Abstract
Objectives
The aim of this study was to monitor rotavirus (RV) infections in adults >18 years with acute gastroenteritis during 2004–2011 national Brazilian RV surveillance. In addition, to characterize the RV group A (RVA) strains in order to gain insight into the supposed vaccine selective pressure imposed to Brazilian children population.
Methods
A total of 2102 convenient fecal specimens were investigated by ELISA, PAGE, and RT-PCR.
Results
RV was detected in 203 (9.6%) of 2102 specimens, and showed a marked peak of detection in September. RVA infection was detected in 9.4% (197/2102) and RV group C (RVC) in 0.3% (6/2102). The most frequent genotypes detected in 2004 and 2005 were G9P[8] (38.5%; 5/13) and G1P[8] (54.5%; 6/11), respectively. The dominant genotype identified from 2006 to 2011 was G2P[4] (64.4%; 116/180). Detection rate varied during the 8-year period of the study from 0.7% to 12.9%.
Conclusion
The high detection rate of G2P[4] in adults provides further evidence that its dominance reflects the seasonality of RVA strains instead of the supposed selective advantage created by vaccination program. It also can be suggested that adult infections may serve as a reservoir to maintain RVA strains in childhood gastroenteritis. Considering the detection rate, the evident reduction of RVA frequency observed in children after vaccine introduction was not present in adults.
Keywords: Rotavirus group A, Rotavirus group C, Adults, Vaccination, Brazil
Introduction
Rotaviruses (RV) are double-stranded RNA (dsRNA) viruses comprising the genus Rotavirus in the family Reoviridae.1 Three of the seven identified groups of RV infect humans. Group A rotavirus (RVA), the most common cause of diarrhea in infants and young children throughout the world, accounts for the large percentage of pediatric hospitalizations.2 As a pathogen of adults, RVA has frequently been overlooked.3, 4 Some authors have reported RVA cases in elderly and immune compromised individuals.5, 6 Both VP7 and VP4, outer proteins layer, are used to further categorize RVA into G (Glycoprotein) and P (Protease) genotypes. The most common G types detected are G1, G2, G3, G4 and G9, while in P typing P[4], P[6] and P[8] are the most frequently isolated.7, 8
RVA infection and circulating genotypes in children in Brazil have been well characterized.9, 10 Nevertheless, limited information is available on the genotypes infecting adults.8, 11 In addition, a high prevalence of G2P[4] was lately reported in Brazil and linked with universal RVA vaccination program using a G1P[8] live oral vaccine suggesting that this monovalent vaccine possibly created conditions in which G2P[4] could acquire selective advantage over P[8] genotypes.12 Nevertheless, a temporal periodicity, within ≈10 year cyclic pattern of G2P[4] has also been observed in Brazil,13 and should have been considered as an alternative explanation to the increased detection of this genotype since 2006. The molecular characterization of RVA genotypes circulating in adults could help to solve this question.
Group B (RVB) and group C (RVC) rotavirus are morphologically identical to RVA, but their dsRNA presents distinct migration patterns on polyacrylamide gels and they are not detected by common enzyme immunoassays (EIAs) for RVA.14 RVB has been associated with outbreaks of diarrhea in adults in China, India, and Bangladesh.15, 16 RVC has been associated with either sporadic diarrheal illness or limited outbreaks of illness in various settings, 15, 17, 18 including Brazil.19, 20, 21, 22
The aim of this work was to monitor RV infections in adults >18 years old with acute gastroenteritis during 2004–2011 national Brazilian RV surveillance. Additionally, to characterize the RVA strains (G- and P-type) circulating in the adult community during this study period in order to gain insight into the supposed vaccine selective pressure imposed to Brazilian children population.
Material and methods
Fecal samples
This is a retrospective and descriptive study conducted from 2004 to 2011 with convenient sampling from surveillance specimen collected from adults >18 years old presenting acute gastroenteritis. Stool samples from patients with acute gastroenteritis were sent to the Enteric Diseases Laboratory of the Adolfo Lutz Institute, regional reference center for rotavirus surveillance, Ministry of Health – Brazil, and a member of Acute Diarrhea Disease Monitoring Program, State Department of Health. The aim of this program is the early detection of outbreaks of diarrhea with national scope. In this study, clinical samples from the following States were used: São Paulo (SP), Mato Grosso (MT), Mato Grosso do Sul (MS), Paraná (PR), Tocantins (TO), Goiás (GO), Santa Catarina (SC), and the Federal District (FD) (Fig. 1).
Fig. 1.
The states highlighted in black collected stool samples from patients with acute gastroenteritis, screened for rotavirus group A infection and sent to the Enteric Diseases Laboratory of the Adolfo Lutz Institute. Adolfo Lutz Institute is Regional Reference Center for rotavirus surveillance and a member of Acute Diarrhea Disease Monitoring Program with national scope.
Rotavirus detection
A total of 2102 stool samples were tested. RVA was detected using commercial immunoenzymatic assays (Premier™ Rotaclone®, Meridian Bioscience, Inc., USA; or RIDASCREEN® Rotavirus, RBiopharm AG, Darmstadt, Germany) performed according to the manufacturer's instructions. RVA ELISA negative samples were tested further for the presence of RVC by polyacrylamide gel electrophoresis (SDS–PAGE) according to standard procedures,23 and confirmed by RT-PCR assay.14
RNA extraction and RT-PCR
RVA and RVC dsRNA was extracted directly from stools using Trizol® reagent (Invitrogen, Carlsbad, CA, USA),24 according to the manufacturer's instructions or by QIAamp® Viral RNA Mini kit (Qiagen, Valencia, CA), and stored at −70 °C. The reverse transcriptase polymerase chain reactions (RT-PCR) for RVA VP7 and VP4 genes were performed according to the protocols previously described.25, 26 RT-PCR protocol used to amplify a fragment of the RVC VP6 gene was described by Gouvea et al.14
Results
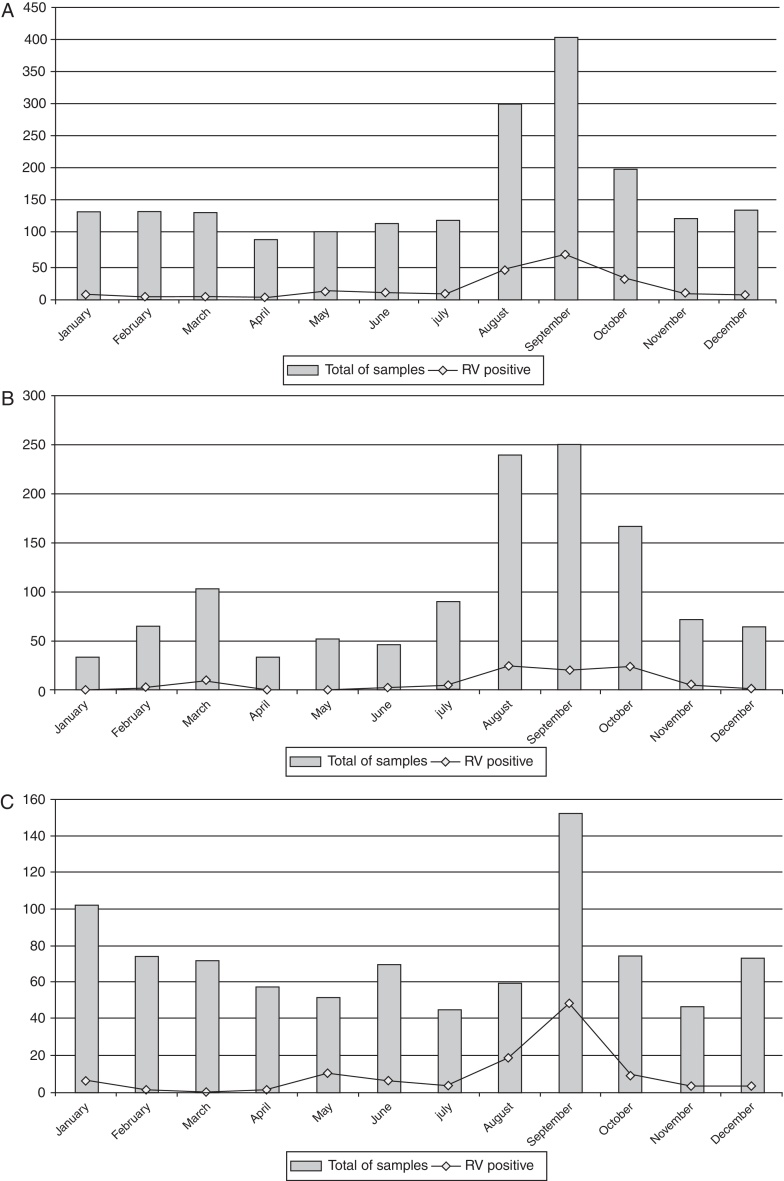
RV was detected in 203 (9.6%) of 2102 specimens collected from adults (Table 1). RV infection was found predominantly in the winter and in drier months, with peak in September (Fig. 2A). Temporal analysis of RV infection allowed the identification of two sections. The first section, between 2004 and 2007, did not reveal clear peak of RV detection, but rather a tendency of RV detection among August, September and, October (Fig. 2B). The second section, between 2008 and 2011, showed a marked peak of RV detection occurring in September (Fig. 2C). RVA infection was detected in 9.4% of the samples (197/2102) and RVC in 0.3% (6/2102). RVC cases were detected only in 2008 (3.4%; 6/178) (Table 1).
Table 1.
Rotavirus genotyping results from adults ≥18 years old presenting acute gastroenteritis, Brazil, 2004–2011.
| Rotavirus genotype | Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2004% (No.) | 2005% (No.) | 2006% (No.) | 2007% (No.) | 2008% (No.) | 2009% (No.) | 2010% (No.) | 2011% (No.) | Total % (No.) | |
| G1P[4] | – | – | 1.9% (1) | 25.0% (2) | – | – | – | – | 1.7% (3) |
| G1P[8] | 30.7% (4) | 54.5% (6) | 7.7% (4) | – | – | – | – | – | 7.8% (14) |
| G2P[4] | – | – | 59.7% (31) | 62.5% (5) | 77.3% (17) | – | 90.4% (57) | 60% (6) | 64.5% (116) |
| G3P[8] | – | 9.1% (1) | – | – | – | – | 4.8% (3) | – | 2.2% (4) |
| G9P[8] | 38.5% (5) | 9.1% (1) | 9.6% (5) | – | – | – | – | 10% (1) | 6.7% (12) |
| G2 + G9P[NT] | – | – | 3.8% (2) | – | – | – | – | – | 1.1% (2) |
| G2 + G3P[4] + [8] | – | – | – | – | – | – | 1.6% (1) | – | 0.5% (1) |
| G9P[NT] | 15.4% (2) | – | – | – | – | – | 1.6% (1) | – | 1.7% (3) |
| G1P[NT] | – | – | 5.8% (3) | – | – | – | – | – | 1.7% (3) |
| G2P[NT] | – | – | 1.9% (1) | – | 4.5% (1) | – | – | 20% (2) | 2.2% (4) |
| GNTP[8] | 7.7% (1) | – | – | – | – | – | – | – | 0.5% (1) |
| GNTP[4] | – | – | – | – | – | – | – | 10% (1) | 0.5% (1) |
| GNTP[NT] | 7.7% (1) | 27.3% (3) | 9.6% (5) | 12.5% (1) | 18.2% (4) | 100% (1) | 1.6% (1) | – | 8.9% (16) |
| Total of genotyped samples | 68.4% (13) | 91.7% (11) | 96.3% (52) | 80.0% (8) | 95.6% (22) | 100% (1) | 92.6% (63) | 100% (10) | 91.4% (180) |
| RT-PCR negative | 31.6% (6) | 8.3% (1) | 3.7% (2) | 20.0% (2) | 4.4% (1) | – | 7.4% (5) | – | 8.6% (17) |
| Total RVA ELISA positive | 7.8% (19) | 4.3% (12) | 11.2% (54) | 4.6% (10) | 12.9% (23) | 0.7% (1) | 20.0% (68) | 4.6% (10) | 9.4% (197) |
| RVC | – | – | – | – | 3.4% (6) | – | – | – | 0.3% (6) |
| Total of samples | 244 | 277 | 484 | 217 | 178 | 146 | 339 | 217 | 2102 |
NT, non-typed.
Fig. 2.
Temporal distribution of RV positive samples from adults ≥18 years old with acute gastroenteritis, Brazil, 2004–2011 (A), 2004–2007 (B), and 2008–2011 (C).
The median age for patients infected with the RVA was 41.5 years old, 63.4% being females. The median age for patients infected with the RVC was 33.3 years old, 66.7% being females. The most frequent genotypes detected in 2004 and 2005 were G9P[8] (38.5%; 5/13) and G1P[8] (54.5%; 6/11), respectively. The dominant genotype identified from 2006 to 2011 was G2P[4] (64.4%; 116/180). Detection rate varied during the 8-year period of the study: 7.8% (19/244) in 2004, 4.3% (12/277) in 2005, 11.2% (54/484) in 2006, 4.6% (10/217) in 2007, 12.9% (23/178) in 2008, 0.7% (1/146) in 2009, 20.0% (68/339) in 2010, and 4.6% (10/217) in 2011 (Table 1).
Discussion
This study was designed to investigate the frequency of RV infection in adults ≥18 years old with acute gastroenteritis and identified the RVA strains in order to investigate possible vaccine impact on the Brazilian pediatric population. In healthy adults, infection causes few or mild symptoms and RV infection is diagnosed via differential diagnosis. However, in immune compromised patients, infection can be severe and persistent.27
RVA spread occurs mainly through the fecal–oral route, with transmission to adults probably due to caring for sick children or ingestion of contaminated food or water.4 The peak in RV infections occurred in winter months as it is usually observed in most of the world28, 29, 30; and also mirror the winter RV seasonality of infection observed in pediatric patients.8, 31, 32, 33, 34
In tropical and subtropical settings, the peak of RV infections occurs in cool dry seasons.28 Strict winter seasonality was also common in the Americas,29 including Brazil's southern area,30 although sporadic RV cases and outbreaks can occur all along the year. However, a particular event was observed. The RV detection rate is usually concentrated between June and August in Brazil8, 31, 32, 33, 34; nevertheless, the frequency of RV detection in this study peaked in September, month that marks the beginning of the rainy season. The seasonal patterns and climatic sensitivities of many infectious diseases are well known35; and global warming is one of the environmental changes now under way. It can be suggested that this climatic variation could promote a range of impacts upon the occurrence of RV infection in the Brazilian population.
The frequency of RVA infection in adults detected in this work (9.4%) was lower than that observed in studies carried out in Japan (14%),3 United States (18%)36 and Paraguay (17.3%),37 but was similar to the frequency found in China (9%).38 A previous study conducted in Brazil showed that RVA rate among adults was 7.1%,8 comparable to the incidence observed in India (7%).39 The overall percentage of RVA detected among children in Brazil has been much higher (∼30%) than that found in adults.8, 9 A significant reduction in the frequency of RVA detection in children with gastroenteritis was observed in Brazil after the RVA vaccine introduction40; nonetheless, the present study showed that this decrease was not evident in adult inhabitants. On the other hand, Anderson et al.41 showed that the prevalence of RVA among adults declined from 4.35% in 2006–2007 (prepediatric impact era) to 2.24% in 2008–2010 (pediatric impact era) in USA, suggesting an indirect protection of adults from RVA by pediatric RVA vaccination.
Considering RVA annual frequency, a low rate of RVA infection was observed in 2009. A similar lack of a RVA season in Brazil during 2009 has been previously described.10, 42, 43 According to the National Meteorology Institute (INMET) records, during the year of 2009, Brazil experienced an atypical raining winter season with higher average rainfall record due to the El Niño phenomenon (http://www.inmet.gov.br). Therefore, this climatic condition could have been involved in the low detection frequency observed.42
A variety of RVA genotypes among adults was described all over the world.11, 14, 44, 45, 46, 47 Throughout the study period, G2P[4] was the most frequent strain in adults (64.5%). G1P[8] and G9P[8] were the most frequent types in 2004–2005, matching to the results of numerous studies focusing RVA G type distribution in many countries, including Brazil.8, 48 However these strains were displaced by G2P[4] during the 2006 season. Recently, a high prevalence of G2P[4] in children was reported in Brazil and linked to universal RV vaccination program using a G1P[8] live oral rotavirus vaccine.12 Nevertheless, the high detection rate of G2P[4] in adults provides further evidence that its predominance reflects the epidemic cycle of RVA strains instead of the supposed selective advantage created by the monovalent G1P[8] vaccination program. On the other hand, it also can be suggested that adult infections may serve as a reservoir to maintain RVA strains in childhood gastroenteritis. In addition, the same strains detected in adults during the study period were known to be circulating in children according to Laboratory records.
G4P[8] strain is one of the most common combinations found in humans worldwide,2 however, it was not detected in this study. At a global level, the weighted prevalence of G4 decreased slightly during 1996–2007.49 The seasonal shifts of RVA strains is a possible mechanism used by the virus to evade herd immunity acquired by previous infections, and thus, ultimately persist in the human population.50 The changing pattern of circulating RVA types associated with greatly reduced diversity of strains may have important implications for the evolution of RVA strains.10
It is worth mentioning that RVA genotyping by RT-PCR presents some limitations. RT-PCR is the method of choice for typing RVA and is regarded as the gold standard. However, the accumulation of point mutations at primer binding sites has been linked with mistyping or failure to identify genotype correctly. Regularly validation of PCR results with sequencing is important,51 especially in mixed RVA infections. In addition, in the present study RVA positive samples could not be genotyped by multiplex RT-PCR. Unfortunately, non-typeable RVA strains have been reported in almost every epidemiological survey around the world, regardless of the methodology employed.52
RVC infection in adults has received negligible attention. Only a few studies documented the RVC occurrence in adults, most of them based on serological evidence.16, 53, 54, 55 In Brazil, RVC was previously detected both in sporadic cases and outbreaks affecting adults.20, 21, 22 The RVC detection rate observed in this work (0.3%) was lower than that observed in a study carried out with adults in Sweden (∼1%).56 It is noted that the RVC incidence and associated disease remain unclear once sensitive tests for its detection are not available to clinical laboratories. The diagnosis is difficult since most of the EIA assays do not recognize the RVC specific VP6 antigen. The RT-PCR using RVC specific primer is a convenient option57; however, it has not been used widely due to the large costs for routine surveillance. The PAGE analysis of dsRNA requires the presence of 108–1010 viral particles/ml for a positive result57; nevertheless, this method has sufficient sensitivity to detect RVC with low costs.42
In conclusion, this study adds further evidence that RV is a minor cause of acute adult gastroenteritis in Brazil. On the other hand, contributes significantly to the understanding of RV infections in mature population. The results also suggest that adults are susceptible to the same types of RVA at the same season of the year as children. It is still unknown whether the burden of RVA in adults will remain unchanged subsequently after widespread impact of RVA vaccination observed in children.41 The finding of different genotypes in the adult population highlights the need for continuing surveillance in both pediatric and mature populations, to monitor the suitability of vaccines and to correlate vaccine efficacy with continuing virus evolution.4 The dynamics between pediatric and adult RVA infection remains unclear, and adult infections may serve as another source of RVA genomic diversity.
Ethical approval
This study was carried out in accordance with the Declaration of Helsinki as revised in 2000, and approved by the Ethics Committee of the Adolfo Lutz Institute, São Paulo. Study participants were not required to provide informed consent as this study was considered by the Ethics Committee to be part of routine surveillance activities.
Author contributions
AL and MCSTT conceived and designed the study; AL, AC, SGM, RCCC acquired the data, performed the immunoenzymatic assays, and the molecular typing of the strains; AL analyzed the data and wrote the manuscript; AC, SGM, RCCC and MCSTT critically revised the manuscript. All authors read and approved the final version. AL and MCSTT are guarantors of the paper.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the Enteric Diseases Laboratory of Adolfo Lutz Institute staff: Adeline M. Fernandes, Ana Paula T. Santos, Camila Goto, Carolina J. Gill, Cibele D. Ribeiro, Fernanda F. Costa, Heloisa R. Vieira, Raquel Guiducci and Samira J. Calux for laboratorial analysis assistance; Antonio Erculiani Junior and Sirlene Henrique Rodrigues Silva for technical assistance. We are grateful to Centers for Surveillance (CVE), São Paulo State Health Department; Public Health Laboratories (LACENs) and, CGLAB/DEVEP/SVS/Ministry of Health, Brasília for assistance in samples collection and epidemiological data. We thank Camila Buoro Auler for critical language review.
References
- 1.Bishop R.F. In: Viral infections of the gastrointestinal tract. 2nd ed. Kapikian A.Z., editor. Marcel Dekker; New York: 1994. Natural history of human rotavirus infections; pp. 131–167. [Google Scholar]
- 2.Parashar U.D., Gibson C.J., Bresse J.S., Glass R.I. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima H., Nakagomi T., Kamisawa T., et al. Winter seasonality and rotavirus diarrhoea in adults. Lancet. 2001;357:1950. doi: 10.1016/S0140-6736(00)05086-8. [DOI] [PubMed] [Google Scholar]
- 4.Gunn L., Feeney S.A., Cashman O., Collins P.J., Coyle P.V., O'Shea H. Molecular characterization of group A rotavirus found in elderly patients in Ireland: predominance of G1P[8], continued presence of G9P[8], and emergence of G2P[4] J Med Virol. 2012;84:2008–2017. doi: 10.1002/jmv.23416. [DOI] [PubMed] [Google Scholar]
- 5.Marshall J., Botes J., Gorrie G., et al. Rotavirus detection and characterisation in outbreaks of gastroenteritis in aged-care facilities. J Clin Virol. 2003;28:331–340. doi: 10.1016/s1386-6532(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 6.Morillo S.G., Luchs A., Cilli A., Carmona R.C., Neme S.N., Timenetsky M.C. Rotavirus genotype G4P[8] and enteric adenovirus in HIV-positive patients with and without diarrhoea in São Paulo State, Brazil. Trans R Soc Trop Med Hyg. 2010;104:165–167. doi: 10.1016/j.trstmh.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Andreasi M.S., Batista S.M., Tozetti I.A., et al. Rotavirus A among hospitalized infants, up to three years of age, with acute gastroenteritis in Campo Grande, State of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2007;40:411–414. doi: 10.1590/s0037-86822007000400008. [DOI] [PubMed] [Google Scholar]
- 8.Carmona R.C., Timenetsky Mdo C., Morillo S.G., Richtzenhain L.J. Human rotavirus serotype G9, São Paulo, Brazil, 1996–2003. Emerg Infect Dis. 2006;12:963–968. doi: 10.3201/eid1206.060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morillo S.G., Luchs A., Cilli A., Costa F.F.C., Carmona R.C.C., Timenetsky M.C.S.T. Characterization of rotavirus strains from day care centers: pre- and post-rotavirus vaccine era. J Pediatr (Rio J) 2010;86:155–158. doi: 10.2223/JPED.1981. [DOI] [PubMed] [Google Scholar]
- 10.Dulgheroff A.C., Figueiredo E.F., Moreira L.P., et al. Distribution of rotavirus genotypes after vaccine introduction in the Triângulo Mineiro region of Brazil: 4-year follow-up study. J Clin Virol. 2012;55:67–71. doi: 10.1016/j.jcv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Timenetsky M.C., Gouvea V., Santos N., Alge M.E., Kisiellius J.J., Carmona R.C. Outbreak of severe gastroenteritis in adults and children associated with type G2 rotavirus. Study Group on Diarrhea of the Instituto Adolfo Lutz. J Diarrhoeal Dis Res. 1996;14:71–74. [PubMed] [Google Scholar]
- 12.Nakagomi T., Cuervas L.E., Gurger R.G., et al. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after introduction of rotavirus vaccine. Arch Virol. 2008;153:591–593. doi: 10.1007/s00705-007-0028-z. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho-Costa F.A., Assis R.M., Fialho A.M., et al. Detection and molecular characterization of group A rotavirus from hospitalized children in Rio de Janeiro, Brazil, 2004. Mem Inst Oswaldo Cruz. 2006;101:291–294. doi: 10.1590/s0074-02762006000300012. [DOI] [PubMed] [Google Scholar]
- 14.Gouvea V., Allen J.R., Glass R.I., et al. Detection of group B and C rotaviruses by polymerase chain reaction. J Clin Microbiol. 1991;29:519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adah M.I., Wade A., Oseto M., Kuzuya M., Taniguchi K. Detection of human group C rotaviruses in Nigeria and sequence analysis of their genes encoding VP4, VP6, and VP7 proteins. J Med Virol. 2002;66:269–275. doi: 10.1002/jmv.2141. [DOI] [PubMed] [Google Scholar]
- 16.Iturriza-Gómara M., Clarke I., Desselberger U., Brown D., Thomas D., Gray J. Seroepidemiology of group C rotavirus infection in England and Wales. Eur J Epidemiol. 2004;19:589–595. doi: 10.1023/b:ejep.0000032381.36658.cb. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M., Banik S., Faruque A.S., et al. Detection and characterization of human group C rotaviruses in Bangladesh. J Clin Microbiol. 2005;43:4460–4465. doi: 10.1128/JCM.43.9.4460-4465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitui M.T., Bozdayi G., Dalgic B., Bostanci I., Nishizono A., Ahmed K. Molecular characterization of a human group C rotavirus detected first in Turkey. Virus Genes. 2009;39:157–164. doi: 10.1007/s11262-009-0420-8. [DOI] [PubMed] [Google Scholar]
- 19.Gabbay Y.B., Mascarenhas J.D., Linhares A.C., Freitas R.B. Atypical rotavirus among diarrhoeic children living in Belém, Brazil. Mem Inst Oswaldo Cruz. 1989;84:5–8. doi: 10.1590/s0074-02761989000100002. [DOI] [PubMed] [Google Scholar]
- 20.Timenetsky Mdo C., Kisielius J.J., Grisi S.J., Escobar A.M., Ueda M., Tanaka H. Rotavirus, adenovirus, astrovirus, calicivirus and small round virus particles in feces of children with and without acute diarrhea, from 1987 to 1988, in the greater São Paulo. Rev Inst Med Trop Sao Paulo. 1993;35:275–280. [PubMed] [Google Scholar]
- 21.Souza D.F., Kisielius J.J., Ueda M., et al. An outbreak of group C rotavirus gastroenteritis among adults living in Valentim Gentil, São Paulo State, Brazil. J Diarrhoeal Dis Res. 1998;16:59–65. [PubMed] [Google Scholar]
- 22.Luchs A., Morillo S.G., Kisielius J.J., Ueda M., Carmona Rde C., Timenetsky Mdo C. Group C rotavirus, detection in Southeastern Brazil after 15 years. J Clin Virol. 2009;46:389–390. doi: 10.1016/j.jcv.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Pereira H.G., Azeredo R.S., Leite J.P., et al. Electrophoretic study of the genome of human rotaviruses from Rio de Janeiro, São Paulo and Pará, Brazil. J Hyg (Lond) 1983;90:117–125. doi: 10.1017/s0022172400063919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Gouvea V., Glass R.I., Woods P., et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentsch J.R., Glass R.I., Woods P., et al. Identification of a group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson E.J., Weber S.G. Rotavirus infection in adults. Lancet Infect Dis. 2004;4:91–99. doi: 10.1016/S1473-3099(04)00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashizume M., Armstrong B., Wagatsuma Y., Faruque A.S., Hayashi T., Sack D.A. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect. 2008;136:1281–1289. doi: 10.1017/S0950268807009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy K., Hubbard A.E., Eisenberg J.N.S. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira H.G., Linhares A.C., Candeias J.A.N., Glass R.I. National laboratory surveillance of viral agents of gastroenteritis in Brazil. Bull Pan Am Health Organ. 1993;27:224–233. [PubMed] [Google Scholar]
- 31.Bányai K., Gentsch J.R., Schipp R., et al. Dominating prevalence of P[8], G1 and P[8], G9 rotavirus strains among children admitted to hospital between 2000 and 2003 in Budapest, Hungary. J Med Virol. 2005;76:414–423. doi: 10.1002/jmv.20372. [DOI] [PubMed] [Google Scholar]
- 32.Gouvea V.S., Domingues A.L., Naveca F.G., Pedro A.R., Bevilacqua C.C. Changing epidemiology of rotavirusrelated hospitalizations in rio de janeiro, Brazil, from 2002 to 2006. Open Virol J. 2007;1:47–50. doi: 10.2174/1874357900701010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro L.R., Giuberti R.S., Barreira D.M., et al. Hospitalization due to norovirus and genotypes of rotavirus in pediatric patients, state of Espírito Santo. Mem Inst Oswaldo Cruz. 2008;103:201–206. doi: 10.1590/s0074-02762008000200013. [DOI] [PubMed] [Google Scholar]
- 34.Gouvea V.S., Dias G.S., Aguiar E.A., et al. Acute gastroenteritis in a pediatric hospital in Rio de Janeiro in pre- and post-rotavirus vaccination settings. Open Virol J. 2009;3:26–30. doi: 10.2174/1874357900903010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patz J.A., Epstein P.R., Burke T.A., Balbus J.M. Global climate change and emerging infectious diseases. JAMA. 1996;275:217–223. [PubMed] [Google Scholar]
- 36.Bresee J.S., Marcus R., Venezia R.A., et al. US Acute Gastroenteritis Etiology Study Team. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis. 2012;205:1374–1381. doi: 10.1093/infdis/jis206. [DOI] [PubMed] [Google Scholar]
- 37.Martínez M., Fariña N., Rodriguez M., Russomando G., Parra G. Presencia de rotavirus en adultos con diarrea en Asunción, Paraguay. Rev Argen Microbiol. 2005;37:99–101. [PubMed] [Google Scholar]
- 38.Wang Y.H., Kobayashi N., Zhou D.J., et al. Molecular epidemiologic analysis of group A rotaviruses in adults and children with diarrhea in Wuhan city, China, 2000–2006. Arch Virol. 2007;152:669–685. doi: 10.1007/s00705-006-0904-y. [DOI] [PubMed] [Google Scholar]
- 39.Tatte V.S., Gentsch J.R., Chitambar S.D. Characterization of group A rotavirus infections in adolescents and adults from Pune, India: 1993–1996 and 2004–2007. J Med Virol. 2010;82:519–527. doi: 10.1002/jmv.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurgel R.Q., Correia J.B., Cuevas L.E. Effect of rotavirus vaccination on circulating virus strains. Lancet. 2008;371:301–302. doi: 10.1016/S0140-6736(08)60164-6. [DOI] [PubMed] [Google Scholar]
- 41.Anderson E.J., Shippee D.B., Weinrobe M.H., et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis. 2013;56:755–760. doi: 10.1093/cid/cis1010. [DOI] [PubMed] [Google Scholar]
- 42.Luchs A., Morillo S.G., de Oliveira C.M., Timenetsky Mdo C. Monitoring of group C rotavirus in children with acute gastroenteritis in Brazil: an emergent epidemiological issue after rotavirus vaccine? J Med Virol. 2011;83:1631–1636. doi: 10.1002/jmv.22140. [DOI] [PubMed] [Google Scholar]
- 43.Cilli A., Luchs A., Morillo S.G., Costa F.F., Carmona Rde C., Timenetsky Mdo C. Characterization of rotavirus and norovirus strains: a 6-year study (2004–2009) J Pediatr (Rio J) 2011;87:445–449. doi: 10.2223/JPED.2122. [DOI] [PubMed] [Google Scholar]
- 44.Yan H., Abe T., Phan T.G., et al. Outbreak of acute gastroenteritis associated with group A rotavirus and genogroup I sapovirus among adults in a mental health care facility in Japan. J Med Virol. 2005;75:475–481. doi: 10.1002/jmv.20292. [DOI] [PubMed] [Google Scholar]
- 45.Pietruchinski E., Benati F., Lauretti F., et al. Rotavirus diarrhea in children and adults in a southern city of Brazil in 2003: distribution of G/P types and finding of a rare G12 strain. J Med Virol. 2006;78:1241–1249. doi: 10.1002/jmv.20686. [DOI] [PubMed] [Google Scholar]
- 46.Feeney S.A., Mitchell S.J., Mitchell F., et al. Association of the G4 rotavirus genotype with gastroenteritis in adults. J Med Virol. 2006;78:1119–1123. doi: 10.1002/jmv.20671. [DOI] [PubMed] [Google Scholar]
- 47.Anderson E.J., Katz B.Z., Polin J.A., Reddy S., Weinrobe M.H., Noskin G.A. Rotavirus in adults requiring hospitalization. J Infect. 2012;64:89–95. doi: 10.1016/j.jinf.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Bányai K., Gentsch J.R., Schipp R., et al. Dominating prevalence of P[8],G1 and P[8],G9 rotavirus strains among children admitted to hospital between 2000 and 2003 in Budapest, Hungary. J Med Virol. 2005;76:414–423. doi: 10.1002/jmv.20372. [DOI] [PubMed] [Google Scholar]
- 49.Bányai K., László B., Duque J., et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(Suppl 1):A122–A130. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- 50.Parra G.I. Seasonal shifts of group A rotavirus strains as a possible mechanism of persistence in the human population. J Med Virol. 2009;81:568–571. doi: 10.1002/jmv.21423. [DOI] [PubMed] [Google Scholar]
- 51.Mitui M.T., Chandrasena T.N., Chan P.K., et al. Inaccurate identification of rotavirus genotype G9 as genotype G3 strains due to primer mismatch. Virol J. 2012;9:144. doi: 10.1186/1743-422X-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honma S., Chizhikov V., Santos N., et al. Development and validation of DNA microarray for genotyping group A rotavirus VP4 (P[4], P[6], P[8], P[9], and P[14]) and VP7 (G1 to G6, G8 to G10, and G12) genes. J Clin Microbiol. 2007;45:2641–2648. doi: 10.1128/JCM.00736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox M.J., James V.L., Azevedo R.S., Massad E., Medley G.F. Infection with group C rotavirus in a suburban community in Brazil. Trop Med Int Health. 1998;3:891–895. doi: 10.1046/j.1365-3156.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 54.Steele A.D., James V.L. Seroepidemiology of human group C rotavirus in South Africa. J Clin Microbiol. 1999;37:4142–4144. doi: 10.1128/jcm.37.12.4142-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukhopadhya I., Anbu D., Iturriza-Gomara M., et al. Anti-VP6 IgG antibodies against group A and group C rotaviruses in South India. Epidemiol Infect. 2010;138:442–447. doi: 10.1017/S0950268809990732. [DOI] [PubMed] [Google Scholar]
- 56.Nilsson M., Svenungsson B., Hedlund K.O., et al. Incidence and genetic diversity of group C rotavirus among adults. J Infect Dis. 2000;182:678–684. doi: 10.1086/315772. [DOI] [PubMed] [Google Scholar]
- 57.Castello A.A., Argüelles M.H., Villegas G.A., et al. Characterization of human group C rotavirus in Argentina. J Med Virol. 2000;62:199–207. doi: 10.1002/1096-9071(200010)62:2<199::aid-jmv11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]