Key Points
Question
How did health and quality of life in US skilled nursing facilities (SNFs) change during the COVID-19 pandemic?
Findings
In this retrospective observational study that included data from 2 985 864 long-term care resident-years in 15 477 SNFs in 2018-2020, SNFs with active COVID-19 cases experienced significant increases in mortality and functional decline during the first year of the pandemic compared with the prepandemic period, while significant increases in weight loss and depressive symptoms occurred in SNFs with active COVID-19 and SNFs with no known COVID-19 cases.
Meaning
Among skilled nursing facilities in the US from January to November 2020, adverse changes occurred in some health and quality of life measures during the first year of the pandemic and prior to the availability of COVID-19 vaccination, compared with the prepandemic period of January to November 2018 and 2019, even among facilities that did not have known COVID-19 cases.
Abstract
Importance
During the COVID-19 pandemic, the US federal government required that skilled nursing facilities (SNFs) close to visitors and eliminate communal activities. Although these policies were intended to protect residents, they may have had unintended negative effects.
Objective
To assess health outcomes among SNFs with and without known COVID-19 cases.
Design, Setting, and Participants
This retrospective observational study used US Medicare claims and Minimum Data Set 3.0 for January through November in each year beginning in 2018 and ending in 2020 including 15 477 US SNFs with 2 985 864 resident-years.
Exposures
January through November of calendar years 2018, 2019, and 2020. COVID-19 diagnoses were used to assign SNFs into 2 mutually exclusive groups with varying membership by month in 2020: active COVID-19 (≥1 COVID-19 diagnosis in the current or past month) or no-known COVID-19 (no observed diagnosis by that month).
Main Outcomes and Measures
Monthly rates of mortality, hospitalization, emergency department (ED) visits, and monthly changes in activities of daily living (ADLs), body weight, and depressive symptoms. Each SNF in 2018 and 2019 served as its own control for 2020.
Results
In 2018-2019, mean monthly mortality was 2.2%, hospitalization 3.0%, and ED visit rate 2.9% overall. In 2020, among active COVID-19 SNFs compared with their own 2018-2019 baseline, mortality increased by 1.60% (95% CI, 1.58% to 1.62%), hospitalizations decreased by 0.10% (95% CI, −0.12% to −0.09%), and ED visit rates decreased by 0.57% (95% CI, −0.59% to −0.55%). Among no-known COVID-19 SNFs, mortality decreased by 0.15% (95% CI, −0.16% to −0.13%), hospitalizations by 0.83% (95% CI, −0.85% to −0.81%), and ED visits by 0.79% (95% CI, −0.81% to −0.77%). All changes were statistically significant. In 2018-2019, across all SNFs, residents required assistance with an additional 0.89 ADLs between January and November, and lost 1.9 lb; 27.1% had worsened depressive symptoms. In 2020, residents in active COVID-19 SNFs required assistance with an additional 0.36 ADLs (95% CI, 0.34 to 0.38), lost 3.1 lb (95% CI, −3.2 to −3.0 lb) more weight, and were 4.4% (95% CI, 4.1% to 4.7%) more likely to have worsened depressive symptoms, all statistically significant changes. In 2020, residents in no-known COVID-19 SNFs had no significant change in ADLs (−0.06 [95% CI, −0.12 to 0.01]), but lost 1.8 lb (95% CI, −2.1 to −1.5 lb) more weight and were 3.2% more likely (95% CI, 2.3% to 4.1%) to have worsened depressive symptoms, both statistically significant changes.
Conclusions and Relevance
Among skilled nursing facilities in the US during the first year of the COVID-19 pandemic and prior to the availability of COVID-19 vaccination, mortality and functional decline significantly increased at facilities with active COVID-19 cases compared with the prepandemic period, while a modest statistically significant decrease in mortality was observed at facilities that had never had a known COVID-19 case. Weight loss and depressive symptoms significantly increased in skilled nursing facilities in the first year of the pandemic, regardless of COVID-19 status.
This study assesses health and quality of life outcomes among long-term residents of skilled nursing facilities before and during the COVID-19 pandemic before vaccines were available.
Introduction
Skilled nursing facilities (SNFs) have been significantly affected by the COVID-19 pandemic.1 SNFs have many vulnerabilities to COVID-19, particularly among long-term care (LTC) residents. These individuals are older, live in close quarters, and many have dementia or other serious illnesses, all risk factors for more severe manifestations of COVID-19. As of February 2022, individuals residing in nursing homes experienced more than 175 000 COVID-19–related deaths.2
In addition to the direct effect of COVID-19, there are concerns about indirect morbidity and mortality from COVID-19–related policies and health system changes.3 From March 2020 to November 2021, federal and state governments imposed a number of measures on SNFs to reduce the spread of COVID-19, including closing their premises to all visitors and eliminating communal dining and other group activities.4 These policies imposed a level of isolation that may have worsened cognitive and physical functioning.5,6,7 The pandemic also led to staffing shortages8 and disruption in the delivery of outpatient,9,10 hospital,11,12 and surgical care13 that may have also affected medically complex LTC residents.
Collectively, these changes imply that even in SNFs without a COVID-19 outbreak, LTC residents faced many new challenges beginning in 2020. Although a growing literature has documented the effect of COVID-19 on nursing homes, less evidence exists on health outcomes among LTC residents who did not contract COVID-19 but nevertheless endured stress and isolation during the pandemic. Understanding the effects of pandemic-related policy change is critical to define the trade-offs involved when facing future pandemic waves.
To address this evidence gap, this study used Medicare administrative claims to assess health and quality of life outcomes among SNFs with no COVID-19 outbreaks, which would reflect policy and health system change alone, and among SNFs during active outbreaks whose changes would reflect COVID-19 combined with policy change.
Methods
Study Sample and Data Sources
This study was approved by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health. Informed consent was waived.
This study used Medicare administrative claims and the Minimum Data Set (MDS) version 3.0 (a federally mandated clinical assessment of all patients in a SNF) from January through November in each of the years 2018 through 2020. Complete December 2020 data for MDS were not yet available at the time of analysis, so we compared the same months in the prepandemic period. SNF residents were eligible for inclusion in the analyzed LTC cohort if they were Medicare beneficiaries enrolled with 12 months of continuous Part A and Part B fee-for-service coverage in a calendar year. The sample identified LTC residents (in contrast to short-stay patients in SNFs for temporary postacute care) using previously published algorithms that rely on SNF entry date and the last recorded assessment in the MDS.14,15 LTC residents were attributed to SNFs based on their SNF of residence as of January 1 in each calendar year. Therefore, each SNF had a fixed cohort of LTC residents in each calendar year that could shrink by mortality. No new LTC residents were allowed to enter the cohort within a year, and any LTC residents who left their facility were retained in the sample with their original SNF attribution. These requirements were imposed to avoid the potential bias of admission or discharge thresholds for LTC residents changing over time in 2020 compared with 2018 and 2019.
Defining COVID-19 Outbreak States
SNFs were assigned into 2 mutually exclusive groups on a monthly basis in 2020: active outbreak (≥1 COVID-19 diagnosis in the current or past month[s]) or no-known outbreak (no COVID-19 diagnosis up to and including that month). Thus, membership in each group varied month by month. A COVID-19 diagnosis was defined as the presence of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code U07.1 in any diagnosis field in a Medicare claim in the inpatient, outpatient, SNF, or carrier files. Once SNFs had their first resident with a COVID-19 diagnosis, they transitioned from no-known to active outbreak status and were not able to return to no-known COVID-19 group status. Compared with data self-reported by SNFs to the federal National Healthcare Safety Network,16,17 the definitions for the 2 outbreak groups intentionally favored classifying SNFs as active to ensure that the no-known COVID-19 outbreak group accurately identified facilities with no active or recent infections (eTable 1 in the Supplement).
SNFs in each of the 2 COVID-19 outbreak groups for each month in 2020 were assigned to the same group for those months in 2018 and 2019. For example, an SNF in the active outbreak group in April 2020 was also categorized in the active group for April 2018 and 2019. The membership in each group differed from month to month, but within each month, SNF membership was identical for 2018, 2019, and 2020.
Study Outcomes
Using Medicare claims, 3 health and utilization outcomes were assessed by month for each SNF group as previously described in the literature18,19: mortality, acute care hospitalization, and emergency department (ED) visits not resulting in hospitalization. Outcomes were calculated as monthly proportions of residents who were hospitalized or had an ED visit at least once across all SNFs in the analysis group.
MDS assessment data were used to measure 3 quality of life outcomes: functional status, weight change, and worsening depressive symptoms. MDS assessments occur at admission, quarterly (including additional information gathered on an annual basis), and with a significant change in clinical status.20 These outcomes were captured as follows: functional status assessed by the number of activities of daily living (ADLs) the residents were unable to perform; resident body weight in pounds (to convert pounds to kilograms, multiply by 0.45); and depressive symptoms identified using the MDS implementation of the Patient Health Questionnaire-9 (PHQ-9),21 a commonly used scale to assess depressive symptoms. For each patient who had an MDS assessment during a given month of a given year, the analysis across SNF patients monthly included the change in each patient’s ADLs requiring assistance (ranging from 0, independent, to 4, fully dependent), change in weight, and whether the PHQ-9 score (ranging from 0, no symptoms, to 27, maximal symptoms) worsened compared with the most recent assessment in the prior year (eg, last assessment in 2019 for a month in 2020). Therefore, only patients with completed MDS assessments in any given month contributed to monthly outcomes for a SNF group.
Study Variables
Patient characteristics included age, race and ethnicity, sex, Medicaid eligibility, number of chronic conditions (measured using 27 flags for individual diagnoses from the Chronic Conditions Data Warehouse research database),22 and indicators for patients who were frail or had dementia (defined in previous publications).23,24 Race and ethnicity, which were self-reported by Medicare beneficiaries at the time of Medicare enrollment, were included to control for potential differences in COVID-19 outcomes associated with race and ethnicity.25 SNF characteristics were measured using data from Nursing Home Compare and the LTCFocus data sets, both nationally comprehensive public data sets on nursing home care.26,27 These characteristics included SNF profit status (for profit, nonprofit, public), geography (urban vs rural, state), number of beds, chain membership, and Medicare overall star rating scale (ranging from 1, poor, to 5, excellent).
Statistical Analysis
Characteristics of SNFs and LTC residents were compared between SNFs with active COVID-19 vs no-known COVID-19 at 2 representative time points, June and November 2020. Unadjusted outcomes were plotted overall and by SNF group, with 95% CIs estimated assuming a normal distribution of the mean outcomes.
To model the difference in outcomes attributable to the pandemic across the year, for each SNF group, separate linear regression models were estimated at the resident-month level. Each model controlled for SNF fixed effects and beneficiary characteristics (age, sex, race and ethnicity, Medicaid eligibility, indicators for 27 chronic conditions). In all models, 2018 and 2019 served as the reference group for indicators of the year, such that each SNF in 2020 was compared with itself in 2018 and 2019 as a control. For the active outbreak group, the coefficient for the key 2020 indicators is interpreted as the change attributable to COVID-19 infection as well as SNF-level policy and health system changes. For the no-known COVID-19 outbreak group, the coefficient can be interpreted as the change attributable to all SNF-level changes in 2020 besides COVID-19 infection.
For health outcomes, the linear regression models spanned 11 calendar months, January through November, and included monthly fixed effects to allow temporal changes during control years. The key quantity of interest was the coefficient for an indicator of the year 2020, which provided an estimate for the mean monthly change in each health outcome attributable to all changes occurring in 2020 vs 2018-2019.
For quality of life outcomes, the same models were estimated but also included a set of interaction terms between calendar month and the year 2020 instead of a single indicator for 2020. The key quantity of interest for quality of life outcomes was the coefficient on the interaction between November 2020 (the latest month of data) and an indicator for 2020 vs 2018-2019. Quality of life outcomes in each month reflect changes compared with the most recent MDS assessment in the prior year; therefore, this quantity of interest reflects the cumulative change during 2020 through November.
There were no missing data; the beneficiaries with an unknown race and ethnicity (<1%) were classified as “other” or “unknown.” Analyses were performed in SAS version 9.4 (SAS Institute Inc). The 95% CI around reported estimates reflects 2-sided estimates (0.025 in each tail) or P ≤ .05. Because of the potential for type I error due to multiple comparisons, findings should be interpreted as exploratory.
Sensitivity Analyses
We performed a series of sensitivity analyses to examine the effect of alternative classifications of outbreak groups on our results. We replicated the analyses above for SNFs grouped as (1) never vs ever had a COVID-19 case, (2) 3 outbreak groups (no COVID-19 case, 1 to 5 months in active status, or ≥6 months in active status) with constant membership month by month, (3) an active group excluding months with 1 or no COVID-19 cases, and (4) the same groupings as the main analysis but split into subgroups by US Census Bureau region. We also examined trends in outcomes for former outbreak SNF-months, defined as no COVID-19 diagnosis for 2 or more months, by definition only possible beginning in March 2020.
Results
Study Sample and COVID-19 Infection Status
There were 44 314 SNF-years with 2 985 864 LTC resident-years from 2018 to 2020 included in the study. In 2020, there were 997 418 residents, of whom 66 291 (66.2%) were women and 755 045 (75.7%) were White non-Hispanic individuals with median age 81 years (IQR, 71-88 years). The characteristics of SNFs and their residents were similar between 2018-2019 and 2020 (eTable 2 in the Supplement).
From January to November 2020, 76 878 SNF-months (41.4%) were categorized as active COVID-19 months, and 97 412 (52.4%) as no-known COVID-19 months. The remaining 11 434 (6.2%) SNF-months were categorized as former COVID-19 months (eTable 3 in the Supplement).
Because SNF COVID-19 status varied by month, characteristics of SNFs were compared in selected months as example cross-sections. In June 2020, the 6935 active COVID-19 SNFs, compared with the 7239 no-known COVID-19 SNFs, had more beds (128.8 vs 89.7), were more often for-profit (75.0% vs 65.4%) and urban (85.5% vs 59.3%), and located in the Northeastern US (25.5% vs 9.5%); Table 1. There were smaller differences in the patient populations between these 2 groups, although patients in active COVID-19 SNFs were less likely to be White than those in no-known COVID-19 SNFs (311 585 [69.0%] vs 286 019 [84.1%]). These patterns were consistent among active COVID-19 and no-known COVID-19 SNFs in November 2020 (eTable 4 in the Supplement).
Table 1. Skilled Nursing Facility and Resident Characteristics by COVID-19 Exposure Status as of June 30, 2020.
| Active outbreak in June 30, 2020 (active COVID-19 SNFs), % | No outbreak by June 30, 2020 (no known COVID-19 SNFs), % | |
|---|---|---|
| SNF characteristics | ||
| SNFs, No. | 6935 | 7239 |
| For profit | 75.0 | 65.4 |
| Not-for-profit | 20.2 | 26.5 |
| Public | 4.7 | 8.1 |
| Urbana | 85.5 | 59.3 |
| No. of beds, median (IQR) | 120 (90-150) | 83 (57-115) |
| Part of a chain | 56.9 | 58.6 |
| Hospital based | 2.2 | 6.1 |
| Region | ||
| South | 35.9 | 33.9 |
| Northeast | 25.5 | 9.5 |
| Midwest | 23.3 | 41.5 |
| West | 15.3 | 15.0 |
| Overall Medicare star rating, mean (SD)b | 3.26 (1.4) | 3.39 (1.4) |
| County-level COVID-19 prevalence per 1000 beneficiaries, mean (SD)c | 1.29 (2.44) | 0.47 (0.62) |
| LTC resident characteristics | ||
| Residents, No. | 451 572 | 340 094 |
| Age, median (IQR), y | 80 (73-89) | 82 (73-89) |
| ≤64 | 11.0 | 9.5 |
| 65-79 | 36.4 | 33.0 |
| ≥80 | 52.6 | 57.4 |
| Women | 64.8 | 67.0 |
| Men | 35.2 | 33.0 |
| Medicaid dual eligible | 66.8 | 61.1 |
| Disabled without ESRD | 10.8 | 9.4 |
| Fraild | 19.4 | 20.3 |
| Dementiae | 56.6 | 55.4 |
| No. of chronic conditions, median (IQR)f | 5 (1-8) | 5 (1-7) |
| Race and ethnicity | ||
| Hispanic | 7.9 | 4.1 |
| Non-Hispanic Black | 19.0 | 8.9 |
| Non-Hispanic White | 69.0 | 84.1 |
| Other or unknowng | 4.1 | 2.9 |
Abbreviations: ESRD, end-stage renal disease; LTC, long-term care; SNF, skilled nursing facility.
Urban was defined as a patient residing in a metropolitan zip code. Urban locations were identified using the Health Resources and Service Administration rural-urban commuting area code database (http://depts.washington.edu/uwruca/index.php).
Medicare star ratings range from 1 (much below average) to 5 (much above average). This score is a composite ranking of individual SNFs that incorporates multiple measures of SNF quality, staffing, and health inspection performance.
COVID-19 cases were defined using publicly available data from the New York Times COVID-19 database (https://github.com/nytimes/covid-19-data). Cumulative county-level cases as of November 2020 were tabulated and normalized per 100 residents in each county.
Frailty was defined using a previously validated claims-based frailty index from Kim et al.23,28 Patients in the top 10% of the frailty index over the entire population of SNF admissions were classified as frail.
Dementia was defined using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes described in Goodman et al.24
The presence of 27 conditions was gathered from the Chronic Condition Data Warehouse (CCW), which uses claims since 1999 to describe Medicare beneficiaries’ accumulated chronic disease burden. Chronic conditions were defined as any condition present by the end of the calendar year. Conditions included Alzheimer disease, Alzheimer disease and related disorders or senile dementia, anemia, asthma, atrial fibrillation, benign prostatic hyperplasia, breast cancer, cataract, chronic kidney disease, chronic obstructive pulmonary disease, colorectal cancer, depression, diabetes, endometrial cancer, glaucoma, heart failure, hip or pelvic fracture, hyperlipidemia, hypertension, hypothyroidism, ischemic heart disease, lung cancer, osteoporosis, prostate cancer, acute myocardial infarction, rheumatoid arthritis, and stroke or transient ischemic attack.
“Other” race options at Medicare enrollment included American Indian or Alaska Native; Asian; Native Hawaiian or Pacific Islander. Less than 1% of beneficiaries are coded as “unknown” race.
Overall Changes in Health Outcomes
Overall, SNFs had significantly higher monthly LTC resident mortality in 2020 than in prior years, with a peak in April 2020 (3.96% [95% CI, 3.92%-3.99%] vs 2.48% [95% CI, 2.33%-2.62%] in 2018 and 2019; Figure 1). However, hospitalization and ED visit rates were significantly lower in 2020 than in prior years. For example, in April 2020, SNFs hospitalized 2.60% (95% CI, 2.57%-2.63%) of their residents, compared with 3.46% (95% CI, 3.32%-3.61%) in April 2018 and April 2019, and sent 1.72% (95% CI, 1.69%-1.74%) of their residents to the ED in April 2020, compared with 3.32% (95% CI, 3.18%-3.46%) in April 2018 and April 2019. However, overall hospitalizations also declined from 3.21% in 2018 (95% CI, 3.18-3.24) to 2.96% (95% CI, 2.92%-2.99%) in 2019 overall, suggesting that the existence of prepandemic trends in hospitalization declined.
Figure 1. Health and Utilization Outcomes for Long-term Care Residents by Skilled Nursing Facility COVID-19 Exposure Status in 2018, 2019, 2020.
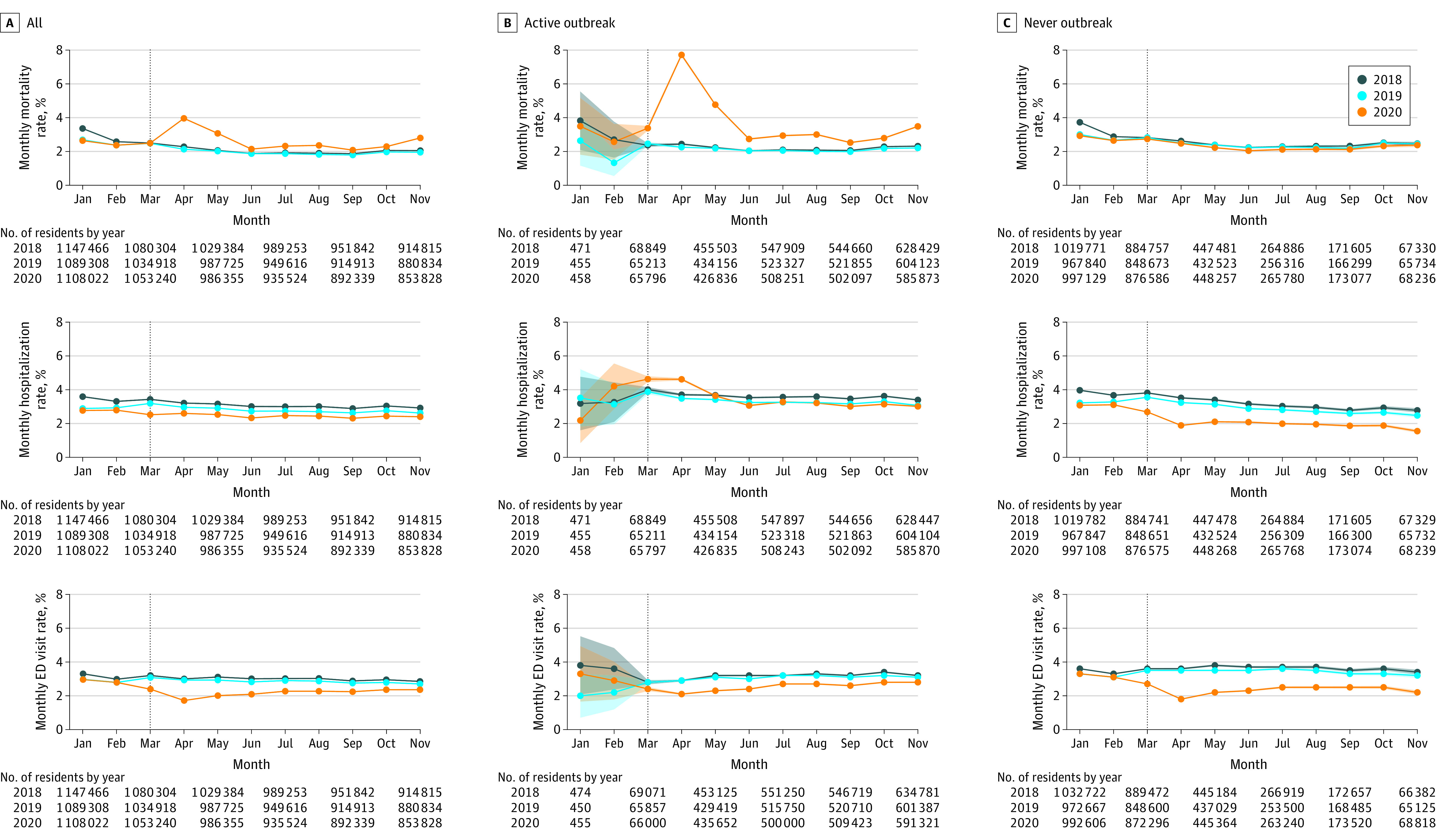
Each figure shows an array of all 15 477 skilled nursing facilities (SNFs) stratified by COVID-19 exposure group vs outcome measured monthly. Within the panels, results are reported for 2018, 2019, and 2020 with 95% CIs (shaded lines; shown only for the time intervals where N is small). The vertical line denotes March 2020, the month when the SNF lockdown policies became effective in the US. For the first 2 rows, outcomes represent average within-individual changes from the current month compared with the most recent assessment from the prior year. Higher scores indicate worse symptoms.
ED indicates emergency department.
Quality of life outcomes were significantly worse in 2020 among LTC residents in SNFs than in prior years. By November 2020, SNF residents required assistance with a greater number of ADLs than in 2018 and 2019, a mean increase of 1.08 (95% CI, 1.06-1.10) in 2020 vs 0.81 (95% CI, 0.80-0.82) in 2018-2019 (Figure 2), compared with the most recent assessment from the prior year.
Figure 2. Quality of Life Outcomes for Long-term Care Residents by Skilled Nursing Facility COVID-19 Exposure Status in 2018, 2019, 2020.
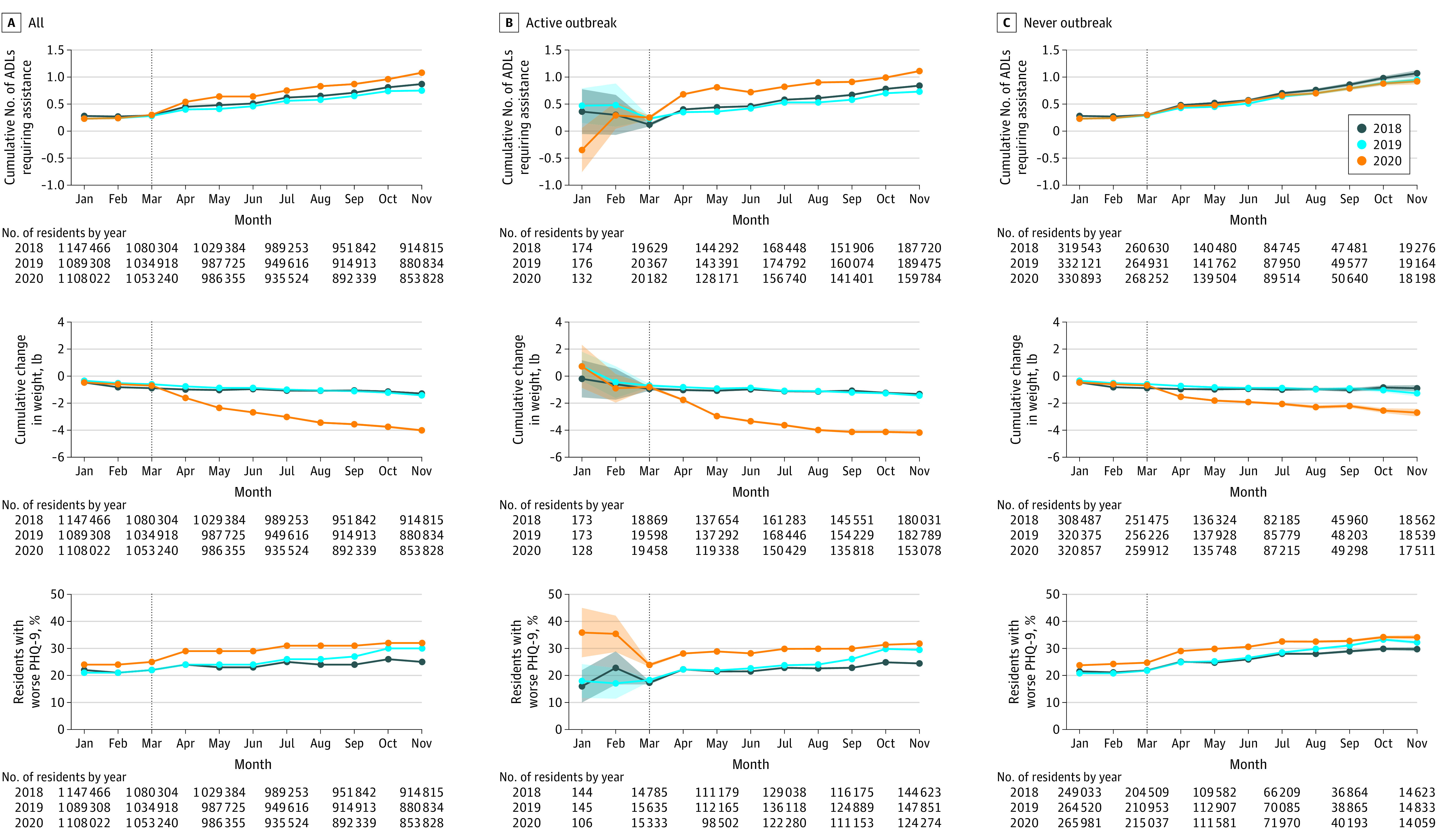
Each panel shows monthly outcomes for all 15 477 skilled nursing facilities (SNFs) in the study sample or SNFs by COVID-19 exposure group in 2018, 2019, and 2020 with 95% CIs (shaded lines; shown only for the time intervals where N is small) assuming normal distribution of mean outcome estimates. The vertical line denotes March 2020, the month when SNF lockdown policies became effective in the US. The quality of life outcomes (activities of daily living [ADLs] requiring assistance, weight change, Patient Health Questionnaire 9 [PHQ-9] symptom worsening) all represent average within-individual changes from the current month compared with the most recent assessment in the prior year. See the Methods section for PHQ-9 definitions. To convert pounds to kilograms, multiply by 0.45.
Similarly, residents had significantly higher weight loss by November 2020 (−4.26 lb [95% CI, −4.36 to −4.16 lb]) compared with 2018 and 2019 (−1.36 lb [95% CI, −1.41 to −1.31 lb]). In November 2020, a significantly higher proportion of SNF residents had worsened depressive symptoms than in their most recent prior year assessment, 32% (95% CI, 31.8%-32.3%) compared with November 2018 and 2019 (27.2% [95% CI 27.1%-27.3%]). However, PHQ-9 scores began to worsen prior to 2020, suggesting prepandemic trends.
Outcomes by COVID-19 Exposure Status
In 2018-2019, mean monthly mortality was 2.2%, mean monthly hospitalization rate was 3.0%, and mean monthly ED visit rate was 2.9% across all SNFs (Figure 2 and Table 2). In 2020, among active COVID-19 SNFs compared with their own 2018-2019 baseline, mortality increased by 1.60% (95% CI, 1.58% to 1.62%), while hospitalization rates decreased by 0.10% (95% CI, −0.12% to −0.09%), and ED visit rates decreased by 0.57% (95% CI, −0.59% to −0.55%). Among no-known COVID-19 SNFs compared with their own 2018-2019 baseline, mortality decreased by 0.15% (95% CI, −0.16% to −0.13%), hospitalizations decreased by 0.83% (95% CI, −0.85% to −0.81%), and ED visits decreased by 0.79% (95% CI, −0.81% to −0.77%). All changes were statistically significant.
Table 2. Adjusted Differential Change in Health and Quality of Life Outcome for Long-term Care Residents by COVID-19 Exposure Status, 2020 vs 2018-2019.
| Outcome | Unadjusted 2018-2019 rate, %b | Change in outcome, January-November 2018-2019c | Adjusted difference, 2020 vs 2018-2019a | |
|---|---|---|---|---|
| Active COVID-19 outbreak (95% CI), % | No-known COVID-19 outbreak (95% CI), % | |||
| Health and utilization | ||||
| Monthly mortality | 2.2 | 1.60 (1.58 to 1.62) | −0.15 (−0.16 to −0.13) | |
| Monthly hospitalization | 3.0 | −0.10 (−0.12 to −0.09) | −0.83 (−0.85 to −0.81) | |
| Monthly ED visits | 2.9 | −0.57 (−0.59 to −0.55) | −0.79 (−0.81 to −0.77) | |
| Quality of life (within-resident change from last assessment in prior year to November annually) | ||||
| Change in No. of ADLs requiring assistance by November (higher is worse) | 0.89 | 0.36 (0.34 to 0.38) | −0.06 (−0.12 to 0.01) | |
| Change in weight by November, lb (lower is worse) | −1.9 | −3.1 (−3.2 to −3.0) | −1.8 (−2.1 to −1.49) | |
| % of residents with worse PHQ-9 by November (higher is worse)d | 27.1 | 4.4 (4.1 to 4.7) | 3.2 (2.3 to 4.1) | |
Abbreviations: ADLs, activities of daily living; ED, emergency department; PHQ-9, Patient Health Questionnaire-9.
Conversion factor: To convert pounds to kilograms, multiply by 0.45.
Adjusted differences represent the difference within the active and no-known COVID-19 groups for each outcome in 2020 vs the average in 2018-2019. Each adjusted difference is estimated using separate linear regression models at the resident-month level. Each model controls for SNF fixed effects (which control for all observable and unobservable SNF characteristics that are time invariant) and beneficiary characteristics (age, sex, race and ethnicity, Medicaid eligibility, indicators for 27 chronic conditions). These results come from coefficients on an indicator for the year 2020 (health and utilization outcomes) or an interaction term between an indicator for year 2020 and November (quality of life outcomes, which are cumulative over the year) for each outcome in adjusted models.
The mean unadjusted monthly rate across all months in 2018-2019. For the quality of life outcomes, the 2018-2019 baseline is the average within-individual change for all assessments by November in 2018 and 2019 vs the prior year.
Mean within-individual cumulative change for all assessments from January to November 2018 and 2019.
The range for the PHQ-9 is 0 to 27 points. Higher scores indicate worse symptoms. This outcome measures whether residents had a worsened PHQ-9 score compared with their latest assessment in the prior year.
In 2018-2019, across all SNFs, residents required assistance with an additional 0.89 ADLs between January and November on average and lost 1.9 lb on average; 27.1% of residents had worsened depressive symptoms. In 2020, residents in active COVID-19 SNFs required assistance with an additional 0.36 ADLs (95% CI, 0.34 to 0.38), lost 3.1 lb (95% CI, −3.2 to −3.0 lb) more weight, and were 4.4% (95% CI, 4.1% to 4.7%) more likely to have worsened depressive symptoms compared with the same SNFs’ 2018-2019 baseline, all statistically significant changes. In 2020, residents in no-known COVID-19 SNFs had no significant change in the number of ADLs requiring assistance (−0.06 [95% CI, −0.12 to 0.01]), but lost 1.8 lb (95% CI, −2.1 to −1.5 lb) more weight and were 3.2% more likely (95% CI, 2.3% to 4.1%) to have worsened depressive symptoms, both statistically significant changes.
Sensitivity Analyses
Comparing 2020 vs 2018-2019, SNFs with no recent cases of COVID-19 (former COVID-19 group) had decreased mortality in the presence of much lower rates of hospitalization and ED visits and had a significant decrease in body weight (eFigure and eTable 5 in the Supplement). Alternative outbreak groupings using a never and ever group and an active COVID-19 group, excluding months with 1 or no cases, showed similar results to the main analysis (eTable 6 in the Supplement). Groupings with constant SNF membership also had qualitatively similar results when comparing the never group and groups that had 6 or more active outbreaks with the active group in the main analysis, with the exception of a larger decline in hospitalization rates (eTable 7 in the Supplement). There were also qualitatively similar results for subgroups of SNFs by census region, with variation in the magnitude of changes by outcome (eTable 8 in the Supplement).
Discussion
In 2020, before the widespread availability of COVID-19 vaccination, mortality for LTC residents in SNFs across the US increased substantially and multiple indicators for quality of life worsened. Although notable decrements in health were seen in residents of SNFs with active COVID-19 outbreaks, both SNFs that never had a documented COVID-19 case and those with only previous cases also experienced significant declines in health, as assessed by weight loss, and to a lesser degree, depressive symptoms.
Overall, these findings suggest that pandemic policy and health system changes in 2020 were associated with negative changes in the physical and mental health of SNF residents not exposed to COVID-19 infection, counterbalanced by lower mortality and hospitalization rates.
When many lockdown policies were implemented early in the COVID-19 pandemic, policy makers and clinical leaders could not foresee that these policies would continue for many months. As the lockdown continued and as many people recognized the importance of protecting medically vulnerable individuals from exposure to COVID-19, there was growing concern about the unintended effects of lockdown on LTC residents. There has been speculation that increased isolation and loneliness from lockdown in nursing homes could worsen SNF residents’ physical and emotional health,3 but there has been little data to quantify this on a national scale. These findings suggest that the aforementioned concerns are plausible and that pandemic-related lockdown policies in SNFs were associated with changes in SNF residents’ well-being, reflected primarily by weight loss and to a smaller degree by depressive symptoms. Weight loss is particularly concerning because frailty and sarcopenia, which can be exacerbated by weight loss in this vulnerable population, are risk factors for increased morbidity and mortality.29,30,31
Unlike the decrements in quality of life, the increased mortality among LTC residents during the pandemic was entirely limited to facilities with active COVID-19 outbreaks, while SNFs without COVID-19 and those with prior COVID-19 had a modest decrease in mortality. The cause of this decrease in mortality is unclear, but some of the decrement may be attributable to tight infection control policy that likely reduced transmission of other nosocomial infections, a chronic problem in SNFs predating the COVID-19 pandemic.32,33,34,35 There are other plausible mechanisms. For example, increased isolation may have also limited resident mobility in nursing homes, leading to both fewer falls and worse quality of life outcomes.
The decrease in mortality occurred in the presence of a substantial decline in both hospitalization and ED use, likely due to a higher threshold to send LTC residents to the hospital during COVID-19 surges.11,12 The observed changes during the pandemic suggest that some hospital use in SNFs has little mortality benefit. This is relevant for initiatives to reduce excessive hospitalization in SNFs, which have been a major focus of nursing home quality-improvement efforts for years.36,37,38
Limitations
This study has several limitations. First, the study population was limited to patients with Medicare fee-for-service coverage, which represents only some of the long-term residents in SNFs. Therefore, these results may not generalize to all SNF residents. Second, the measure of COVID-19 exposure at the SNF level may not have captured all cases because the sample included only observed COVID-19 diagnoses in Medicare claims, rather than directly observed test results or other indicators. There may also be bias from possible mismatch between the time a COVID-19 diagnosis appeared in the claims compared with true onset of symptoms. Also, this analysis does not incorporate data on SNF staff member infections, which may influence SNF group membership. Third, the quality of life measures relied on within-resident differences between MDS assessments, which could vary in time and be heterogenous. Fourth, as with any observational analysis, there is a risk of unmeasured confounding, primarily related to differences between facilities that did vs did not have COVID-19 outbreaks. However, using each SNF as its own control and observing the similarity of the patterns among no-known COVID-19 and former COVID-19 SNFs strengthen the possibility that these findings are not driven by confounding. Also, the decline in outcomes among former COVID-19 SNFs suggests that differences between SNFs with active and no-known COVID-19 were not entirely driven by unobserved confounding because all SNFs in the former COVID-19 group were once in the active outbreak group by definition. Fifth, the clinical relevance of some of the smaller changes observed, such as higher rates of worsened PHQ-9 symptoms, is still unclear. Sixth, the data and analyses are based on a time frame during the pandemic without use of vaccination and without clear definition of the role of other therapies (antivirals, steroids, monoclonal antibodies). Because of these early SARS-CoV-2 variants, it is uncertain whether or how these findings may be applicable to COVID-19 infections in 2022. Seventh, this analysis is not able to isolate which specific policy or policies may have been most harmful or exclude the possibility that other health system changes (eg, reduced staffing or increased staff turnover) may be driving some of these results. However, this analysis provides evidence on the trade-offs that may be inherent in aggressive infection control policy when considering guidance for future pandemics.
Conclusions
Among skilled nursing facilities in the US during the first year of the COVID-19 pandemic and prior to the availability of COVID-19 vaccination, mortality and functional decline significantly increased at facilities with active COVID-19 cases compared with the prepandemic period, while a modest statistically significant decrease in mortality was observed at facilities that had never had a known COVID-19 case. Weight loss and depressive symptoms significantly increased in skilled nursing facilities in the first year of the pandemic, regardless of COVID-19 status.
eTable 1. Comparison of Outbreak Status Classification Between Medicare Claims and National Healthcare Safety Network Data
eTable 2. Characteristics of SNFs in 2018-2019 vs 2020
eTable 3. SNF Outbreak Classification by Month in 2020
eTable 4. Characteristics of Active and Never SNFs in November, 2020
eTable 5. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in Former SNFs, 2020 vs 2018-2019
eTable 6. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in SNFs with Alternate Group Definitions, 2020 vs 2018-2019
eTable 7. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in SNFs with Constant Membership Groups, 2020 vs 2018-2019
eTable 8. Adjusted Differential Change in Health and Quality of Life Outcome for LTC Residents in SNFs by US Census Region, 2020 vs 2018-2019
eFigure. Health and Quality of Life Outcomes in Former-SNFs
References
- 1.Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1(3):e200369-e200369. doi: 10.1001/jamahealthforum.2020.0369 [DOI] [PubMed] [Google Scholar]
- 2.Shen K, Loomer L, Abrams H, Grabowski DC, Gandhi A. Estimates of COVID-19 cases and deaths among nursing home residents not reported in federal data. JAMA Netw Open. 2021;4(9):e2122885. doi: 10.1001/jamanetworkopen.2021.22885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levere M, Rowan P, Wysocki A. The adverse effects of the COVID-19 pandemic on nursing home resident well-being. J Am Med Dir Assoc. 2021;22(5):948-954.e2. doi: 10.1016/j.jamda.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidance for infection control and prevention of Coronavirus disease (COVID-19) in nursing homes. Centers for Medicare & Medicaid Services . 2020;Updated March 10, 2021. Accessed June 21, 2022. https://www.cms.gov/files/document/qso-20-14-nh-revised.pdf
- 5.Abbasi J. Social isolation—the other COVID-19 threat in nursing homes. JAMA. 2020;324(7):619-620. doi: 10.1001/jama.2020.13484 [DOI] [PubMed] [Google Scholar]
- 6.Paulin E. Is isolation killing America’s nursing home residents? AARP. September 3, 2020. Accessed June 21, 2022. https://www.aarp.org/caregiving/health/info-2020/covid-isolation-killing-nursing-home-residents.html
- 7.Simard J, Volicer L. Loneliness and isolation in long-term care and the COVID-19 pandemic. J Am Med Dir Assoc. 2020;21(7):966-967. doi: 10.1016/j.jamda.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarry BE, Grabowski DC, Barnett ML. Severe staffing and personal protective equipment shortages faced by nursing homes during the COVID-19 pandemic: study examines staffing and personal protective equipment shortages faced by nursing homes during the COVID-19 pandemic. Health Aff (Millwood). 2020;39(10):1812-1821. doi: 10.1377/hlthaff.2020.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff (Millwood). 2021;40(2):349-358. doi: 10.1377/hlthaff.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern Med. 2021;181(3):388-391. doi: 10.1001/jamainternmed.2020.5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smulowitz PB, O’Malley AJ, Khidir H, Zaborski L, McWilliams JM, Landon BE. National trends in ED visits, hospital admissions, and mortality for Medicare patients during the COVID-19 pandemic. Health Aff (Millwood). 2021;40(9):1457-1464. doi: 10.1377/hlthaff.2021.00561 [DOI] [PubMed] [Google Scholar]
- 13.Prasad NK, Englum BR, Turner DJ, et al. A nation-wide review of elective surgery and COVID-surge capacity. J Surg Res. 2021;267:211-216. doi: 10.1016/j.jss.2021.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories. Health Serv Res. 2011;46(1 pt 1):120-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. doi: 10.1186/s12913-017-2318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long-term care facilities (LTCF) component. National Healthcare Safety Network, Centers for Disease Control and Prevention. Accessed June 21, 2022. https://www.cdc.gov/nhsn/ltc/index.html
- 17.COVID-19 nursing home data. Centers for Medicare & Medicaid Services. Accessed June 21, 2022. https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/
- 18.Barnett ML, Joynt Maddox KE, Orav EJ, Grabowski DC, Epstein AM. Association of skilled nursing facility participation in a bundled payment model with institutional spending for joint replacement surgery. JAMA. 2020;324(18):1869-1877. doi: 10.1001/jama.2020.19181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. 2019;380(3):252-262. doi: 10.1056/NEJMsa1809010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minimum data set (MDS) 3.0 resident assessment instrument (RAI) manual. Centers for Medicare & Medicaid Services. Accessed June 21, 2022. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual
- 21.Jenny Wei Y-J, Chen C, Fillingim RB, et al. Uncontrolled pain and risk for depression and behavioral symptoms in residents with dementia. J Am Med Dir Assoc. 2021;22(10):2079-2086.e5. doi: 10.1016/j.jamda.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronic conditions data warehouse. Centers for Medicare & Medicaid Services. Accessed June 21, 2022. https://www.ccwdata.org/.
- 23.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement. 2017;13(1):28-37. doi: 10.1016/j.jalz.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care. 2020;58(1):e1-e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Find & compare nursing homes, hospitals and other providers near you. Medicare.gov. Accessed June 21, 2022. https://www.medicare.gov/NursingHomeCompare/Data/About.html
- 27.Shaping long-term care in America Project. Yale University School of Public Health. Accessed June 21, 2022. https://ltcfocus.org.
- 28.Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. How do frail Medicare beneficiaries fare under bundled payments? J Am Geriatr Soc. 2019;67(11):2245-2253. doi: 10.1111/jgs.16147 [DOI] [PubMed] [Google Scholar]
- 29.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309-323. doi: 10.1146/annurev.nutr.22.010402.102715 [DOI] [PubMed] [Google Scholar]
- 30.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBlanc ES, Rizzo JH, Pedula KL, et al. ; Study of Osteoporotic Fractures Research Group . Long-Term weight trajectory and risk of hip fracture, falls, impaired physical function, and death. J Am Geriatr Soc. 2018;66(10):1972-1979. doi: 10.1111/jgs.15532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall AJ, Wikswo E, Pringle K, Gould LH, Parashar UD. Vital signs: foodborne norovirus outbreaks—United States, 2009–2012. MMWR Morb Mortal Wkly Rep. 2014;63(22):491-495. [PMC free article] [PubMed] [Google Scholar]
- 33.Salem-Schatz S, Griswold P, Kandel R, et al. A statewide program to improve management of suspected urinary tract infection in long-term care. J Am Geriatr Soc. 2020;68(1):62-69. doi: 10.1111/jgs.16261 [DOI] [PubMed] [Google Scholar]
- 34.Ponnada S, Guerrero DM, Jury LA, et al. Acquisition of Clostridium difficile colonization and infection after transfer from a Veterans Affairs hospital to an affiliated long-term care facility. Infect Control Hosp Epidemiol. 2017;38(9):1070-1076. doi: 10.1017/ice.2017.140 [DOI] [PubMed] [Google Scholar]
- 35.Karanika S, Grigoras C, Flokas ME, et al. The attributable burden of Clostridium difficile infection to long-term care facilities stay: a clinical study. J Am Geriatr Soc. 2017;65(8):1733-1740. doi: 10.1111/jgs.14863 [DOI] [PubMed] [Google Scholar]
- 36.Kane RL, Huckfeldt P, Tappen R, et al. Effects of an intervention to reduce hospitalizations from nursing homes: a randomized implementation trial of the INTERACT program. JAMA Intern Med. 2017;177(9):1257-1264. doi: 10.1001/jamainternmed.2017.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingber MJ, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff (Millwood). 2017;36(3):441-450. doi: 10.1377/hlthaff.2016.1310 [DOI] [PubMed] [Google Scholar]
- 38.Grabowski DC, O’Malley AJ. Use of telemedicine can reduce hospitalizations of nursing home residents and generate savings for Medicare. Health Aff (Millwood). 2014;33(2):244-250. doi: 10.1377/hlthaff.2013.0922 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of Outbreak Status Classification Between Medicare Claims and National Healthcare Safety Network Data
eTable 2. Characteristics of SNFs in 2018-2019 vs 2020
eTable 3. SNF Outbreak Classification by Month in 2020
eTable 4. Characteristics of Active and Never SNFs in November, 2020
eTable 5. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in Former SNFs, 2020 vs 2018-2019
eTable 6. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in SNFs with Alternate Group Definitions, 2020 vs 2018-2019
eTable 7. Adjusted Differential Change in Health and Quality of Life Outcomes for LTC Residents in SNFs with Constant Membership Groups, 2020 vs 2018-2019
eTable 8. Adjusted Differential Change in Health and Quality of Life Outcome for LTC Residents in SNFs by US Census Region, 2020 vs 2018-2019
eFigure. Health and Quality of Life Outcomes in Former-SNFs


