie et al.7 in 2016 reported that omeprazole(most commonly prescribed) administration reduced the overall osteoclast number in the healing site of bone resulting in impaired osseointegration. Further concluded that dose of 5 mg/kg and 10 mg/kg of omeprazole had no influence on osseointegration. On contrary Teskin et al.18 in 2021 observed no such association in their study on rat tibia. Wu et al.15 in 2016 in their retrospective cohort study observed negative association between the PPIs and bone metabolism and concluded that the administration of PPIs increase the risk of dental implant osseointegration failure.
According to Altay et al.17 in 2018 dental implant placed in PPIs users showed 4.3 times more likely to fail prior to loading. Chrcanovic et al.16 in 2017 reported a statistically significant difference in failure rates of dental implant between PPIs users and non users.
The result of the present systematic review suggests that administration of PPIs negatively affects the dental implant biomechanical efficiency and should be considered as a risk factor for dental implant failure.
9. Conclusion-
The biomechanical efficiency of dental implant depends on the successful osseointegration and proton pump inhibitors adversely affecting the osseointegration results in dental implant loss. Interaction of PPIs, osseointegration of implant and bone regeneration are related strongly to the metabolism of bone. PPIs therapy affects bone regeneration and osseointegration causing a defective bone metabolism, impaired bone healing and increased risk of bone fracture. The clinicians and patients should avoid the unnecessary use of proton pump inhibitors. Further studies are required to determine whether dietary and pharmacologic strategies can be used successfully to manage the risk of osseointegrated dental implant failure among PPIs users. Alternatives medication for PPIs can be prescribed for that patient who has to undergo the surgery for dental implant. Well designed, prospective, randomized controlled trials should be carried out on a large population including the users and non-users to determine the treatment strategies for patients on PPIs undergoing implant therapy. Animal models should be developed to understand the histopathological changes caused by PPIs on bone and an assay can be developed to screen the suitable patients for implant therapy.
Declaration of competing interest
None.
References
- 1.Mangano F., Mortellaro C., Mangano N., Mangano C. Is low serum vitamin D associated with early dental implant failure? A retrospective evaluation on 1625 implants placed in 822 patients? Mediat Inflamm. 2016;2016 doi: 10.1155/2016/5319718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrektsson T., Brånemark P.I., Hansson H.A., Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 4.Olmedo-Gaya M.V., Manzano-Moreno F.J., Cañaveral-Cavero E., de Dios Luna-del Castillo J., Vallecillo-Capilla M. Risk factors associated with early implant failure: a 5-year retrospective clinical study. J Prosthet Dent. 2016;115:150–155. doi: 10.1016/j.prosdent.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Koldsland O.C., Scheie A.A., Aass A.M. Prevalence of implant loss and the influence of associated factors. J Periodontol. 2009;80:1069–1075. doi: 10.1902/jop.2009.080594. [DOI] [PubMed] [Google Scholar]
- 6.Apostu D., Lucaciu O., Lucaciu G.D., et al. Systemic drugs that influence titanium implant osseointegration. Drug Metab Rev. 2017;49:92–104. doi: 10.1080/03602532.2016.1277737. [DOI] [PubMed] [Google Scholar]
- 7.Al Subaie A., Emami E., Tamimi I., et al. Systemic administration of omeprazole interferes with bone healing and implant osseointegration: an in vivo study on rat tibiae. J Clin Periodontol. 2016;43:193–203. doi: 10.1111/jcpe.12506. [DOI] [PubMed] [Google Scholar]
- 8.Targownik L.E., Lix L.M., Metge C.J., Prior H.J., Leung S., Leslie W.D. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ (Can Med Assoc J) 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalili H., Huang E.S., Jacobson B.C., Camargo C.A., Jr., Feskanich D., Chan A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372. doi: 10.1136/bmj.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu E.W., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngamruengphong S., Leontiadis G.I., Radhi S., Dentino A., Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–1218. doi: 10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- 12.Ito T., Jensen R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, Vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Hoorn M.M., Tett S.E., de Vries O.J., Dobson A.J., Peeters G.M. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: a prospective cohort study. Bone. 2015;81:675–682. doi: 10.1016/j.bone.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Yu E.W., Blackwell T., Ensrud K.E., et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X., Al-Abedalla K., Abi-Nader S., Daniel N.G., Nicolau B., Tamimi F. Proton pump inhibitors and the risk of osseointegrated dental implant failure: a cohort study. Clin Implant Dent Relat Res. 2017;19:222–232. doi: 10.1111/cid.12455. [DOI] [PubMed] [Google Scholar]
- 16.Chrcanovic B.R., Kisch J., Albrektsson T., Wennerberg A. Intake of proton pump inhibitors is associated with an increased risk of dental implant failure. Int J Oral Maxillofac Implants. 2017;32:1097–1102. doi: 10.11607/jomi.5662. [DOI] [PubMed] [Google Scholar]
- 17.Altay M.A., Sindel A., Özalp Ö., Yıldırımyan N., Kocabalkan B. Proton pump inhibitor intake negatively affects the osseointegration of dental implants: a retrospective study. J Korean Assoc Oral Maxillofac Surg. 2019;45:135–140. doi: 10.5125/jkaoms.2019.45.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ursomanno B.L., Cohen R.E., Levine M.J., Yerke L.M. Effect of proton pump inhibitors on bone loss at dental implants. Int J Oral Maxillofac Implants. 2020 Jan 1;35(1) doi: 10.11607/jomi.7800. [DOI] [PubMed] [Google Scholar]
- 19.Tekin S., Dundar S., Demirci F., et al. Biomechanical and biochemical evaluation of the effect of systemic application of omeprazole on the osseointegration of titanium implants. Int J Oral Maxillofac Implants. 2021;7:1–7. doi: 10.1186/s40729-021-00310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedeka R.R., Souza R.F., Spechler S.J. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imhann F., Bonder M.J., Vich Vila A., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:74–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Rodrigues J., Reis S., Teixeira S., Lopes S., Fernandes M.H. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J. 2013;280:5052–5064. doi: 10.1111/febs.12478. [DOI] [PubMed] [Google Scholar]
- 23.Ito T., Jensen R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu E.W., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selhub J., Morris M.S., Jacques P.F. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentration. Proc Natl Acad Sci USA. 2007;104:19995–20000. doi: 10.1073/pnas.0709487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhonukshe Rutten RA., Pluijm S.M., de Groot L.C., Lips P., Smit J.H., Van Staveren W.A. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation and fractures in healthy elderly people. J Bone Miner Res. 2005;20:921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 27.Van Meurs J.B., Dhonukshe, Rutten R.A., et al. Homocysteine levels and risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 28.Bauer D.C., Gluer C.C., Cauley J.A., et al. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women.A prospective study. Study of osteporotic fractures Research Group. Arch Intern Med. 1997;157:629–634. [PubMed] [Google Scholar]
- 29.Hoorn E.J., van der Hoek J., Rob A., Kuipers E.J., Bolwerk C., Zietse R.A. Case series of proton pump inhibitor–induced hypomagnesemia. Am J Kidney Dis. 2010;56:112–116. doi: 10.1053/j.ajkd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Maggio M., Lauretani F., Ceda G.P., et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone. 2013;57:437–442. doi: 10.1016/j.bone.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoorn E.J., van der Hoek J., Rob A., Kuipers E.J., Bolwerk C., Zietse R.A. Case series of proton pump inhibitor–induced hypomagnesemia. Am J Kidney Dis. 2010;56:112–116. doi: 10.1053/j.ajkd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Jefferies K.C., Cipriano D.J., Forgac M. Function, structure and regulation of the vacuolar (H1)-ATPases. Arch Biochem Biophys. 2008;476:33–42. doi: 10.1016/j.abb.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuukkanen J., V€a€an€anen H. Omeprazole, a specific inhibitor of H12 K1-ATPase, inhibits bone resorptionin vitro. Calcif Tissue Int. 1986;38:123–125. doi: 10.1007/BF02556841. [DOI] [PubMed] [Google Scholar]
- 34.Roberts S., Narisawa S., Harmey D., Mill_an J.L., Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle–mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 35.Histing T., Stenger D., Scheuer C., et al. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif Tissue Int. 2012;90:507–514. doi: 10.1007/s00223-012-9601-x. [DOI] [PubMed] [Google Scholar]
- 36.Mizunashi K., Furukawa Y., Katano K., Abe K. Effect of omeprazole, an inhibitor of H1, K1-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 37.Cottrell J.A., Vales F.M., Schachter D., et al. Osteogenic activity of locally applied small molecule drugs in a rat femur defect model. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/597641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodde E.W., Kowalski R.S., Spauwen P.H., Jansen J.A. No increased bone formation around alendronate or omeprazole loaded bioactive bone cements in a femoral defect. Tissue Eng. 2008;14(1):29–39. doi: 10.1089/ten.a.2007.0086. [DOI] [PubMed] [Google Scholar]
- 39.Hasanin A.H. Impact of omeprazole on bone remodeling in normal and ovariectomized Wistar rats. Eur Rev Med Pharmacol Sci. 2014;18(13):1948–1956. [PubMed] [Google Scholar]
- 40.Histing T., Stenger D., Scheuer C., et al. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif Tissue Int. 2012;90:507–514. doi: 10.1007/s00223-012-9601-x. [DOI] [PubMed] [Google Scholar]