Abstract
Background
It is well known that toll-like receptor 2 (TLR2) mediates responses of both innate and adaptive immunity to microbial pathogen, including mycobacteria. Single-nucleotide polymorphisms (SNPs) in the TLR2 gene that impair its function may be associated with the development of pulmonary tuberculosis (PTB). The aim of this study was to evaluate the possible association between TLR2 Arg677Trp and 597T/C polymorphisms and PTB in a sample of Iranian population.
Materials and methods
This case–control study was performed on 174 PTB and 177 healthy subjects. Tetra amplification refractory mutation system-polymerase chain reaction (T-ARMS-PCR) was used to detect the SNPs.
Results
There was no significant difference in the polymorphism of Arg677Trp of the TLR2 gene among PTB and control groups (p > 0.05). The results showed that there was a significant difference between case and control groups regarding 597T/C polymorphism (χ2 = 12.21, p = 0.002). The TC and CC genotypes were found to be associated with the risk of PTB (OR = 2.13, 95% CI = 1.25–3.62, p = 0.005 and OR = 4.88, 95% CI = 1.56–15.26, p = 0.007, respectively).
Conclusion
Our data suggest that 597T/C polymorphism, but not Arg677Trp polymorphism, of the TLR-2 gene is a risk factor for susceptibility to PTB in a sample of Iranian population.
Keywords: Tuberculosis, TLR2, Single nucleotide polymorphism
Introduction
Tuberculosis, caused by Mycobacterium tuberculosis, is a global public health problem throughout the world especially in Asia and Africa. Approximately nine million new cases were reported in 2008 worldwide (with an incidence of 139/100,000 inhabitants), of which more than one million died.1 It can affect almost all of the body, including the brain, the kidneys and the bones, but predominately manifests itself in the lungs where it is named pulmonary tuberculosis. One-third of the earth's population is infected with tuberculosis (TB) but only 10% of infected individuals will develop the disease. Increasing evidence indicates that host genetic factors play an important role in susceptibility to TB.2
Toll-like receptors (TLRs) are important mediators of the inflammatory response in the first line of host defense by recognition of many pathogen-related molecules and endogenous proteins associated with immune activation.3, 4, 5, 6, 7 TLRs are classified as members of the IL-1R super-family based on a shared cytoplasmic region known as the TIR (Toll/IL-1R) domain.8 TLRs are cell-surface receptors that induce a signal in the affected cell, involving a number of proteins, such as MyD88, IL-1 receptor-associated with extracellular leucine-rich domains and an intracellular signaling domain and are found in monocytes, macrophages and neutrophils.9 TLRs have been implicated in the activation of macrophage by a variety of chemically diverse bacterial products (lipopolysaccharide (LPS), lipoproteins and peptidoglycans)10, 11 and kinase and p38 mitogen-activated protein kinase (MAPK).12, 13, 14, 15 This signaling cascade leads to NF-κB activation, which induces the secretion of several proinflammatory cytokines16 and has been reported to be the chief mediator of macrophage activation in response to mycobacteria.17 In vitro studies have revealed that TLR2 activation directly leads to intracellular killing of M. tuberculosis by alveolar macrophages.18 High susceptibility to M. tuberculosis infection of TLR2-deficient mice19, 20 suggests that mutations affecting TLR2 expression may impair host response to this pathogen. In contrast, Sugawara et al. have found that TLR2 does not play an important role in the pathogenesis of murine tuberculosis, though it is important for defense against mycobacterial infection.21 In addition it has been reported that MyD88 deficiency did not influence the development of murine tuberculosis.22
To date, there is little and controversial data regarding the impact of TLR2 polymorphism and susceptibility to PTB. It has been shown that TLR2 gene (Arg677Trp, Arg753Gln) polymorphisms were not associated with susceptibility to TB.23 Ben-Ali et al.24 have found that TLR2 Arg677Trp polymorphism is a predisposing risk factor for PTB. A significant association between TLR2 597T/C polymorphism and tuberculosis has been found.25 Therefore, the present study aimed to evaluate the possible association between Arg677Trp and 597T/C polymorphisms of TLR2 and pulmonary tuberculosis in a sample of Iranian population.
Materials and methods
This case–control study was performed in the Research Center for Infectious Diseases and Tropical Medicine, Bou-Ali Hospital, Zahedan, Iran. A total of 174 patients with pulmonary tuberculosis and 177 unrelated healthy subjects were enrolled in the study. The groups were matched for sex and age. The subjects who underwent treatment for PTB and newly diagnosed PTB cases were enrolled in the study within the case group. The diagnosis of PTB was based on clinical, radiological, sputum Acid Fast Bacillus (AFB) smear positivity, culture, and response to antituberculosis chemotherapy as described previously.26, 27 Controls were selected from the Zahedan population who participated in a metabolic syndrome project and have no recent signs, symptoms or history of pulmonary tuberculosis.
The project was approved by the local Ethics Committee of the Zahedan University of Medical Sciences, and written informed consent was taken from all participants. Blood samples were collected in Na-EDTA tubes from patients and healthy controls and stored at −20 °C until DNA extraction.
Genomic DNA extraction from blood samples was carried out as described previously.28 SNPs genotyping of TLR2 Arg677Trp and 597T/C polymorphisms were performed using tetra-ARMS-PCR, which is a simple and rapid method for detection of SNP.29, 30, 31, 32 In this method two external primers (control band) and two inner primers (allele specific primers) are used as shown in Table 1.
Table 1.
Primers used for polymorphism determination of TLR2.
| Primers | C2029T (Arg677Trp) | 597T/C (rs3804099) |
|---|---|---|
| Forward inner | 5′-CCCTTCAAGTTGTGTCTTCATACGT-3′ | 5′-CCAAAAAGTTTGAAGTCAATTCAGCAT-3′ |
| Reverse inner | 5′-TTGCCAGGAATGAAGTCACG-3′ | 5′-TCATATGAAGGATCAGATGACTTCCG-3′ |
| Forward outer | 5′-CTGTGCTCTGTTCCTGCTGATC-3′ | 5′-ATTGCAAATCCTGAGAGTGGGAA-3′ |
| Reverse outer | 5′-TGAGAATGGCAGCATCATTGTT-3′ | 5′-CAAACTTTCATCGGTGATTTTCACA-3′ |
For TLR2 Arg677Trp polymorphism the product sizes were: 199 bp for T allele, 264 bp for C allele and 419 bp for two outer primers (control band). The product sizes for detection of 597T/C polymorphism were: 173 bp for C allele, 228 bp for T allele and 349 bp for control band.
Polymerase chain reaction (PCR) was performed using commercially available PCR premix (AccuPower PCR PreMix, BIONEER, Daejeon, South Korea) according to the manufacturer recommended protocol. Into a 0.2-mL PCR tube containing the AccuPower PCR Pre-Mix, 1 μL template DNA (∼100 ng/μL), 1 μL of each primer (10 μM) and 15 μL DNase-free water were added.
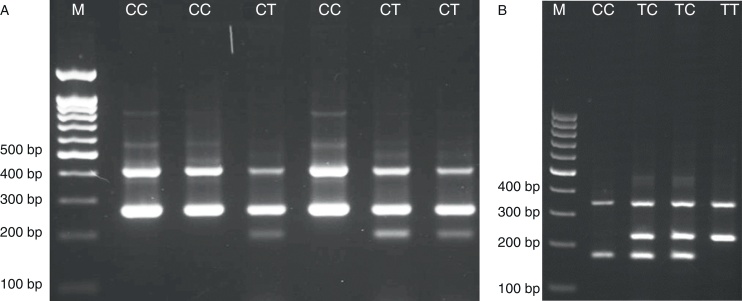
The PCR cycling condition for 597T/C was 5 min at 95 °C followed by 30 cycles of 30 s at 95 °C, 30 s at 53 °C and 30 s at 72 °C. The PCR condition for detection of Arg677Trp polymorphism was 95 °C for 5 min followed by 30 cycles, consisting of denaturation at 95 °C for 40 s, annealing at 55 °C for 20 s, and extension at 72 °C for 45 s with a final step at 72 °C for 10 min to allow for complete extension of all PCR fragments. The PCR products were analyzed by electrophoresis on a 2% agarose gel containing 0.5 μg/ml ethidium bromide and visualized by transillumination with UV light and photograph was taken (Fig. 1). We regenotyped approximately 20% of the samples to verify the initial results. The check confirmed the previous genotyping results by 100%.
Fig. 1.
Photograph of the PCR products of the TLR2 Arg677Trp (rs1695 A/G) gene (A) and 597T/C polymorphism (B). M, DNA marker.
Statistical analysis
The statistical analysis of the data was performed using the SPSS 18.0 software. Demographics and biochemical parameters between the groups were analyzed by independent sample t-test for continuous data and χ2 test for categorical data. The associations between genotypes and PTB were estimated by computing the odds ratio (OR) and 95% confidence intervals (95% CI) from logistic regression analyses.
Results
Of the 174 study patients, 110 (63.2%) were female and 64 (36.8%) male. In uninfected group, 97 (54.8%) were female and 80 (45.2%) were male. The mean ± SD ages for cases and controls were 50.17 ± 20.47 and 46.88 ± 15.45, respectively. There was no significant difference among the groups regarding sex and age (p > 0.05).
The genotype and allele frequencies of TLR2 polymorphisms in patient and control groups were summarized in Table 2.
Table 2.
The genotypes and allele distribution of TLR2 polymorphism in case (pulmonary tuberculosis) and control groups.
| Polymorphism | Case, n (%) | Control, n (%) | OR (95% CI) | p | aOR (95% CI) | p |
|---|---|---|---|---|---|---|
| 2029C/T (Arg677Trp) | ||||||
| CC | 134 (77.0) | 148 (83.6) | Reference | Reference | ||
| CT | 40 (23.0) | 29 (16.4) | 1.52 (0.90–2.59) | 0.121 | 1.51 (0.88–2.58) | 0.135 |
| TT | 0 (0) | 0 (0) | – | – | – | – |
| Alleles | ||||||
| C | 308 (88.5) | 325 (91.8) | ||||
| T | 40 (11.5) | 29 (8.2) | 1.45 (0.88–2.41) | 0.163 | ||
| 597T/C | ||||||
| Codominant | ||||||
| TT | 27 (15.5) | 52 (29.4) | Reference | Reference | ||
| TC | 134 (77.0) | 120 (67.8) | 2.15 (1.27–3.64) | 0.004 | 2.13 (1.25–3.62) | 0.005 |
| CC | 13 (7.5) | 5 (2.8) | 5.0 (1.62–15.52) | 0.005 | 4.88 (1.56–15.26) | 0.007 |
| Dominant | ||||||
| TT | 27 (15.5) | 52 (29.4) | Reference | Reference | ||
| TC + CC | 147 (84.5) | 125 (70.6) | 2.27 (1.34–3.82) | 0.002 | 2.24 (1.32–3.79) | 0.003 |
| Recessive | ||||||
| TT + TC | 161 (92.5) | 172 (97.2) | Reference | Reference | ||
| CC | 13 (7.5) | 5 (2.8) | 2.78 (0.97–7.97) | 0.057 | 2.72 (0.94–7.86) | 0.065 |
| Alleles | ||||||
| T | 188 (54.0) | 224 (63.2) | ||||
| C | 160 (46.0) | 130 (36.8) | 1.5 (1.1–2.0) | 0.014 | ||
Adjusted for age and sex.
The wild-type genotype 677 Arg/Arg was observed in 134 (47.0%) of the patients, whereas 40 (23.0%) were heterozygous (677 Arg/Trp). In the control group, the frequencies of genotypes were 148 (83.6%) for Arg/Arg and 29 (16.4%) for Arg/Trp. No one in either group was homozygous for the mutant genotype (Trp/Trp). No significant association was found between the groups regarding 677 Arg/Trp (C/T) polymorphism of TLR2 (OR = 1.52, 95% CI = 0.88–2.58, p = 0.135).
Our finding showed that there was a significant difference between case and control groups regarding TLR2 597T/C polymorphism (χ2 = 12.21, p = 0.002). The TLR2 597T/C polymorphism was a risk predisposing factor for PTB in codominant (OR = 2.13, 95%CI = 1.25–3.62, p = 0.005, TT vs TC; OR = 4.88, 95% CI = 1.56–15.26, p = 0.007, TT vs CC) and dominant (OR = 2.24, 95% CI = 1.32–3.79, p = 0.003, TT vs TC-CC) tested inheritance models (Table 2).
Allele frequencies for T and C were found to be 0.54 and 0.46 in the PTB group and 0.63 and 0.37 in the control group, respectively. The C allele was a predisposing risk factor for PTB (OR = 1.5, 95% CI = 1.1–2.0, p = 0.014).
Discussion
In the present study, we have investigated the impact of TLR2 polymorphisms at position 597T/C and missense mutation affecting the intracellular domain of human TLR2 Arg677Trp (C2029T) and susceptibility to PTB in a sample of Iranian population. A significant difference was found between patients with PTB and healthy controls regarding TLR2 597T/C polymorphism genotype and allelic distribution. No significant difference was found between the groups concerning Arg677Trp (C2029T) polymorphism in our population. In agreement with our finding Xue et al.23 have found no association between Arg677Trp polymorphism of TLR2 and PTB in the southeastern Chinese population. On the other hand, Schroder et al.33 have shown that no individual was identified carrying the Arg677Trp polymorphism in Germany. In contrast to our findings Ben-Ali et al.24 have found that TLR2 Arg677Trp polymorphism (C2029T) is a predisposing risk factor for PTB. In line with the present study, they found no subject in either group to be homozygous for the mutant genotype (Trp/Trp).
Concerning the 597T/C, Thuong et al.25 have found that 597CC genotype was associated with tuberculosis in Vietnam. Our results are consistent with their finding. The 597TC and CC genotypes were found to be associated with PTB risk in our population.
Caws et al.34 have found that individuals with the 597C allele of TLR-2 were more likely to have tuberculosis caused by the East-Asian/Beijing genotype than other individuals.
Lorenz et al.35 reported a single nucleotide polymorphism in the TLR2 gene (arginine to glutamine) substitution at residue 753 (Arg753Gln) that leads to a decreased response of macrophages to bacterial peptides, resulting in an attenuated immune response in the host. It has previously been demonstrated that the TLR2 polymorphism results in a decreased ability of macrophages to respond to several bacterial peptides.35
Ogus et al.36 have found that Arg753Gln polymorphism of the TLR2 gene influences the risk of developing tuberculosis. There is no clear explanation for these discrepancies, but may reflect differences in genetic background among the study populations.
Tuberculosis kills more people each year than any other single infectious disease. It has been proposed that development of tuberculosis disease depends on interaction between the pathogen, environment, and the host. Many studies support that the differences in host immune genes accompanies susceptibility/resistance to TB.37, 38
The host's immune response to M. tuberculosis is mainly mediated by a TLR2 signaling in macrophages, which leads to direct bactericidal effect by suppressing the proliferation of M. tuberculosis or induction of apoptosis of infected macrophages.39
The findings of this study might be limited by the relatively small sample size and therefore our limited statistical power analysis.
In conclusion, our findings in a sample of Iranian population provide evidence that 597T/C polymorphism, but not Arg677Trp polymorphism, of the TLR-2 gene is a risk factor for susceptibility to pulmonary tuberculosis.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by dissertation grant from Zahedan University of Medical Sciences. The authors thank the patients and healthy subjects who willingly participated in the study.
References
- 1.Orcau A., Cayla J.A., Martinez J.A. Present epidemiology of tuberculosis. Prevention and control programs. Enferm Infecc Microbiol Clin. 2011;29(Suppl 1):2–7. doi: 10.1016/S0213-005X(11)70011-8. [DOI] [PubMed] [Google Scholar]
- 2.Stein C.M. Genetic epidemiology of tuberculosis susceptibility: impact of study design. PLoS Pathog. 2011;7:e1001189. doi: 10.1371/journal.ppat.1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Underhill D.M., Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Schnare M., Barton G.M., Holt A.C., et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 7.Ozinsky A., Underhill D.M., Fontenot J.D., et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misch E.A., Hawn T.R. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 10.Means T.K., Jones B.W., Schromm A.B., et al. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 11.Supajatura V., Ushio H., Nakao A., et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 13.Sabroe I., Jones E.C., Usher L.R., et al. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 14.Sabroe I., Parker L.C., Wilson A.G., et al. Toll-like receptors: their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy. 2002;32:984–989. doi: 10.1046/j.1365-2745.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R., Preston-Hurlburt P., Kopp E., et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 16.Vancurova I., Miskolci V., Davidson D. NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J Biol Chem. 2001;276:19746–19752. doi: 10.1074/jbc.M100234200. [DOI] [PubMed] [Google Scholar]
- 17.Underhill D.M., Ozinsky A., Smith K.D., et al. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoma-Uszynski S., Stenger S., Takeuchi O., et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 19.Drennan M.B., Nicolle D., Quesniaux V.J., et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiling N., Holscher C., Fehrenbach A., et al. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara I., Yamada H., Li C., et al. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47:327–336. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugawara I., Yamada H., Mizuno S., et al. Mycobacterial infection in MyD88-deficient mice. Microbiol Immunol. 2003;47:841–847. doi: 10.1111/j.1348-0421.2003.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y., Zhao Z.Q., Wang H.J., et al. Toll-like receptors 2 and 4 gene polymorphisms in a southeastern Chinese population with tuberculosis. Int J Immunogenetics. 2010;37:135–138. doi: 10.1111/j.1744-313X.2009.00892.x. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ali M., Barbouche M.R., Bousnina S., et al. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin Diagn Lab Immunol. 2004;11:625–626. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thuong N.T., Hawn T.R., Thwaites G.E., et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8:422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 26.Naderi M., Hashemi M., Kouhpayeh H., et al. The status of serum procalcitonin in pulmonary tuberculosis and nontuberculosis pulmonary disease. J Pak Med Assoc. 2009;59:647–648. [PubMed] [Google Scholar]
- 27.Naderi M., Hashemi M., Mehdizadeh A., et al. Serum adenosine deaminase activity and total antioxidant capacity of plasma in pulmonary tuberculosis and non-tuberculosis pulmonary disease. Turk J Med Sci. 2010;40:701–706. [Google Scholar]
- 28.Hashemi M., Moazeni-Roodi A.K., Fazaeli A., et al. Lack of association between paraoxonase-1 Q192R polymorphism and rheumatoid arthritis in southeast Iran. Genet Mol Res. 2010;9:333–339. doi: 10.4238/vol9-1gmr728. [DOI] [PubMed] [Google Scholar]
- 29.Hashemi M., Moazeni-Roodi A.K., Fazaeli A., et al. The L55M polymorphism of paraoxonase-1 is a risk factor for rheumatoid arthritis. Genet Mol Res. 2010;9:1735–1741. doi: 10.4238/vol9-3gmr893. [DOI] [PubMed] [Google Scholar]
- 30.Ye S., Dhillon S., Ke X., et al. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:E88–E98. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naderi M., Hashemi M., Karami H., et al. Lack of association between rs1024611 (−2581 A/G) Polymorphism in CC-chemokine ligand 2 and susceptibility to pulmonary tuberculosis in Zahedan. Southeast Iran Prague Med Rep. 2011;112:272–278. [PubMed] [Google Scholar]
- 32.Hashemi M., Hoseini H., Yaghmaei P., et al. Association of polymorphisms in glutamate-cysteine ligase catalytic subunit and microsomal triglyceride transfer protein genes with nonalcoholic fatty liver disease. DNA Cell Biol. 2011;30:569–575. doi: 10.1089/dna.2010.1162. [DOI] [PubMed] [Google Scholar]
- 33.Schroder N.W., Hermann C., Hamann L., et al. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J Mol Med (Berl) 2003;81:368–372. doi: 10.1007/s00109-003-0443-x. [DOI] [PubMed] [Google Scholar]
- 34.Caws M., Thwaites G., Dunstan S., et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz E., Mira J.P., Cornish K.L., et al. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68:6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogus A.C., Yoldas B., Ozdemir T., et al. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 37.Sharma S., Kumar V., Khosla R., et al. Association of P2X7 receptor +1513 (AC) polymorphism with tuberculosis in a Punjabi population. Int J Tuberc Lung Dis. 2010;14:1159–1163. [PubMed] [Google Scholar]
- 38.Hashemi M., Sharifi-Mood B., Nezamdoost M., et al. Functional polymorphism of interferon-gamma (IFN-gamma) Gene +874T/A polymorphism is associated with pulmonary tuberculosis in Zahedan. Southeast Iran Prague Med Rep. 2011;112:38–43. [PubMed] [Google Scholar]
- 39.Yoshida A., Inagawa H., Kohchi C., et al. The role of toll-like receptor 2 in survival strategies of Mycobacterium tuberculosis in macrophage phagosomes. Anticancer Res. 2009;29:907–910. [PubMed] [Google Scholar]