Abstract
This cohort study assesses the presence of neutralizing antibodies in the serum samples of children in different age groups during and after SARS-CoV-2 infection.
Epidemiologic data indicate that SARS-CoV-2 infection in children is usually mild, which contrast with high rates of morbidity and mortality in older adults.1,2 Data on the strength and durability of antibodies generated after SARS-CoV-2 infection in children remain limited.3,4 Such data are critical in understanding disease severity, identifying risk of reinfection, and establishing herd immunity and vaccination policy. In this study, we analyzed the dynamics of neutralizing antibodies in a cohort of children and adolescents after SARS-CoV-2 infection. The study period covered the emergence of the original SARS-CoV-2 Wuhan strain up to and including the Delta variant.
Methods
We recruited individuals aged 0 to 16 years with SARS-CoV-2 infection confirmed by polymerase chain reaction test with nasopharyngeal swabs at KK Women’s and Children’s Hospital in Singapore from February 1, 2020, to September 30, 2021. Participant follow-up included blood sampling or collection of residual blood sample from routine clinical care when available at various time points up to 16 months after infection. Ethical approval for this study was granted by Singhealth Centralised Institutional Review Board. Written informed consent was obtained for blood collection, and informed consent for public health research for collection of residual samples was waived. We followed the STROBE reporting guideline.
Serum of blood samples was tested with a surrogate viral neutralizing assay to detect neutralizing antibodies to SARS-CoV-2.5 We performed a biochemical measurement of the amount of neutralizing antibodies present in the test serum samples using inhibition enzyme-linked immunosorbent assay. We performed temporal distribution of neutralizing antibody levels since infection, with adjustment for age, sex, and symptom status.
Significance level was set at 2-sided P < .05. Data analysis was performed using R (R Core Team).
Results
Of the 126 study participants (mean [range] age, 7.4 years [1 month to 16 years]; 74 boys [58.7%], 52 girls [41.3%]), 38 (30%) completed 2 to 4 visits or blood sampling. All symptomatic cases (91 [72%]) were mild, with fever, cough, and runny nose as commonly reported symptoms. No cases were moderate or severe or developed into multisystem inflammatory syndrome in children.
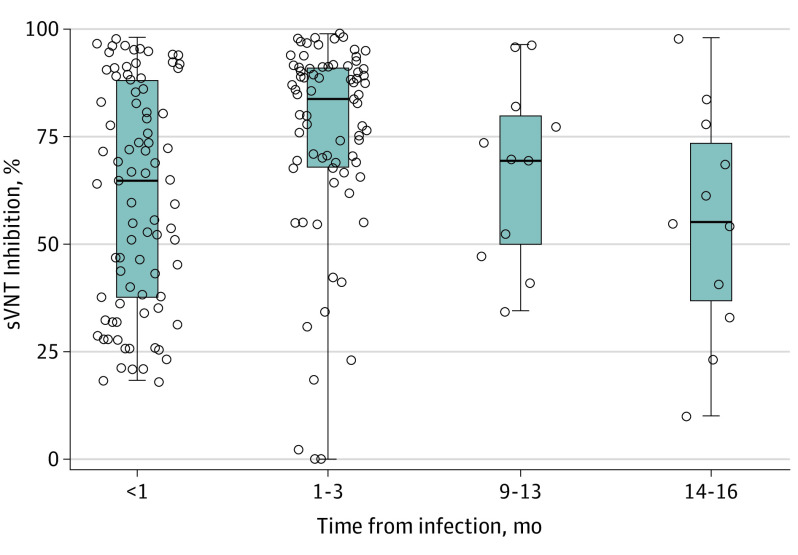
Peak neutralizing antibody levels were reached at a median of 84% approximately 1 to 3 months after infection (Figure 1). Neutralizing antibody levels remained reasonably high with a median of 69.8% at 9 to 13 months after infection. In the adjusted analysis, neutralizing antibody levels by postinfection time were not associated with patient characteristics, such as sex and symptom status (Figure 2). However, during the acute phase of infection (<1 month), neutralizing antibody levels were highest in those younger than 5 years (71.6%; 95% CI, 58.5%-84.6%) and lowest in the 12 to 16 years group (49.9%; 95% CI, 41.3%-58.6%). Neutralizing antibodies in participants younger than 5 years remained little changed in the point estimates up to 16 months after infection.
Figure 1. Total Neutralizing Antibody Levels in Children and Time Since SARS-CoV-2 Infection.
Circles represent the neutralizing antibody level in each blood sample, the center line within boxes represents median, the outer bounds of boxes represent lower and upper quartiles, and whiskers represent range. sVNT indicates surrogate virus neutralization test.
Figure 2. Adjusted Mean Neutralizing Antibody Level Over Time in Children After SARS-CoV-2 Infection.
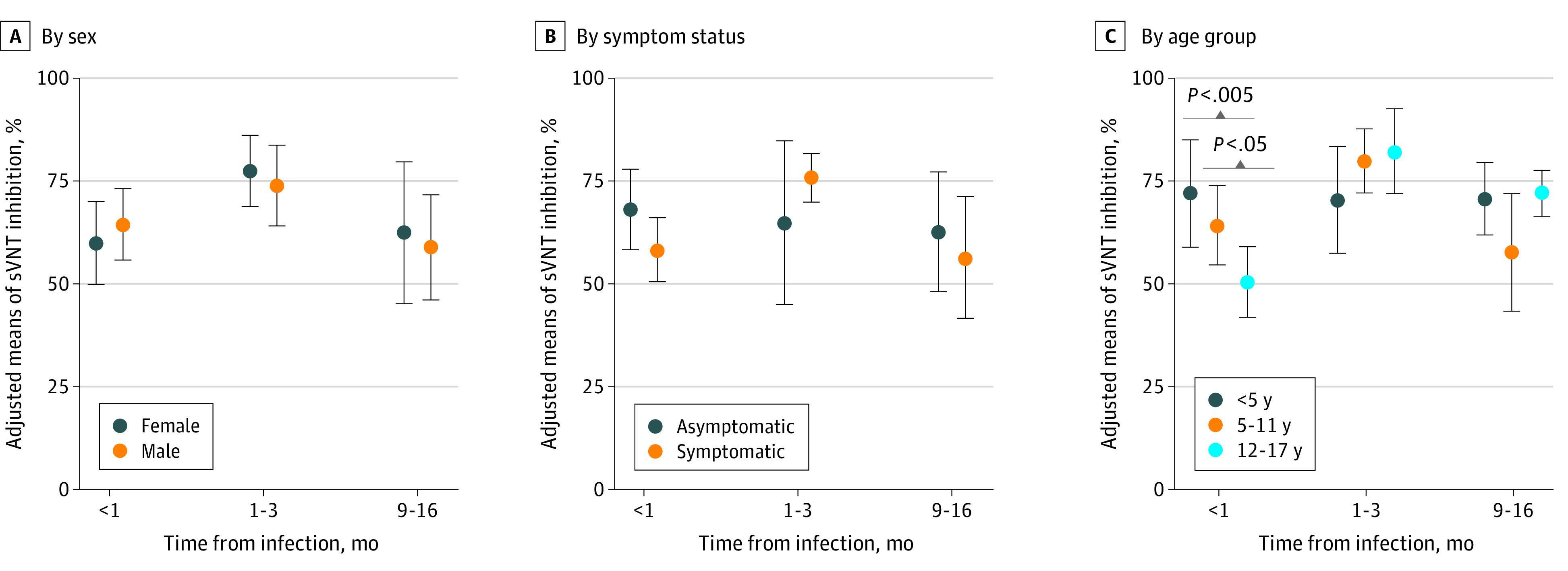
Error bars represent 95% CIs. sVNT indicates surrogate virus neutralization test.
Discussion
This study provided evidence of the durability of neutralizing antibodies in children up to 16 months after infection. There were no differences in level and duration of neutralizing antibodies by sex or symptom status. However, younger age (<5 years) was associated with significantly rapid generation of neutralizing antibody levels during the acute phase of infection and less degradation over time, compared with older age.
Study limitations included the assessment of neutralizing antibodies specific to viral spike protein receptor-binding domain as they were highly associated with protection.6 The sample size also decreased 9 months after infection, but all age groups were still represented.
The findings suggest that risk of SARS-CoV-2 reinfection in younger children is lower than in adults, which has important implications for scheduling COVID-19 vaccination after infection. The findings also broaden the understanding about less severe clinical disease in younger children.
References
- 1.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143-156. doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Thoon KC, Chong CY, et al. Comparative analysis of symptomatic and asymptomatic SARS-CoV-2 infection in children. Ann Acad Med Singap. 2020;49(8):530-537. doi: 10.47102/annals-acadmedsg.2020257 [DOI] [PubMed] [Google Scholar]
- 3.Dowell AC, Butler MS, Jinks E, et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol. 2022;23(1):40-49. doi: 10.1038/s41590-021-01089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mensah AA, Campbell H, Stowe J, et al. Risk of SARS-CoV-2 reinfections in children: prospective national surveillance, January 2020 to July 2021, England. medRxiv. Posted December 11, 2021. doi: 10.1101/2021.12.10.21267372 [DOI] [PMC free article] [PubMed]
- 5.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073-1078. doi: 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 6.Lumley SF, O’Donnell D, Stoesser NE, et al. ; Oxford University Hospitals Staff Testing Group . Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533-540. doi: 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]