Abstract
Nocardia spp. are a group of aerobic actinomycetes widely distributed in soil, and associated with severe opportunistic infections, essentially pulmonary infections.
We report the first case of disseminated infection associated with urinary tract infection caused by Nocardia veterana. The diagnosis was difficult; despite the presence of pulmonary nodules, the lung biopsies remained negative while only one aerobic blood culture and the urine culture were positive for N. veterana, identified after a 16S rDNA gene sequence analysis.
Few cases of clinical importance due to N. veterana have been published since its characterization. The bacteriological diagnosis of nocardiosis can be difficult to establish because of the delayed growth and the specific techniques that are required. This case illustrates the necessity of performing specific investigations in immunocompromised patients who present with infectious disease because the severity of this infection requires early diagnosis and quick initiation of appropriate antibiotic therapy.
Abbreviations: CAPD, continuous ambulatory peritoneal dialysis; BAL, bronchoalveolar lavage; CT, computed tomography; FDG, deoxyglucose labeled with fluorine 18; PAS, periodic acid Schiff; PCR, polymerase chain reaction; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Keywords: Nocardia veterana, Urinary tract infection, MALDI-TOF MS system, 16S rDNA gene sequencing
Case presentation
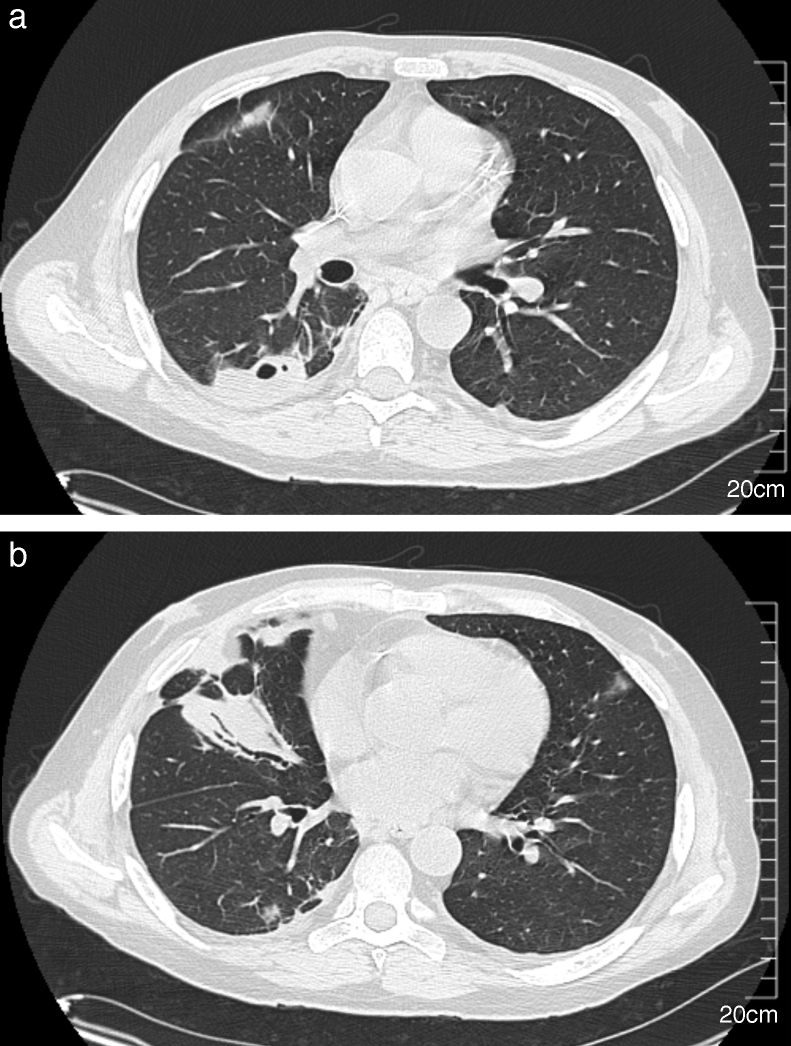
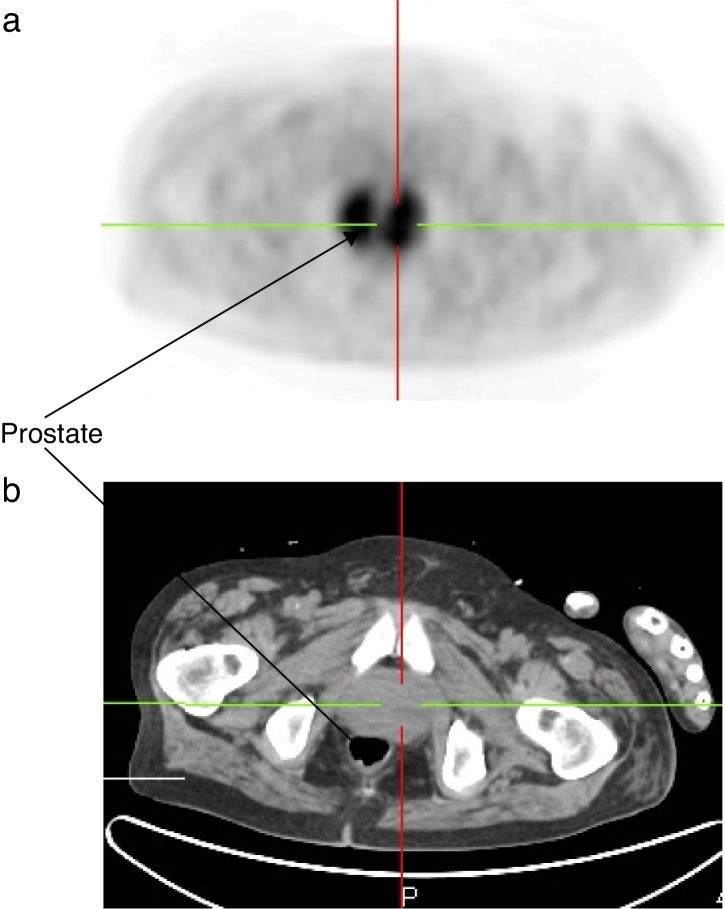
A 51-year-old man was admitted to the hospital to investigate his chest pain and weight loss (8 kg in two months). His past medical history included a grade 4 left temporal glioblastoma, diagnosed 11 months before, and multicystic kidney disease. The patient underwent surgery and radiation therapy, followed by first line chemotherapy with temozolomide and second line chemotherapy with lomustine, vincristine, and procarbazin, due to disease progression. He was given 32 mg of methylprednisolone per day starting at the beginning of the chemotherapy. Multicystic kidney disease caused renal terminal insufficiency, which required continuous ambulatory peritoneal dialysis (CAPD) for more than four months. The patient presented with a progressive shortness of breath, right-sided chest pain, and dysuria. He had no fever, and a physical exam showed right basal crackles. The laboratory test results were as follows: hemoglobin 9.7 g/dL; neutrophils 12 × 109/L; lymphocytes 4.2 × 109/L with a CD4 lymphocyte count of 1.64 × 109/L; serum creatinine 630 μmol/L, and C-reactive protein 113.5 mg/L (reference value <10 mg/L). Additionally, two sets of blood cultures and urine culture were performed. Urine analysis revealed 1.3 × 106 leukocytes/mL (normal range <103/mL) and Gram-positive short bacilli at microscopic examination. Chest computed tomography (CT) showed multiple sub-pleural nodules in the right lower lobe that were adjacent to pleural thickening without pleural effusion, and consolidation in the middle lobe (Fig. 1). One of the nodules was excavated. The PET imaging performed to demonstrate deep infection showed an intense and diffuse accumulation of the deoxyglucose labeled with fluorine 18 (FDG) in the prostate (Fig. 2) revealing prostatitis.
Fig. 1.
Chest CT scan showing right-sided multiple pulmonary nodules with cavitation (A) and parenchymal consolidation in the middle lobe (B).
Fig. 2.
PET scan showing an intense and diffuse accumulation of the FDG in the prostate (A: PET imaging; B: scanned imaging of the prostate).
A bronchoalveolar lavage (BAL) and a CT-guided needle lung biopsy of the posterior nodule were performed. In the laboratory, tissue analysis found acute suppurative inflammation, with negative PAS, Grocott and Giemsa staining. Gram and acid-fast staining were processed and were negative on both samples. Standard culture performed on BAL was non-contributive, with polymicrobial non-pathogenic nasopharyngeal flora; mycobacterial and mycological cultures remained negative. Standard culture was not undertaken on the pulmonary biopsy because of its insufficient amount. Both samples were tested for tuberculosis using polymerase chain reaction (PCR) on the MTB/RIF test platform (GenExpert®; Cepheid, Sunnyvale, CA, USA) and were negative. PCR amplification and sequencing of 16S rDNA and 18S rDNA for the detection of bacteria and fungi, respectively, were performed on the pulmonary biopsy after dewaxing, and were negative. Four days after the patient's admission, growth was identified in one aerobic blood culture using the BacT Alert 3D® culture system (BioMérieux, Marcy l’Etoile, France). The bacterium was a non-motile, Gram-positive organism with branching filaments. Small, chalky white, rough colonies grew within 48 h when inoculated on boiled blood agar plates that were incubated at 37 °C in 5% CO2. They were partially acid-fast and were considered to likely represent Nocardia spp. A Gram-positive bacterium was observed simultaneously in the urine culture, with more than 100,000 colonies/mL. The two strains, from blood and urine cultures, were identified to the genus level as Nocardia spp. using the MALDI-TOF MS (matrix-assisted laser desorption–ionization time-of-flight mass spectrometry) system (Bruker Daltonik, Bremen, Germany) after an extraction procedure. The identification to the species level as Nocardia veterana was obtained using a 16S rDNA gene sequence analysis (99.9% sequence matching with the type strain N. veterana DSM 44445T).
Prior to the isolation of Nocardia, the patient was empirically treated with intravenous trimethoprim-sulfamethoxazole (400 mg/80 mg, twice daily) and ceftriaxone (750 mg twice daily). Doses were adapted to renal function, with hemodialysis performed three times per week. Antimicrobial susceptibility was assessed by the French Observatory of Nocardiosis (Lyon, France) by broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI0 methods and breakpoints).1 The profile revealed resistance to amoxicillin + clavulanate (MIC of 32/16 μg/mL), ciprofloxacin (MIC >4 μg/mL) and doxycycline (MIC = 8 μg/mL); intermediate susceptibility to gentamicin (MIC of 8 μg/mL) and minocyclin (MIC = 2 μg/mL); and susceptibility to cefotaxime (≤4 μg/mL), ceftriaxone (MIC = 8 μg/mL), imipenem (MIC ≤ 2 μg/mL), trimethoprim-sulfamethoxazole (MIC ≤ 1/19 μg/mL) and linezolid (MIC ≤ 4 μg/mL). After initiation of treatment, the respiratory symptoms slowly improved, and dysuria was completely resolved. The biological inflammatory syndrome decreased gradually, and a chest CT scan performed after one month of antibiotic therapy showed a moderate decrease in nodules size. Accordingly, the urinary and blood culture controls showed no remaining Nocardia. Unfortunately, due to glioblastoma progression, the patient's neurological status declined, and he died two months after the initial admission.
Nocardiosis infections are caused by the saprophytic aerobic, Gram-positive, branching and filamentous bacilli belonging to the genus Nocardia. The identification of Nocardia isolates at the species level is critical for defining the spectrum of diseases that are caused by each species and to predict antimicrobial susceptibility.2 To date, approximately 90 species have been described (NCBI taxonomy for Nocardia), and a third of them have been implicated in human disease.3 If isolates grow well on blood agar plates, the routine identification of Nocardia strains using conventional phenotypical methods is a fastidious and time-consuming process that is now restricted to reference centers.2 In recent years, 16S rDNA gene sequence analysis has become accessible to clinical laboratories for definitive species identification.4 In our case, the strain of N. veterana was confirmed at the species level by almost complete 16S rDNA gene sequencing (1315 nt) by using SQ1 (5′-AGAGTTGATCMTGGCTCAG-3′) and SQ6 (5′-CGGTGTGTACAAGGCCC-3′) primers, according to previous published data.5 The sequences obtained presented a 99.9% sequence similarity (one difference out of 1315 nt, excluding the primers) to the type strain N. veterana DSM 44445T. The MALDI-TOF MS identification systems are based on the comparison of the tested isolate mass spectrum with reference databases. Previous studies reported that the Bruker MALDI-TOF MS system accurately identified species from the genus Nocardia spp.6 but a preliminary extraction step was mandatory to obtain satisfactory results. In our case, the identification was obtained to the genus level after an extraction procedure in two steps (boiling followed by ethanol–formic acid extraction). At first, the identification by the direct colony method failed to identify the bacterium, although it has been recently published with other MALDI-TOF MS systems.7
N. veterana is a species that was characterized in 2001.8 The difficulties in the identification of this species, until recently, may imply that it is more common as a human pathogen than previously reported.9 Indeed, little is known about N. veterana because few cases of clinical importance have been published. One isolate was from a brain abscess10; three were reported in patients with pulmonary infections2, 11, 12, 13, 14; one isolate was recovered in a bowel abscess and was associated with colon carcinoma15; two cases of mycetomas from Japan were published16, 17; one isolate has been recovered from ascitic fluid,8 one was responsible for an endogenous endophtalmitis,18 and recently, a case of N. veterana causing nodular lymphangitis was reported.19 Bloodstream infection due to N. veterana is a rare condition. In our case, this pathogen was isolated from blood and urine cultures, and this is the first reported case of urinary tract infection due to N. veterana. This implies that the infectious spectrum of N. veterana could be relatively broad.
Like other species of Nocardia spp., N. veterana causes infections mainly in immunocompromised hosts. As predisposing factors, the patient was administered long-term corticosteroid and chemotherapy (including temozolomide) for neoplastic disease. However, in a recent study of nocardiosis and AIDS, it was shown that despite deep immunodepression, nocardiosis was an uncommon complication. Trimethoprim-sulfamethoxazole, which is used in primary prophylaxis for toxoplasmosis, could be effective against nocardiosis as well.
It is unclear how our patient became infected by N. veterana. Pulmonary infection is the most common site of nocardiosis, and it is usually acquired by inhalation of sporulated fragmented nocardial mycelia found in the environment.11, 20 Most of the time, diagnosis is based on the isolation of Nocardia from respiratory samples. In our case, we identified a disseminated N. veterana infection with the presence of this bacterium in peripheral blood culture and urine. The hypothesis of airborne contamination, with an infection that was initially limited to the lung and a secondary hematological dissemination, sometimes to the central nervous system and soft tissue,8 may be considered. However, we have not proved the presence of N. veterana in the chest, although there were several indications of its presence, including the patient's pulmonary presentation, chest CT scan features with nodules and parenchymal consolidation12 (Fig. 1) and the negativity of all bacteriological chest sampling for other pathogens. The contamination of the BAL by non-pathogenic nasopharyngeal flora of the upper respiratory tract could have interfered with culturing of Nocardia spp. We have no explanation for the negativity of PCR amplification of 16S rDNA performed on the pulmonary biopsy after dewaxing; the low sensitivity of PCR amplification could explain the result. Furthermore, the role of CAPD must be considered, as several cases of peritoneal nocardiosis have been reported.21 Our patient had daily home- and self-made CAPD, which could have been a predisposing factor for this infection because of non-optimal skills. We did not perform bacteriological testing of ascitic fluid because our patient had no abdominal pain, which makes the hypothesis of CAPD-induced contamination less likely.
The antimicrobial agents to be used against N. veterana remain controversial due to discrepancies regarding methodological variations, differences in interpretations concerning breakpoints and, therefore, susceptibilities. Based on limited in vitro studies of antimicrobial agents against this microorganism, the empiric drug of choice for this pathogen would be trimethoprim-sulfamethoxazole9, 22 at a dose of 10–15 mg/kg daily. In our case, N. veterana remained sensitive to trimethroprim-sulfamethoxazole and ceftriaxone, which was the initial empirical treatment. The treatment of nocardiosis must be extended by considering many factors, such as the severity of infection or the immunizing status. In the previously published cases, the duration of treatment ranged from a few weeks to a number of years based on expert opinion on the treatment of other Nocardia infections.10 Because the patient died two months after the initial admission, we could not evaluate his response to the antibiotic treatment.
Conclusion
In conclusion, this is the first reported case of urinary tract infection due to N. veterana. Nocardiosis due to N. veterana is a rare disease, but its incidence will most likely increase in the coming years because of the growing population of immunocompromised hosts. This case illustrates the difficulty of establishing a bacteriological diagnosis of nocardiosis and the necessity of performing specific investigations in immunocompromised patients who present with infectious disease because the severity of this infection requires early diagnosis and quick initiation of appropriate antibiotic therapy.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Clinical and Laboratory Standards Institute Document M-A . 2nd ed. 2011. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes – approved standard. CLSI document. [PubMed] [Google Scholar]
- 2.Liu W., Lai C., Hsiao C., et al. Bacteremic pneumonia caused by Nocardia veterana in an HIV-infected patient. Int J Infect Dis. 2011;15:430–432. doi: 10.1016/j.ijid.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Kong F., Wang H., Zhang E., et al. secA1 gene sequence polymorphisms for species identification of Nocardia species and recognition of intraspecies genetic diversity. J Clin Microbiol. 2010;48:3928–3934. doi: 10.1128/JCM.01113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wellinghausen N., Pietzcker T., Kern W.V., Essig A., Marre R. Expanded spectrum of Nocardia species causing clinical nocardiosis detected by molecular methods. Int J Med Microbiol. 2002;292:277–282. doi: 10.1078/1438-4221-00208. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Nava V., Couble A., Molinard C., Sandoval H., Boiron P., Laurent F. Nocardia mexicana sp. nov., a new pathogen isolated from human mycetomas. J Clin Microbiol. 2004;42:4530–4535. doi: 10.1128/JCM.42.10.4530-4535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verroken A., Janssens M., Berhin C., et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Nocardia species. J Clin Microbiol. 2010;48:4015–4021. doi: 10.1128/JCM.01234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.farfour E., Leto J., Barritault M., et al. Evaluation of the Andromas matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of aerobically growing Gram-positive bacilli. J Clin Microbiol. 2012;50:2702–2707. doi: 10.1128/JCM.00368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godreuil S., Didelot M., Perez C., et al. Nocardia veterana isolated from ascitic fluid of a patient with human immunodeficiency virus infection. J Clin Microbiol. 2003;41:2768–2773. doi: 10.1128/JCM.41.6.2768-2773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conville P., Fischer S., Cartwright C., Witebsky F. Identification of Nocardia species by restriction endonuclease analysis of an amplified portion of the 16S rRNA gene. J Clin Microbiol. 2000;38:158–164. doi: 10.1128/jcm.38.1.158-164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arends J., Stemerding A., Vorst S., de Neeling A., Weersink A. First report of a brain abscess caused by Nocardia veterana. J Clin Microbiol. 2011;49:4364–4365. doi: 10.1128/JCM.01062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson M., Kuzniar T.J. Pulmonary nocardiosis in a patient with chronic obstructive pulmonary disease – case report and literature review. Pneumonol Alergol Pol. 2012;80:565–569. [PubMed] [Google Scholar]
- 12.Tsujimoto N., Saraya T., Kikuchi K., et al. High-resolution CT findings of patients with pulmonary nocardiosis. J Thorac Dis. 2012;4:577–582. doi: 10.3978/j.issn.2072-1439.2012.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gürtler V., Smith R., Mayall B., Pötter-Reinemann G., Stackebrandt E., Kroppenstedt R. Nocardia veterana sp. nov., isolated from human bronchial lavage. Int J Syst Evol Microbiol. 2001;51:933–936. doi: 10.1099/00207713-51-3-933. [DOI] [PubMed] [Google Scholar]
- 14.Pottumarthy S., Limaye A., Prentice J., Houze Y., Swanzy S., Cookson B. Nocardia veterana, a new emerging pathogen. J Clin Microbiol. 2003;41:1705–1709. doi: 10.1128/JCM.41.4.1705-1709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlebusch S., Nimmo G., Carter R. Bowel abscess with Nocardia veterana associated with colon carcinoma. Pathology. 2010;42:306–307. doi: 10.3109/00313021003633144. [DOI] [PubMed] [Google Scholar]
- 16.Kashima M., Kano R., Mikami Y., et al. A successfully treated case of mycetoma due to Nocardia veterana. Br J Dermatol. 2005;152:1349–1352. doi: 10.1111/j.1365-2133.2005.06551.x. [DOI] [PubMed] [Google Scholar]
- 17.Kano R., Hattori Y., Murakami N., et al. The first isolation of Nocardia veterana from a human mycetoma. Microbiol Immunol. 2002;46:409–412. doi: 10.1111/j.1348-0421.2002.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 18.Scott M., Mehta S., Rahman H.T., Grossniklaus H.E., Yeh S. Nocardia veterana endogenous endophthalmitis in a cardiac transplant patient. J Ophthalmic Inflamm Infect. 2013;3:44. doi: 10.1186/1869-5760-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dua J., Clayton R. First case report of Nocardia veterana causing nodular lymphangitis in an immunocompromised host. Australas J Dermatol. 2014;55:48–50. doi: 10.1111/ajd.12043. [DOI] [PubMed] [Google Scholar]
- 20.Al-Tawfiq J., Al-Khatti A. Disseminated systemic Nocardia farcinica infection complicating alefacept and infliximab therapy in a patient with severe psoriasis. Int J Infect Dis. 2010;14:153–157. doi: 10.1016/j.ijid.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Kendrick-Jones J., Ratanjee S., Taylor S., Marshall M. Nocardia asteroides peritoneal dialysis-related peritonitis: a case of successful treatment and return to peritoneal dialysis. Nephrol Dial Transplant. 2008;23:2693–2694. doi: 10.1093/ndt/gfn252. [DOI] [PubMed] [Google Scholar]
- 22.Ansari S., Safdar A., Han X., O’Brien S. Nocardia veterana bloodstream infection in a patient with cancer and a summary of reported cases. Int J Infect Dis. 2006;10:483–486. doi: 10.1016/j.ijid.2006.03.005. [DOI] [PubMed] [Google Scholar]