Abstract
Human actinomycosis with involvement of the spine is a rare condition although it has been first described a long time ago. It is probably underrecognized since its clinical presentation is often misleading and accurate bacteriological diagnosis is challenging. We herein report a rare case of cervical actinomycosis with paravertebral abscess and spondylitis imputed to an infection by Actinomyces meyeri in a 52-year-old immunocompetent Caucasian man. A. meyeri should be considered as a potential cause for subacute or chronic spondylitis, even in immunocompetent subjects. Modern diagnostic tools such as Matrix-Assisted Laser Desorption–Ionization Time of Flight mass spectrometry and 16S rRNA sequencing are efficient for accurate microbiological identification.
Keywords: Actinomyces, Spondylitis, MALDI–TOF mass spectrometry, 16S rRNA
Introduction
Actinomycosis is a chronic suppurative infection caused by Actinomyces spp., which are facultative anaerobic, branching Gram positive, acid-fast negative bacilli, belonging to the normal flora of the oropharyngeal cavity. It is characterized by abscess formation, tissue fibrosis and draining sinuses.1, 2 The most classical presentation of the disease is the cervico-facial form, accounting for approximately half the cases, followed by thoracic, abdomino-pelvic and cerebral localizations.1 Spinal involvement is a rare feature, although it has been described for a long time in the literature.1, 2, 3, 4, 5 The diagnosis of actinomycosis is quite challenging given the insidious evolution, mimicking other diseases such as malignancies, and the specific procedures required for accurate microbiological identification of the pathogen. We herein report a rare case of cervical actinomycosis with paravertebral abscess and spondylitis of the sixth cervical vertebra associated with Actinomyces meyeri isolation from blood and prevertebral pus cultures in an adult immunocompetent male patient.
Case presentation
A 52-year-old man was admitted to our hospital with a 3-week history of inflammatory cervical pain, irradiating to both shoulders. Previous therapeutic management with NSAID systemic administration and a short-course of corticotherapy had failed to improve the clinical picture and symptoms even worsened. At admission, physical examination was characterized by fever up to 40 °C and exacerbation of the pain while pressing on the cervical spine and paravertebral muscles. A critical orodental presentation was evidenced. Biological tests revealed the following abnormalities: hyper leucocytosis at 21 G/L (normal <10) with 90% of polymorphonuclear cells and a marked inflammatory syndrome with a C-reactive protein level at 347 mg/L (normal <5) and a fibrinogen at 11.5 g/L (normal <5.0). Human immunodeficiency virus (HIV) infection was ruled out by serological testing as well as hypogammaglobulinemia by serum electrophoresis. Glycemia was within normal range. There was no past or recent history of alcohol intake. A magnetic resonance imaging (MRI) of the cervical spine revealed a spondylitis of the sixth cervical vertebra surrounded by a prevertebral abscess neighboring the vertebras C5 to C7, completed by a significant infiltrative lesion involving the soft tissues and muscular compartment invading the overall cervical site (Fig. 1). Following a computed tomography (CT) guided sampling of the abscess and blood puncture for microbiological investigations, an empiric antibiotic combination with intravenous amoxicillin (4 g every 6 h) and gentamicin (10 mg/kg once a day) was started. Patient condition dramatically improved following the initiation of the anti-infectious course. Concurrently, microbiological investigations were contributive with initial identification of a methicillin susceptible Staphylococcus epidermidis and the consecutive isolation of an Actinomyces sp. strain using BacT/Alert SN® anaerobic medium (BioMerieux, Craponne, France) and pricking out the broth on Columbia agar supplemented with 5% plain sheep blood (BioMerieux, Craponne, France). The latter (named CRL1) was identified as A. meyeri using Matrix-Assisted Laser Desorption–Ionization Time of Flight (MALDI–TOF) mass spectrometry. Subsequently, a sequence analysis of the 16S rRNA gene was carried out. A total of 1441 continuous nucleotides were determined using the Sanger method. The sequence of isolate CRL1 (GenBank accession number KC522297) was compared to all bacterial sequences available from the GenBank database using the BLAST program (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Sequences with more than 90% homology were selected to build a phylogenetic tree using the neighbor-joining algorithm6 with the Mega5 software7 (Fig. 2). Our isolate was found to have 99.4% homology with the A. meyeri strain TTE10 (GU561321) and 99.9% homology with the A. meyeri strain Prevot 2477B (NR029286). Thus, isolate CRL1 was definitely identified as A. meyeri. Therefore, we considered S. epidermidis as a contaminant. The ongoing treatment was replaced by an oral combination of amoxicillin (4 g per day) and clindamycin (600 mg every 8 h) for a three-month course, followed by amoxicillin alone for six months more. In addition, the patient underwent an avulsion of teeth 46 and 47, presumed to be the entry point of the infectious process. A cervical MRI performed five months after the initiation of treatment showed disappearance of the prevertebral abscess and of the infiltrative presentation surrounding soft tissues. Three years later, the improvement in the general condition was confirmed and the patient considered as cured.
Fig. 1.
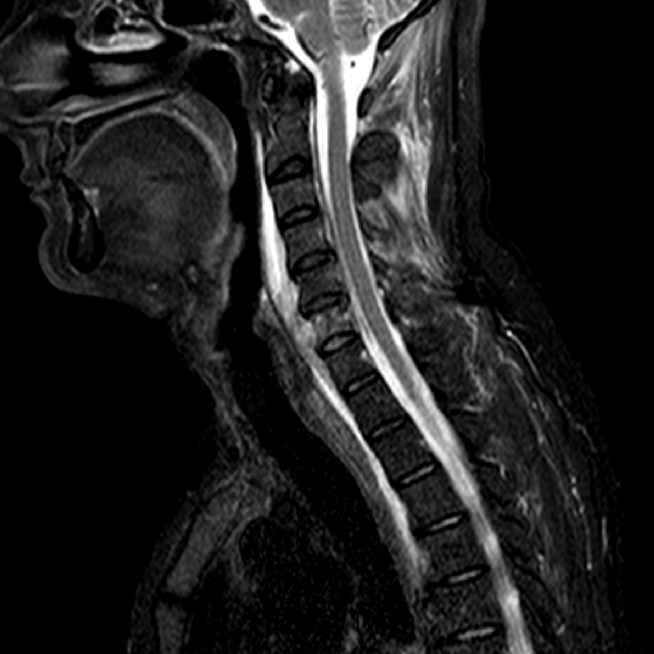
MRI sagittal slide of the cervical spine (STIR sequence) showing spondylitis of the sixth cervical vertebra with prevertebral abscess and infiltration of the neighboring soft tissues and muscular compartment in a 52-year-old immunocompetent man.
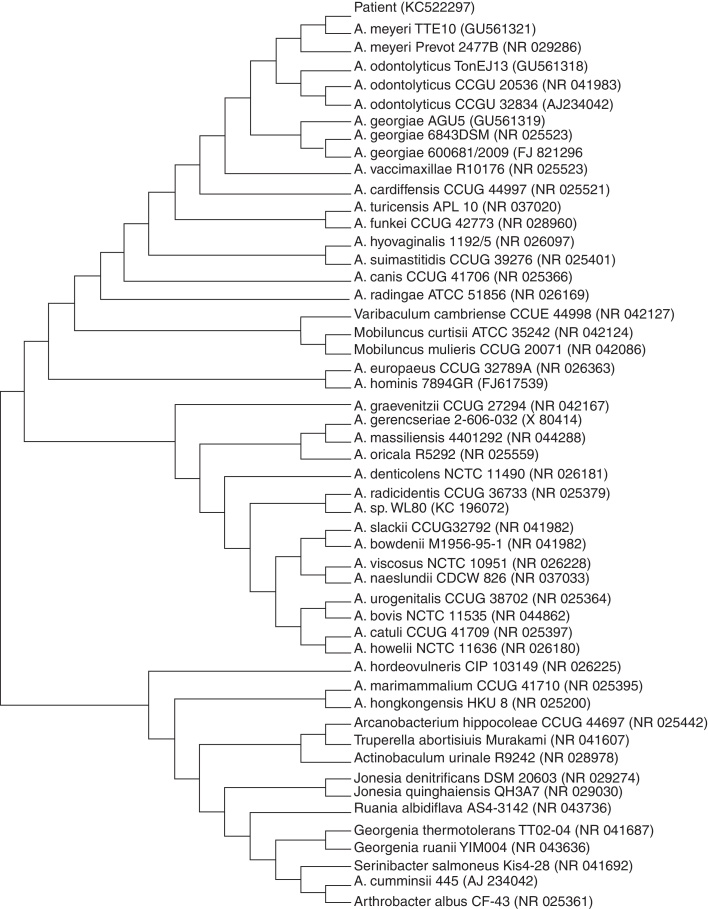
Fig. 2.
Phylogenetic tree of 16S rRNA sequences of patient isolate and non-redundant strains with at least 90% homology found in the GenBank database, constructed by using the neighbor-joining method. GenBank accession numbers are given in parentheses.
Discussion
Members of Actinomyces genus are fastidious, facultative, anaerobic, Gram positive and acid-fast negative branching bacilli. They are closely related to the aerobic Nocardia spp., and classified as higher prokaryotic bacteria as well as the Mycobacteriacae family, which belongs to the same order Actinomycetales. Numerous Actinomyces species may cause disease in human. The most frequently reported is Actinomyces israelii and was first isolated in 1891 from a lung abscess.3 Actinomyces spp. considered an opportunistic pathogen in humans belongs to the normal flora of the oropharyngeal cavity. It can result in infection either in immunocompetent or immunocompromised persons, notably when a break in the mucosa of the gastrointestinal tract occurs, wherever between the oral cavity and the rectum.1 Thus, actinomycosis has been conditioned by numerous risk factors such as dental care and sepsis, abdominal surgery, appendicitis, diverticulitis, or the use of intrauterine and intravaginal devices.3 In our patient, entry of infectious agent was attributed to the detrimental dental and periodontal condition. Actinomycosis is classically described through several clinico-anatomical features. The cervico-facial form accounts for approximately half the cases. Thoracic and abdomino-pelvic localizations are found in 15% and 20% of the cases, respectively. Lastly, invasion of the central nervous system is considered as a very rare condition.1 Spinal involvement is an exceptional feature, which represents less than 5% of the concerned sites. It is usually secondary to infection of contiguous tissues rather than to hematogenous spread.2, 3 Differential diagnoses include all chronic and suppurative infectious processes. The main differential diagnoses are nocardiosis, tuberculosis, spondyloarthritis and primary or secondary malignancies. Unlike spinal tuberculosis, actinomycosis usually spares intervertebral discs and involves the adjacent pedicles and transverse processes, as well as the neighboring ribs.2 Microbiological diagnosis requires appropriate clinical specimens, such as pus, and enriched culture media. They best grow at 37 °C in an atmosphere containing 6–10% carbon dioxide. Classically, Actinomyces spp. appears as “molar-tooth” colonies on agar or as “bread-crumb” colonies in suspension in broth-media.1 Identification of species is difficult. The procedure relies on complex phenotypic testing and, when insufficient, on mass spectrometry or 16S rRNA sequencing. In tissues, Actinomyces spp. grows in microscopic or macroscopic clusters of tangled filaments. When grossly visible, these clusters may exude from soft tissues through sinuses, taking a yellowish or brownish color and are called “sulfur granules”. Of note, their identification is not specific of the actinomycosis occurrence and their absence cannot rule out the diagnosis.1 Other bacteria such as Actinobacillus actinomycetemcomitans, Eikenella corrodens, Fusobacterium spp., Bacteroides spp., Capnocytophaga spp., Staphylococcus spp., Streptococcus spp. and Enterococcus spp. may be concomitantly isolated from the same clinical specimens but their role in pathogenicity remains definitely unclear. They could hypothetically facilitate the growth of Actinomyces spp. by reducing the oxygen tension.1, 3 Penicillin G is the cornerstone of the treatment of actinomycosis. However, Actinomyces species are susceptible to various antimicrobials including tetracyclines, erythromycin, clindamycin, cephalosporins, carbapenems, streptomycin and chloramphenicol.1 The most commonly reported alternative in penicillin-sensitive patients is clindamycin, especially in severe cases, such as in our patient.8, 9 A long duration of treatment is generally suitable to prevent relapses and a combined medico-surgical approach is to be required for complicated forms of the disease.1
Conclusion
Spondylitis due to Actinomyces spp. is an exceptional feature. However, this diagnosis must be considered in case of an insidious spondylitis in a patient with consistent exposure conditions. In addition, it must be kept in mind that co-infection with other bacteria may be possible and challenging in microbiological diagnosis issues. Accurate identification of species relies on 16S rRNA sequencing and analysis. However, this case shows that MALDI–TOF mass spectrometry could represent an emerging and valuable alternative to the latter as the result can be promptly provided.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to Dr Emilie Bessede for her generous help in isolating and identifying the CRL1 (KC522297) strain.
References
- 1.Smego R.A, Jr., Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1261. doi: 10.1086/516337. [DOI] [PubMed] [Google Scholar]
- 2.Voisin L., Vittecoq O., Mejjad O., et al. Spinal abscess and spondylitis due to actinomycosis. Spine. 1998;23:487–490. doi: 10.1097/00007632-199802150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Honda H., Bankowski M.J., Kajioka E.H.N., et al. Thoracic vertebral actinomycosis: Actinomyces israelii and Fusobacterium nucleatum. J Clin Microbiol. 2008;46:2009–2014. doi: 10.1128/JCM.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cope V.Z. Actinomycosis of bone with special reference to infection of vertebral column. J Bone Joint Surg Br. 1951;33B:205–214. [PubMed] [Google Scholar]
- 5.Simonet-Lamm A., Binois R., Bador J., et al. A meyeri spondylodiscitis. Med Mal Infect. 2011;41:619–620. doi: 10.1016/j.medmal.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 7.Tamura K., Peterson D., Peterson N., et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fass R.J., Scholand J.F., Hodges G.R., et al. Clindamycin in the treatment of serious anaerobic infections. Ann Intern Med. 1973;78:853–859. doi: 10.7326/0003-4819-78-6-853. [DOI] [PubMed] [Google Scholar]
- 9.Lerner P.I. Susceptibility of pathogenic actinomycetes to antimicrobial compounds. Antimicrob Agents Chemother. 1974;5:302–309. doi: 10.1128/aac.5.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]