Abstract
Background & Aims
Although EASL guidelines recommend anti-HDV testing in all HBsAg-positive individuals, HDV infection remains an underdiagnosed condition. We describe the impact of an HDV screening program by reflex anti-HDV testing in all HBsAg-positive samples and compare the results before and after its implementation.
Methods
In total, 2,236 HBsAg-positive determinations were included from January 2018 to December 2021. Only the first sample from each participant was evaluated: 1,492 samples before reflex anti-HDV testing (2018–2020) and 744 samples after (2021). Demographic and clinical characteristics of anti-HDV-positive patients were collected.
Results
Before reflex testing, anti-HDV had been tested in 7.6% (114/1492) of HBsAg-positive individuals: 23% (91/390) attended in an academic hospital and only 2% (23/1,102) in primary care centres. After reflex testing was established, 93% (691/744) of HBsAg-positive cases were evaluated for anti-HDV: 91% (533/586) in the academic hospital and 100% (158/158) in primary care. The anti-HDV-positive prevalence was similar before and after reflex testing: 9.6% (11/114) and 8.1% (56/691), respectively. However, the absolute number of anti-HDV-positive patients increased. Most anti-HDV-positive patients were young, HBeAg-negative, Caucasian males. HDV-RNA was detectable in 35 (65%) of 54 tested, HBV-DNA was undetectable in 64%, and alanine aminotransferase levels were normal in 48%.
Conclusions
Anti-HDV reflex testing quintupled the absolute number of diagnoses of chronic hepatitis D infection. Before the reflex test, a large percentage of HBsAg-positive individuals had not undergone any anti-HDV determination. Implementation of reflex testing increases the diagnosis of patients with chronic hepatitis D.
Lay summary
Chronic hepatitis delta (CHD) is a viral disease caused by HDV, which requires the presence of HBV to propagate. HDV infection can cause rapid progression to cirrhosis, among other severe complications. The prevalence of CHD worldwide is controversial, and the infection often goes unrecognised, mainly because of unawareness among physicians. Use of reflex testing in other viral hepatitis has proven to increase detection and linking-to-care of infected patients. Implementation of anti-HDV testing in all HBsAg-positive patients has led to a 5-fold increase in the number of HDV diagnoses in an academic hospital and primary care centres.
Keywords: Chronic hepatitis D, Anti-HDV reflex testing, Anti-HDV screening, HDV diagnosis
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; APASL, Asian-Pacific Association for the Study of the Liver; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; FIB-4, fibrosis-4; GGT, gamma glutamyl transferase; PWID, people who inject drugs
Graphical abstract
Highlights
-
•
HDV testing rates in daily clinical practice are low despite EASL guidelines recommending universal screening.
-
•
Implementation of HDV reflex testing led to a 5-fold increase in the number of HBV cases diagnosed with hepatitis D.
-
•
Risk factors were unknown in 60% of anti-HDV positive cases, supporting systematic anti-HDV reflex testing in all HBsAg-positive patients.
-
•
It would be of great value to assess the cost-effectiveness of anti-HDV reflex testing.
Introduction
HDV is a hepatotropic single-stranded RNA virus that requires the presence of HBV to propagate. HDV causes one of the most severe forms of chronic viral hepatitis, being associated with a 2- to 3-fold higher risk of developing liver cirrhosis and hepatocellular carcinoma than infection by HBV alone.1,2 Implementation of vaccination programs against HBV in the nineties undoubtedly had an impact on HDV prevalence in young populations.3 In high-income countries, the current burden of HDV mainly encompasses ageing patients with advanced liver fibrosis and young immigrants from endemic areas.4 Still, HDV infection is believed to be underestimated owing to a lack of, or suboptimal, screening programs in HBsAg-positive individuals.5
Anti-HDV testing in all HBsAg-positive patients is recommended in the 2017 EASL guidelines on the management of HBV infection,6 as well as the 2016 Asian-Pacific guidelines on the management of chronic hepatitis B (APASL, Asian-Pacific Association for the Study of the Liver).7 However, the 2018 American Association for the Study of Liver Diseases (AASLD) guidelines recommend anti-HDV testing only in HBsAg-positive individuals who are at risk, such as people who inject drugs, men who have sex with men, individuals at risk of acquiring sexually transmitted diseases, and immigrants from areas where HDV is highly endemic.8 Although anti-HDV testing in all HBsAg-positive individuals is suggested in the European and Asian-Pacific guidelines, it is believed that many cases remain untested.
The aims of the present study were to determine the anti-HDV testing rates in all HBsAg-positive samples in a large population over a 3-year period, to assess the impact of anti-HDV reflex testing to diagnose HDV in HBsAg-positive cases, and to describe the epidemiology and clinical characteristics of anti-HDV-positive individuals in our setting.
Materials and methods
Study design
The study was divided into 2 parts, including a retrospective and a prospective analysis. In the retrospective part, anti-HDV requests were evaluated in all HBsAg-positive samples processed in a central laboratory attending a catchment area of 450,000 inhabitants in the northern part of Barcelona (Spain) from January 2018 to December 2020.
The central laboratory received samples from 1 academic hospital and 17 primary care centres. All HBsAg-positive samples were reviewed by 1 of the investigators to determine whether an anti-HDV test was subsequently requested at any time, and whether HDV-RNA had been determined in anti-HDV-positive cases. A database was constructed to record baseline demographics and the clinical and laboratory characteristics of anti-HDV-positive patients. The overall and annual percentages of anti-HDV testing and HDV-RNA testing were analysed.
In the prospective part of the study, anti-HDV reflex testing was manually added to all HBsAg-positive serum samples over a 1-year period (January–December, 2021). Demographics and clinical characteristics, including HDV-RNA, were recorded in all anti-HDV-positive patients, as was done in the retrospective part.
HBV serological markers (HBsAg and HBeAg) and anti-HCV antibodies were tested using commercial electrochemiluminescent immunoassays on a COBAS 8000 instrument (Roche Diagnostics, Rotkreuz, Switzerland). Anti-HDV antibodies were analysed with the Liaison XL murex Anti-HDV kit (DiaSorin, Saluggia, Italy), and anti-HIV antibodies with the Liaison XL murex HIV Ab/Ag kit (DiaSorin, Saluggia, Italy). HBV-DNA was quantified by real-time PCR with a detection limit of 10 IU/ml (COBAS 6800, Roche Diagnostics, Mannheim, Germany). HDV-RNA was quantified with an in-house 1-step quantitative RT-PCR using the HDV-RNA international standard of the World Health Organization (1st World Health Organization International Standard for Hepatitis D Virus RNA for Nucleic Acid Amplification Technique-based Assays; PEI code number: 7657/12),9 with a detection limit of 100 IU/ml and a quantification limit of 600 IU/ml. Liver fibrosis stage was assessed by transient elastography: the cut-offs were >7.5 kPa for advanced fibrosis and >13.5 kPa for liver cirrhosis.
Statistics
Normally distributed quantitative variables were compared with the Student t test and expressed as the mean ± SD. Variables with a non-normal distribution were analysed with the Mann-Whitney U test and expressed as the median and inter-quartile range (IQR). Categorical variables were compared using the Chi-square test or Fisher's exact test when frequencies were <5% and expressed as frequencies and percentages. Results were considered statistically significant at a p-value <0.05. All statistical analyses were carried out using IBM SPSS, 20 (SPSS Inc., Armonk, NY, USA).
Results
Routine hepatitis D screening
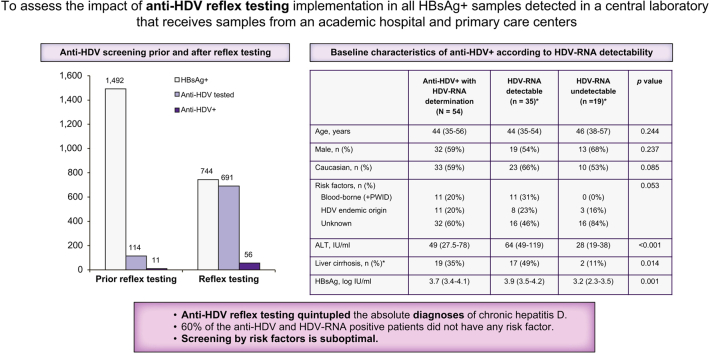
In total, 1492 HBsAg-positive samples were sent to the central laboratory between January 2018 and December 2020 and all were reviewed. Anti-HDV testing had been performed in 114 (7.6%) cases, and 11 (9.6%) were anti-HDV positive.
Anti-HDV determination had been requested in 91 (23%) of 390 samples from the academic hospital, and in only 23 (2%) of 1,102 samples from the primary care centres (p <0.001).
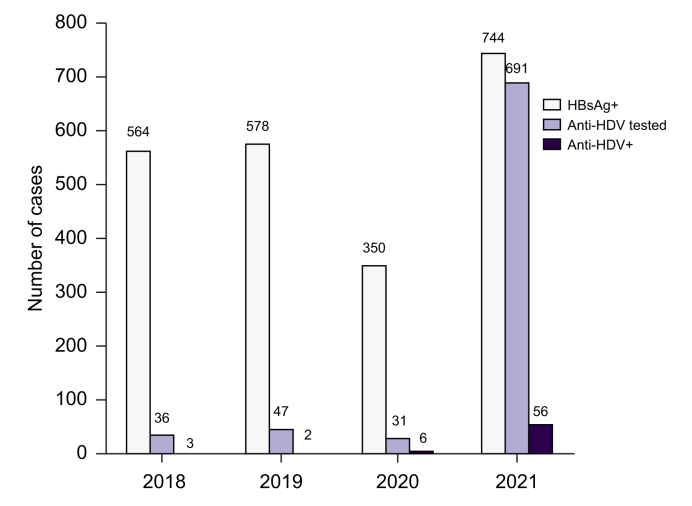
The percentage of HBsAg-positive samples tested for anti-HDV was similar across the years: 6.4% (36/564) in 2018, 8.1% (47/578) in 2019, and 8.9% (31/350) in 2020, with most anti-HDV determinations being requested by the academic hospital (32/36, 38/47, and 21/31, respectively).
Implementation of anti-HDV reflex testing
Reflex anti-HDV testing was established in January 2021, and data were collected from January 2021 to December 2021. In this prospective analysis, 93% (691/744) of HBsAg-positive samples were tested for anti-HDV: 91% (533/586) from the academic hospital and 100% (158/158) from primary care.
The overall prevalence of anti-HDV was 8.1% (56/691). This value was similar to the 9.6% (11/114) prevalence observed before the start of reflex testing (2018–2020). However, the absolute number of patients diagnosed with HDV increased from 11 cases in the 3 years before reflex testing to 56 cases in 2021 (Fig. 1).
Fig. 1.
Number of anti-HDV testing and positive cases among HBsAg-positive samples.
Anti-HDV, anti-hepatitis D virus antibodies.
Epidemiological and clinical characteristics of anti-HDV-positive individuals in our setting
HDV-RNA was determined in 54 of the 67 anti-HDV-positive patients, and 35 (65%) of them had detectable HDV-RNA. Among the total of anti-HDV-positive patients, most were young Caucasian males, 48% had normal alanine aminotransferase (ALT) levels, 60% advanced fibrosis, and 34% liver cirrhosis. Nine patients were co-infected with HCV, and 1 had detectable HCV-RNA. Four patients were co-infected with HIV; all were receiving antiretroviral therapy. The main epidemiological, clinical, serological, and virological characteristics of anti-HDV-positive individuals are shown in Table 1. Those with detectable HDV-RNA had significantly lower platelet levels, higher ALT, aspartate aminotransferase, and gamma glutamyl transferase levels, and more often advanced fibrosis and liver cirrhosis than those with undetectable HDV-RNA. In addition, patients with detectable HDV-RNA showed statistically lower HBsAg levels and had undetectable HBV-DNA more often. In addition, a considerable pool of patients (39%) had detectable but unquantifiable HBV-DNA (<20 IU/ml).
Table 1.
Demographic, serologic, virologic, and clinical data of all anti-HDV-positive cases with HDV-RNA determination.
| Anti-HDV-positive with HDV-RNA determination (N = 54) |
HDV-RNA detectable (n = 35)∗ |
HDV-RNA undetectable (n = 19)∗ |
p value | |
|---|---|---|---|---|
| Age, years | 44 (35–56) | 44 (35–54) | 46 (38–57) | 0.244 |
| Male, n (%) | 32 (59) | 19 (54) | 13 (68) | 0.237 |
| Ethnicity, n (%) | 0.085 | |||
| Caucasian | 33 (59) | 23 (66) | 10 (53) | |
| African | 15 (28) | 7 (20) | 8 (42) | |
| Asian | 6 (11) | 5 (14) | 1 (5) | |
| Risk factors, n (%) | 0.053 | |||
| Blood-borne (including PWID) | 11 (20) | 11 (31) | 0 (0) | |
| HDV endemic country | 11 (20) | 8 (23) | 3 (16) | |
| Unknown | 32 (60) | 16 (46) | 16 (84) | |
| Platelets, ×109/L | 167 (115.5–217.5) | 147 (104–187) | 208 (145–251) | 0.013 |
| ALT, IU/ml | 49 (27.5–78) | 64 (49–119) | 28 (19–38) | <0.001 |
| Normal ALT, n (%) | 23 (43) | 8 (23) | 15 (79) | <0.001 |
| AST, IU/ml | 41 (28–83) | 63 (39–115) | 28 (23–32) | <0.001 |
| GGT, IU/ml | 43 (25.5–75) | 56 (28–83) | 28 (22–47) | 0.003 |
| Non-invasive markers | ||||
| Transient elastography, kPa | 9.5 (6.1–15) | 12 (8–16.4) | 7.8 (5.6–11) | 0.024 |
| FIB-4 | 1.7 (1.3–3.9) | 2.1 (1.3–5.0) | 1.4 (0.9–1.9) | 0.028 |
| APRI | 0.7 (0.4–1.8) | 1.3 (0.6–3.2) | 0.4 (0.3–0.6) | <0.001 |
| Advanced fibrosis, n (%)∗ | 17 (32) | 10 (29) | 7 (37) | 0.044 |
| Liver cirrhosis, n (%)∗ | 19 (35) | 17 (49) | 2 (11) | 0.014 |
| HBsAg, log IU/ml | 3.7 (3.4–4.1) | 3.9 (3.5–4.2) | 3.2 (2.3–3.5) | 0.001 |
| HBeAg, n (%) | 4 (7) | 4 (11) | 0 (0) | 0.193 |
| HBV-DNA, IU/ml | 3.1 (2.5–4.5) | 2.7 (2.4–4.2) | 3 (2.5–4.1) | 0.602 |
| HBV-DNA undetectable, n (%) | 15 (28) | 9 (26) | 6 (32) | 0.043 |
| HBV-DNA detectable not quantifiable, n (%) | 22 (63) | 20 (57) | 2 (11) | |
| HBV-DNA detectable, n (%) | 17 (31) | 6 (17) | 11 (58) | <0.001 |
| HDV-RNA, log IU/ml∗ | 4.9 (4.5–5.7) | n.a. | n.a. | n.a. |
Quantitative variables are expressed are median and inter-quartile range and qualitative variables as number and percentage.
ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; FIB-4, fibrosis-4; GGT, gamma glutamyl transferase; n.a., not applicable, PWID, people who inject drugs.
Determined in 50 patients.
Discussion
Analysis of laboratory testing from 2018 to 2020 found that screening for HDV infection was low in our setting. Anti-HDV antibodies were tested in only a small proportion of HBsAg-positive samples: <10% in a catchment population of 450,000 inhabitants in Barcelona. Most screening requests (∼80%) came from the academic hospital, with very few requests from primary care centres, even though these tests were available. This suggests that awareness of HDV infection is low among primary care physicians. The HDV screening rate was similar over the 3 years analysed except during the COVID-19 pandemic, in which the number of HBsAg-positive samples decreased, particularly during the first wave of the infection, when a lockdown of more than 3 months was declared (15 March–21 June). Significant decreases in HBV and HCV testing during the start of the COVID-19 pandemic have also been reported.10
The low HDV screening rate diverged from the 2017 EASL recommendations for the management of hepatitis B, which encourage anti-HDV testing in all HBsAg-positive cases regardless of the presence of risk factors or severity of the liver disease. In a previous oral communication, our group reported that the HDV testing rate before publication of the EASL guidelines was even lower in our setting, with only a slight increase after the guidelines emerged (7.5% vs. 9.4%, p = 0.015).11
Better results were seen in the large Bordeaux Metropolis Without Viral Hepatitis programme. The anti-HDV testing rate was 49% in 988 HBsAg-positive patients, and 10% (50/483) of them were anti-HDV positive. HDV-RNA was tested in 39 cases. The higher HDV screening rate in the Bordeaux study compared with that of our review before reflex testing is likely attributable to the existence of an already established programme in Bordeaux for this purpose, whereas HDV testing was requested at the physicians’ discretion in our hospital. However, despite the programme, half the HBsAg-positive samples in the Bordeaux study remained untested and potentially undiagnosed.
A study performed in a London centre with clinic-led anti-HDV testing reviewed the clinical and laboratory data of 168 HBsAg-positive patients and reported a 40% (67/168) prevalence of anti-HDV-positive status.12 These reports, along with our findings, highlight the need for health policy initiatives aimed at improving HDV screening, especially relevant since the arrival of new HDV drugs.
There was a considerable difference in the diagnostic yield in our setting following implementation of HDV reflex testing, which led to a 5-fold increase in the number of HBV cases diagnosed with hepatitis D. The increase was similar in both the academic hospital and primary care centres. Reflex testing has been evaluated in other viral infections such as hepatitis C, particularly for viraemia detection. With this approach, additional blood drawn for HCV-RNA testing and an appointment for the results can be avoided in anti-HCV-positive cases.13 A study in the emergency department of an academic hospital showed that reflex testing enables detection of active HCV infection in a single sample.14 The authors reported a 0.7% prevalence of active HCV infection, a value almost 3-fold higher than that seen in the general population. Anti-HDV reflex testing has been evaluated in a single centre in London, but the results obtained were not compared with prior routine testing.12
The profile of our anti-HDV-positive patients is in line with the findings from other European cohorts.[15], [16], [17] A considerable number were migrants from countries where HDV infection is endemic (20%) or patients with blood-borne risk factors (20%). Risk factors were unknown in the remaining 60%, which further supports the rationality of systematic anti-HDV reflex testing in all HBsAg-positive patients, rather than testing according to risk factors. In addition, those with detectable HDV-RNA showed higher ALT levels and more often had cirrhosis,18 and viraemic patients had lower HBV-DNA levels, supporting the theory of HDV suppression over HBV.19
The main limitation of our study is the scarce data regarding risk factors for hepatitis D in HBsAg-positive cases, making it difficult to assess whether HDV testing was mainly requested in high-risk groups, as recommended in the AASLD guidelines. However, the increase in the number of HDV cases detected by reflex testing suggests that not all patients with HDV risk factors had been routinely screened. Finally, it would be of value to assess the cost-effectiveness of establishing reflex testing for this purpose.
In summary, HDV testing rates in daily clinical practice are low despite EASL guidelines recommending universal screening. HDV infection is associated with significant liver-related mortality; hence, the importance of establishing anti-HDV reflex testing algorithms for an early HDV diagnosis.
Financial support
This study received support in part from the Instituto de Salud Carlos III (PI20/01692).
Authors’ contributions
Guarantor of the article and responsibility for the integrity of the work as a whole, from inception to published article: MB. Study design: MB, RE, FRF. Collected the clinical and laboratory data: AP, AR, EV, JV, ABD. Performed laboratory testing: AR, BP. Carried out the analysis and interpretation of data: MRB, MB, AP, AR. Drafted the manuscript: MB, AP. Reviewed the manuscript: MB, MRB, RE, FRF. Approved the final version of the article: all authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of interest
MB has served as a speaker and advisory board member for Gilead, Roche, and Arbutus. RE has served as a speaker and advisory board member for Gilead. MRB has served as a speaker for Abbvie and Gilead.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100547.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Farci P., Niro G.A. Clinical features of hepatitis D. Semin Liver Dis. 2012;32:228–236. doi: 10.1055/s-0032-1323628. [DOI] [PubMed] [Google Scholar]
- 2.Puigvehí M., Moctezuma-Velázquez C., Villanueva A., Llovet J.M. The oncogenic role of hepatitis delta virus in hepatocellular carcinoma. JHEP Rep. 2019;1:120–130. doi: 10.1016/j.jhepr.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzetto M., Ciancio A. Epidemiology of hepatitis D. Semin Liver Dis. 2012;32:211–219. doi: 10.1055/s-0032-1323626. [DOI] [PubMed] [Google Scholar]
- 4.Rizzetto M., Hamid S., Negro F. The changing context of hepatitis D. J Hepatol. 2021;74:1200–1211. doi: 10.1016/j.jhep.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Husa P., Linhartová A., Nemecek V., Husová L. Hepatitis D. Acta Virol. 2005;49:219–225. [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Sarin S.K., Kumar M., Lau G.K., Abbas Z., Chan H.L.Y., Chen C.J., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyne M.T., Mallory M.A., Xie H.B., Mei Y., Schlaberg R., Hillyard D.R. Sequencing of the hepatitis D virus RNA WHO international standard. J Clin Virol. 2017;90:52–56. doi: 10.1016/j.jcv.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Mandel E., Peci A., Cronin K., Capraru C.I., Shah H., Janssen H.L.A., et al. The impact of the first, second and third waves of Covid-19 on hepatitis B and C testing in Ontario, Canada. J Viral Hepat. 2021;29:205–208. doi: 10.1111/jvh.13637. [DOI] [PubMed] [Google Scholar]
- 11.Palom A., Rando-Segura A., Riveiro-Barciela M., Barreira-Diaz A., Rodríguez-Frías F., Esteban-Mur E., et al. Low adherence to guidelines recommendation for testing hepatitis D in HBsAg-positive patients leads to a high rate of undiagnosis. Oral presentation. Hepatology. 2021;74 148A–9A. [Google Scholar]
- 12.El Bouzidi K., Elamin W., Kranzer K., Irish D.N., Ferna B., Kennedy P., et al. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol. 2015;66:33–37. doi: 10.1016/j.jcv.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Pawlotsky J.M., Negro F., Aghemo A., Berenguer M., Dalgard O., Dusheiko G., et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Llaneras J., Barreira-Díaz A., Rando-Segura A., Bañares J., Meza B., Ruiz L., et al. Simultaneous hepatitis B and C screening in an emergency department: a broad linkage to care strategy. Poster presentation. Hepatology. 2021;74:761A. [Google Scholar]
- 15.Stroffolini T., Sagnelli E., Sagnelli C., Russello M., De Luca M., Rosina F., et al. Hepatitis delta infection in Italian patients: towards the end of the story? Infection. 2017;45:277–281. doi: 10.1007/s15010-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 16.Ordieres C., Navascues C.A., Gonzalez-Dieguez M.L., Rodríguez M., Cadahía V., Varela M., et al. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: a report from Northern Spain. Eur J Gastroenterol Hepatol. 2017;29:277–283. doi: 10.1097/MEG.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 17.Sperle I., Steffen G., Leendertz S.A., Sarma N., Beerman S., Thamm R., et al. Prevalence of Hepatitis B, C, and D in Germany: results from a scoping review. Front Public Health. 2020;8:424. doi: 10.3389/fpubh.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palom A., Rodríguez-Tajes S., Navascués C.A., García-Samaniego J., Riveiro-Barciela M., Lens S., et al. Long-term clinical outcomes in patients with chronic hepatitis delta: the role of persistent viraemia. Aliment Pharmacol Ther. 2020;51:158–166. doi: 10.1111/apt.15521. [DOI] [PubMed] [Google Scholar]
- 19.Huang C., Lo S.J. Hepatitis D virus infection, replication and cross-talk with the hepatitis B virus. World J Gastroenterol. 2014;20:14589–14597. doi: 10.3748/wjg.v20.i40.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.