This systematic review and meta-analysis evaluates the Ages and Stages Questionnaire's utility as a screening or diagnostic tool to identify developmental delay in children aged 12-60 months.
Key Points
Question
Does the Ages and Stages Questionnaire (ASQ) have the potential to identify developmental delay in children aged 12 to 60 months?
Findings
ASQ scores more than 2 SDs below the mean had moderate sensitivity and specificity to predict any delay, severe delay, motor delay, and cognitive delay.
Meaning
If a child aged 12-60 months passes all ASQ domains, there is a moderate probability that they do not have severe developmental delay; if they fail the ASQ motor or cognitive domain, there is a moderate probability that they have motor or cognitive delay, respectively.
Abstract
Importance
The Ages and Stages Questionnaire (ASQ) is a commonly used developmental screening tool, but its utility is debated.
Objectives
To conduct a a systematic review and meta-analysis to evaluate ASQ’s utility as a screening or diagnostic tool to identify developmental delay in children aged 12-60 months.
Data Sources
Medline, EMBASE, CINAHL, PsycINFO, and Mednar were searched from inception until December 2021.
Study Selection
Studies meeting both criteria were included. ASQ was performed at age 12 to 60 months or where the median age at ASQ was at least 12 months and formal developmental assessments were done within 2 months of ASQ.
Data Extraction and Synthesis
True positive, false positive, false negative, and true negatives from individual studies were extracted. Meta-analysis was conducted with Stata version 16.1. Risk of bias was assessed using the QUADAS-2 tool. Certainty of evidence (COE) was assessed using GRADE guidelines.
Main Outcomes and Measures
Ability of ASQ scores more than 2 SDs below the mean in more than 1 domain (ASQ-2SD) to identify any developmental delay or severe delay. Based on generally accepted interpretation of likelihood ratio (LR) values, a positive LR (PLR) more than 5 and a negative LR (NLR) of 0.2 or less were considered necessary to rule in or rule out developmental delay, respectively, with at least moderate probability.
Results
Initial search yielded 5777 citations of which 43 were included in the review. Of them, 36 were included in the meta-analysis. The pooled sensitivity, specificity, PLR, and NLR are as follows: ASQ-2SD to predict any delay in 1 or more domain (n = 16), 0.77 (95% CI, 0.64-0.86), 0.81 (95% CI, 0.75-0.86), 4.10 (95% CI, 3.17-5.30), and 0.28 (95% CI, 0.18-0.44); ASQ-2SD to predict severe delay in 1 or more domain (n = 15), 0.84 (95% CI, 0.75-0.90), 0.77 (95% CI, 0.71-0.82), 3.72 (95% CI, 2.98-4.64), and 0.20 (95% CI, 0.13-0.32); ASQ-2SD motor domain to predict motor delay (n = 7), 0.41 (95% CI, 0.26-0.57), 0.94 (95% CI, 0.87-0.97), 6.5 (95% CI, 3.8-11.1), and 0.63 (95% CI, 0.50-0.81); and ASQ-2SD cognitive domain to predict cognitive delay (n = 2), 0.44 (95% CI, 0.24-0.65), 0.93 (95% CI, 0.81-0.95), 6.4 (95% CI, 2.4-16.8), and 0.61 (95% CI, 0.43-0.86). The COE was low/very low.
Conclusions and Relevance
If a child aged 12 to 60 months passes all ASQ domains, there is a moderate probability that they do not have severe developmental delay (low COE). If a child aged 12-60 months fails the motor or cognitive domain of ASQ, there is a moderate probability that they have some motor or cognitive delay, respectively (very low COE).
Trial Registration
PROSPERO (CRD42021268543).
Introduction
Early identification of developmental delay in children is essential to enable timely intervention.1 Formal developmental assessments, such as the Bayley Scales of Infant and Toddler Development (BSID), are considered gold standards. These assessments are time consuming, expensive, and need the physical attendance of the child and caregivers, and thus may not be feasible in resource-limited settings or in pandemic conditions.
An alternative is to use screening tests to decide who will need further evaluation. A common screening tool is the Ages and Stages Questionnaire (ASQ), a parent-completed questionnaire at ages 1 to 66 months. It consists of questions on communication (language), gross motor, fine motor, problem-solving (cognitive), and personal-adaptive skills.2,3 There are 2 cutoff scores for each of the 5 domains, a refer zone (>2 SDs below the mean) and a monitoring zone (1-2 SDs below the mean). The advantages of ASQ are that it requires less administration time (10-15 minutes), is relatively inexpensive, and helps establish a sense of parental involvement.4,5
Many studies have evaluated ASQ’s ability to identify developmental delays. While there are many systematic reviews6,7,8,9,10,11,12,13 on developmental screening tools, they either included only 1 to 4 studies comparing ASQ vs concurrently performed formal tests or addressed a different question. Hence, we conducted a systematic review and meta-analysis to evaluate the accuracy of ASQ vs concurrently performed formal developmental tests in young children. Our aims were to evaluate (1) whether ASQ is useful as a screening tool that facilitates triaging decisions about further formal assessments and (2) whether ASQ is useful as a diagnostic tool so that one could use it to diagnose developmental delay to facilitate early intervention.
Methods
This systematic review was conducted using Cochrane guidelines and other relevant resources14,15,16 and reported using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.17 It was registered on PROSPERO (CRD42021268543).
Inclusion Criteria
Studies meeting the following criteria were included: ASQ performed between 12 and 60 months of age or where the median age at ASQ was at least 12 months and formal developmental assessments (reference tests) were completed within 2 months of ASQ (before or after).
Exclusion Criteria
The following studies were excluded: studies assessing the ability of ASQ to predict future outcomes (eg, ASQ at 1 year and BSID, Third Edition [BSID-III] at 3 years), studies in which only those who failed ASQ underwent formal tests, studies using only ASQ social emotional (SE), studies that did not use the conventional ASQ scoring system, and studies conducted exclusively in infants younger than 12 months (a special age group for early childhood development that would need its own systematic review instead of being diluted in the 0-5 years age range).
Outcomes of Interest
For this meta-analysis, 2 thresholds were used to define a failure on ASQ. ASQ scores more than 2 SDs below the mean in 1 or more domain (ASQ-2SD) and ASQ scores more than 1 SD below the mean (ASQ-1SD) in 1 or more domain. Similarly, 2 thresholds were used on formal developmental assessments: (1) any developmental delay was defined as scores more than 1 SD below the mean in 1 or more domain and (2) severe developmental delay was defined as scores more than 2 SDs below the mean in 1 or more domain. Studies that had provided at least 1 comparison were included.
Primary Outcomes
ASQ-2SD in any domain to predict any developmental delay on reference tests.
ASQ-2SD in any domain to predict severe developmental delay on reference tests.
Secondary Outcomes
ASQ-2SD in the motor domain to predict (1) any motor delay or (2) severe motor delay on the corresponding domain of reference tests.
ASQ-2SD in the cognitive domain to predict (1) any cognitive delay or (2) severe cognitive delay on the corresponding domain of reference tests.
ASQ-1SD in any domain to predict (1) any delay or (2) severe delay on reference tests.
ASQ-1SD in the motor domain to predict (1) any motor delay or (2) severe motor delay on the corresponding domain of reference tests.
ASQ-1SD in the cognitive domain to predict (1) any cognitive delay or (2) severe cognitive delay on the corresponding domain of reference tests.
For the above comparisons, gross and/or fine motor domains on the ASQ or reference tests were considered to represent the motor domain. Problem-solving and/or communication domains on ASQ were considered to represent the cognitive domain. Similarly, cognitive and/or language domains on reference tests were considered to represent the cognitive domain.
Literature Search and Study Selection
PubMed, EMBASE, CINAHL, PsycINFO, and Mednar were searched from inception until December 2021. PubMed was searched using the terms “ASQ” or “Ages and Stages Questionnaire.” EMBASE was searched using “Ages and Stages Questionnaire,” “mp” (mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word), or “ASQ.mp.” Other databases were searched using similar terminology.
Data Extraction
The numbers of true positive (TP), false positive (FP), false negative (FN), and true negatives (TN) from the studies were extracted. Where necessary, authors were contacted to provide this information; 3 provided additional information or clarified published data.18,19,20 If studies had reported sensitivity and specificity without providing TPs, FPs, FNs, and TNs, we reconstructed a contingency table and used these formulae to derive them.15
Assessment of Risk of Bias and Applicability Concerns
The quality of studies was assessed using the QUADAS-2 tool.21 The RevMan V.5.4 software was used to generate risk of bias graphs.
Statistical Analysis
Stata SE version 16 (StataCorp; 2019) with MIDAS command22 was used to derive pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and respective 95% CIs. A summary receiver operating characteristic (ROC) curve was generated to display the results of studies in the ROC space.16 The I2 statistic values of more than 25%, more than 50%, and more than 75% were considered to indicate low, moderate, and high heterogeneity, respectively.23 Fagan nomograms were used to calculate posttest probabilities.24 Deeks method was used to test publication bias.25
Since likelihood ratios (LRs) are considered more useful clinically,26 we based our conclusions on generally accepted interpretation of LR values.26,27,28,29 A PLR27,28,29 is more than 10. A high likelihood target condition is present the PLR is more than 5 to 10. A moderate likelihood target condition is present when the PLR is more than 2 to 5. A low likelihood target condition is present when the PLR is 1 to 2. An NLR27,28,29 is 0.1 or less. A high likelihood target condition is absent when the NLR is more than 0.1 to 0.2. A moderate likelihood target condition is absent when the NLR is more than 0.2 to 0.5. A low likelihood target condition is absent when the NLR is more than 0.5. Based on the above information, we considered PLR more than 5 and NLR of 0.2 or less as minimum requisites to rule in or rule out developmental delay, respectively, with at least moderate probability. The certainty of the evidence (COE) was assessed using GRADE guidelines.30,31 GradPro software was used to generate the summary of findings table.32 At all stages of the review, a minimum 2 independent reviewers were involved. Differences in opinion were resolved by discussion among all reviewers (S.M., D.W., J.T., S.R., and M.B.). The level of significance was P = .10 and P values were 2-tailed.
Results
Initial search yielded 5777 citations of which 43 were included in the systematic review3,4,18,19,20,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70 Of them 36 were included in the meta-analysis.3,4,18,19,20,33,34,35,37,39,40,43,44,45,46,47,48,49,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69 eFigure 1 in the Supplement gives the PRISMA flow diagram. Three studies used ASQ-1, 15 used ASQ-2, 24 used ASQ-3, and 1 was unspecified. Age at assessment varied from 12 to 60 months (eTables 1 and 2 in the Supplement). A total of 77% (33 of 43) had low risk of bias in patient selection domain, 49% (21 of 43) in the index test, 47% (20 of 43) in the reference standard, and 63% (27 of 43) in flow and timing. There were no applicability concerns in 86% (37 of 43) (eFigures 2 and 3 in the Supplement).
ASQ-2SD to Diagnose Any Developmental Delay
The pooled (16 studies; 18 comparisons) sensitivity was 0.77 (95% CI, 0.64-0.86; I2 = 94.9%) and specificity was 0.81 (95% CI, 0.75-0.86; I2 = 96.6%) (Figure 1). The summary area under ROC curve was 0.86 (95% CI, 0.83-0.89) (eFigure 4 in the Supplement) and DOR 14.62 (95% CI, 8.3-25.7; I2 = 100%) (eFigure 5 in the Supplement). The PLR was 4.10 (95% CI, 3.17-5.30; I2 = 89.8%), and NLR was 0.28 (95% CI, 0.18-0.44; I2 = 95.3%) (Figure 2). The P value of .44 on the Deeks test indicated that publication bias was unlikely (eFigure 6 in the Supplement).
Figure 1. Sensitivity and Specificity of Ages and Stages Questionnaire Score More Than 2 SDs Below the Mean in 1 or More Domain to Predict Any Delay.
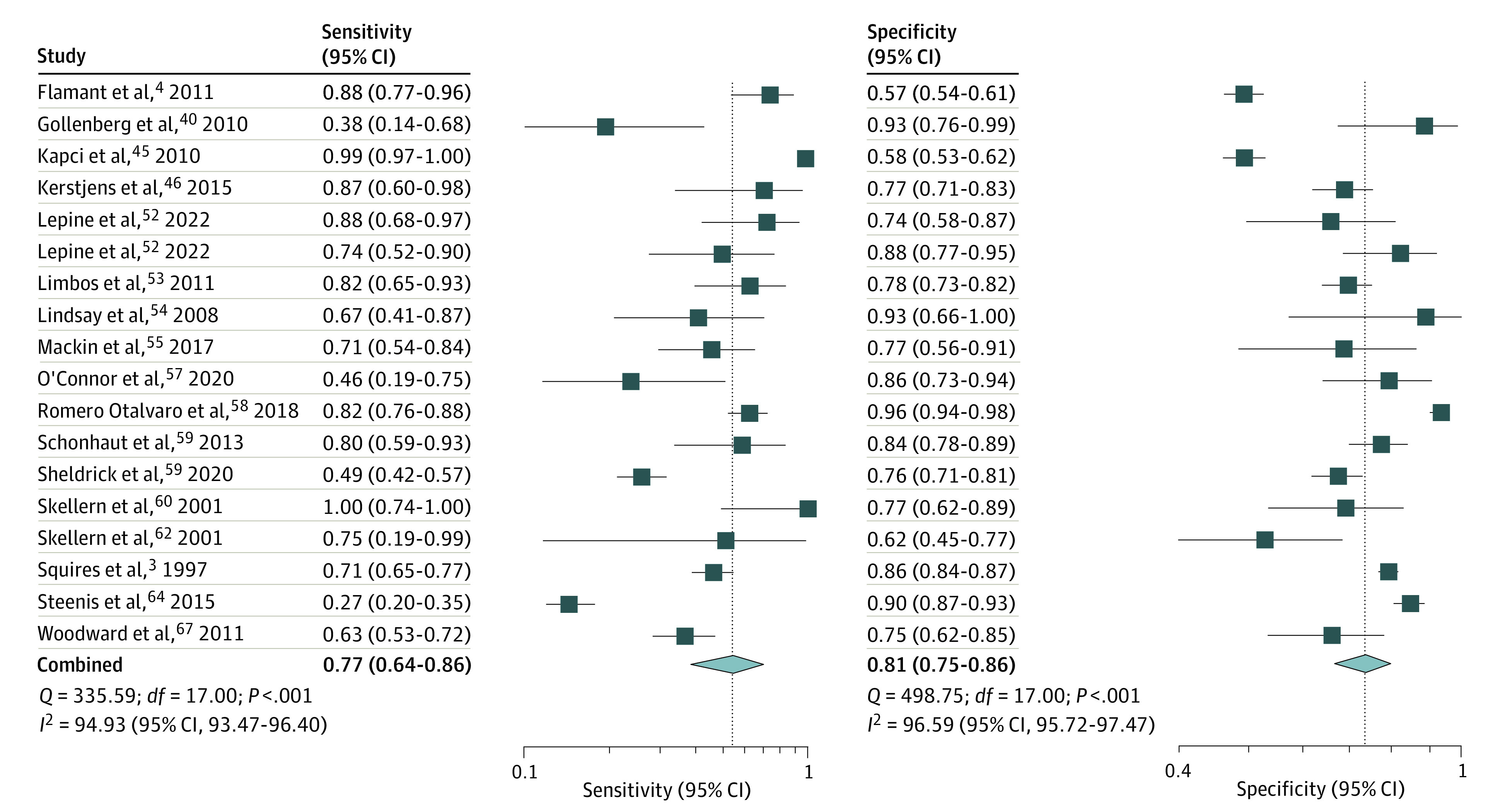
Figure 2. Likelihood Ratios of Ages and Stages Questionnaire Score More Than 2 SDs Below the Mean in 1 or More Domain to Predict Any Delay.
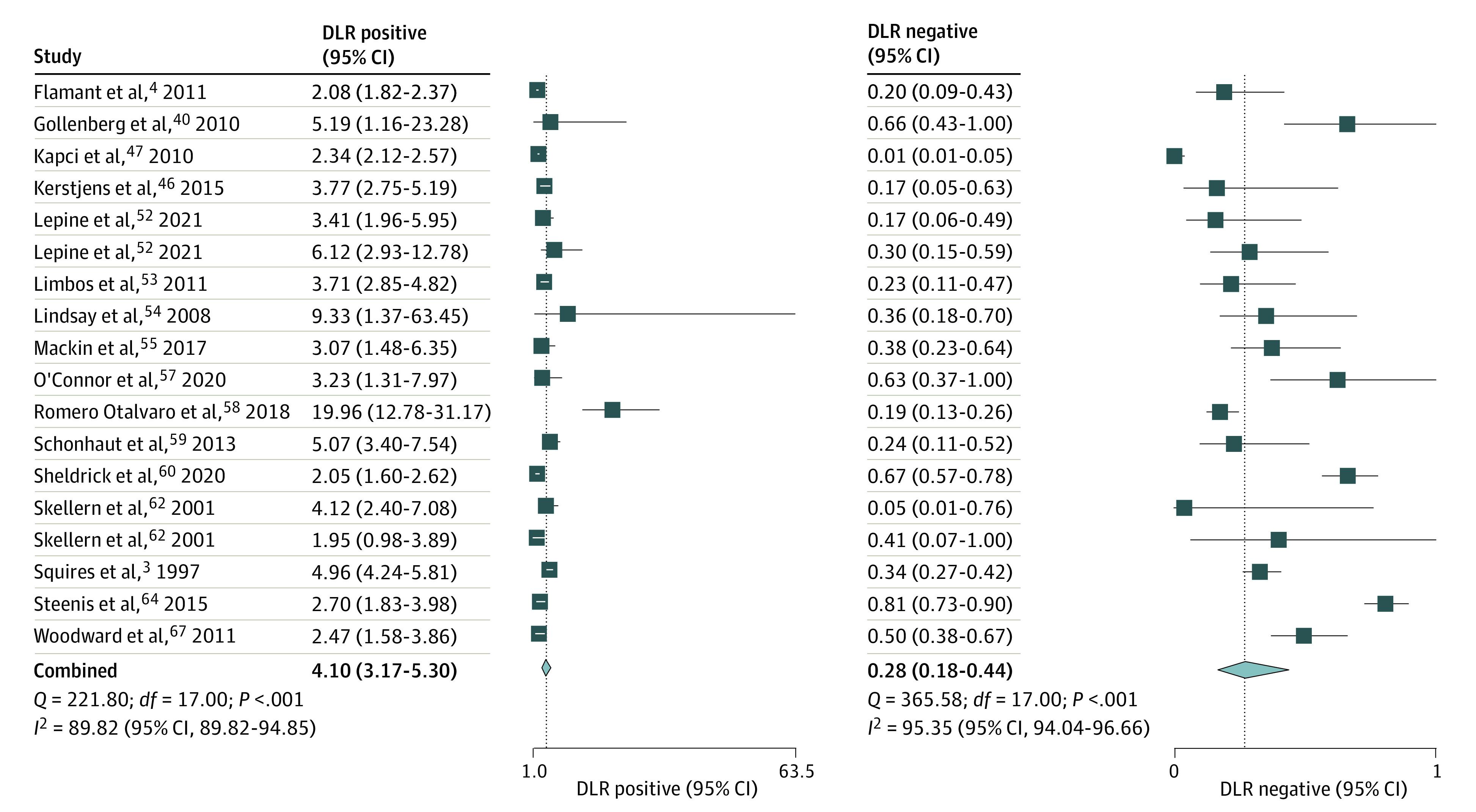
DLR indicates diagnostic likelihood ratio.
Posttest Probabilities for Any Developmental Delay Based on Results of ASQ-2SD
Fagan nomogram (eFigure 7 in the Supplement) illustrates that if the baseline prevalence of any developmental delay is 5%, and a child from that population fails ASQ-2SD, the posttest probability of having any developmental delay increases to 18% for that child. On the other hand, if a child from that population passes ASQ-2SD, the posttest probability of having any developmental delay decreases from 5% to 1%. eTable 3 in the Supplement gives posttest probabilities for various baseline prevalence rates.
Accuracy of ASQ-2SD in Any Domain to Diagnose Severe Developmental Delay
The pooled estimate (15 studies; 17 comparisons) for sensitivity was 0.84 (95% CI, 0.75-0.90; I2 = 85.3%) and for specificity, 0.77 (95% CI, 0.71-0.82; I2 = 95.8%) (Figure 3). The summary area under ROC curve was 0.87 (95% CI, 0.84-0.90) (eFigure 8 in the Supplement). The DOR was 18.2 (95% CI, 11.1-30.0; I2 = 100%) (eFigure 9 in the Supplement). The PLR was 3.72 (95% CI, 2.98-4.64; I2 = 92.1%), and NLR 0.20 (95% Ci, 0.13-0.32; I2 = 86.9%) (Figure 4). The P value of .88 on the Deeks test indicated that publication bias was unlikely (eFigure 10 in the Supplement).
Figure 3. Sensitivity and Specificity of Ages and Stages Questionnaire Score More Than 2 SDs Below the Mean in 1 or More Domain to Predict Severe Delay.
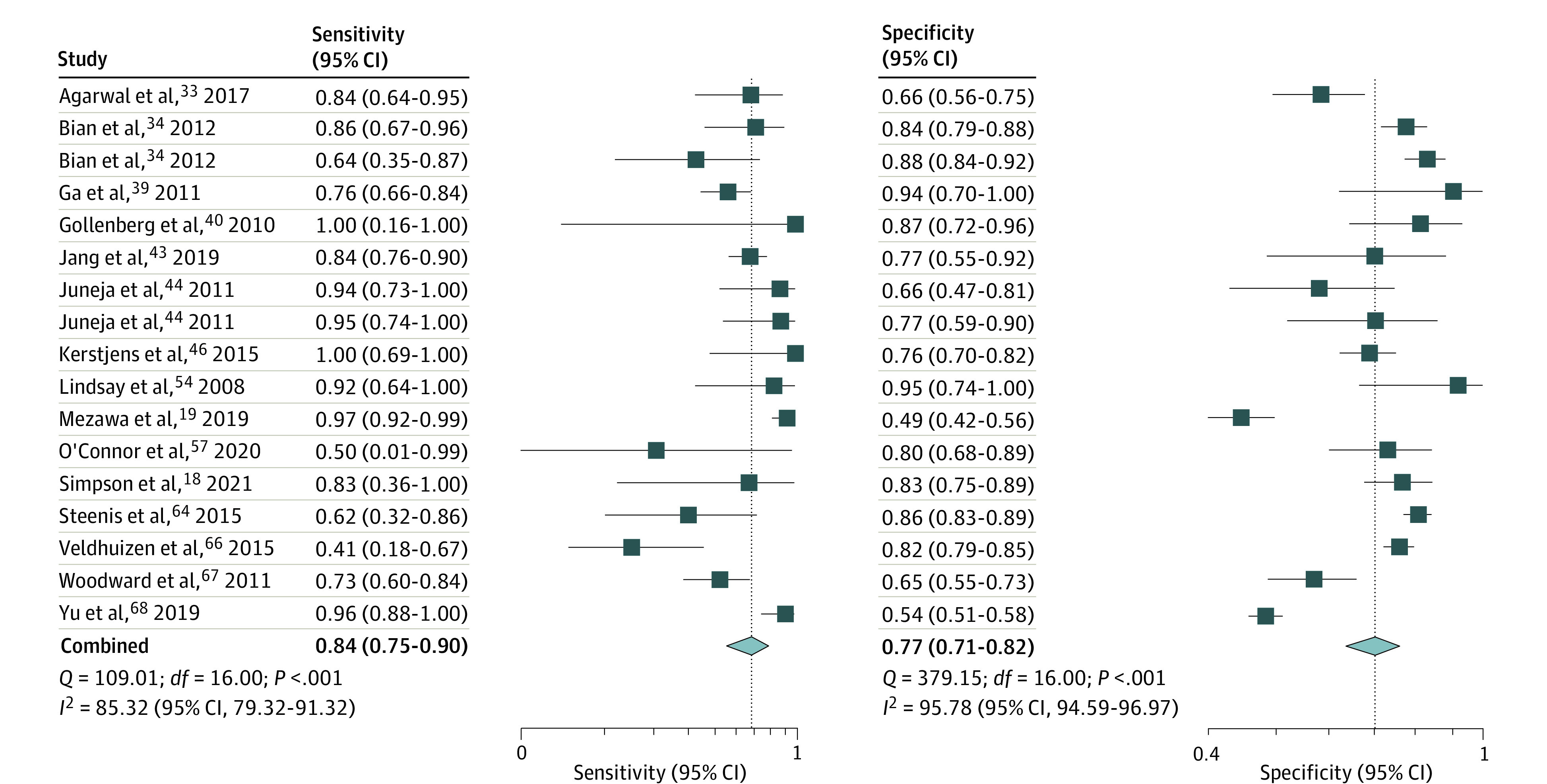
Figure 4. Likelihood Ratios of Ages and Stages Questionnaire Score More Than 2 SDs Below the Mean in 1 or More Domain to Predict Severe Delay.
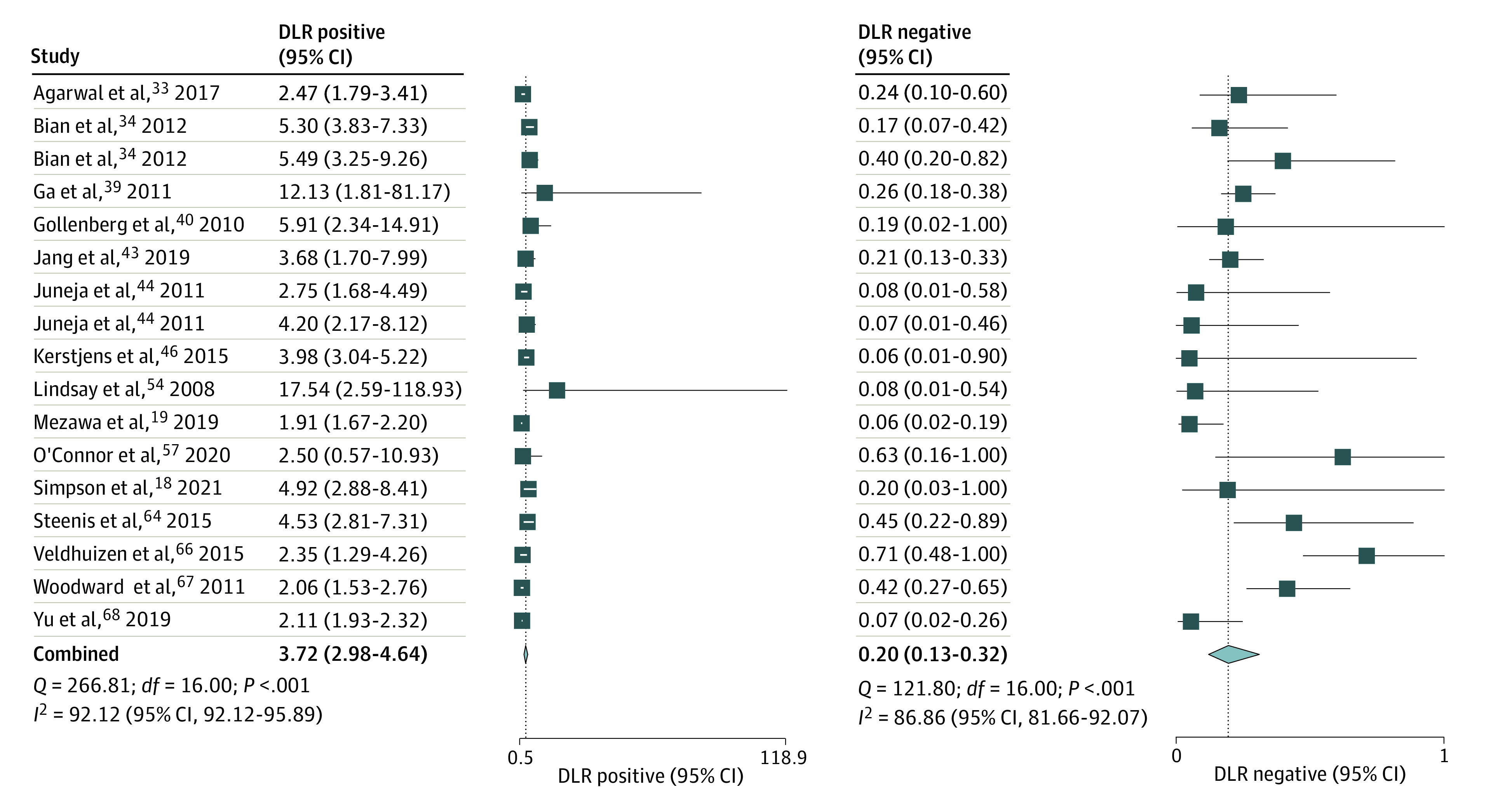
DLR indicates diagnostic likelihood ratio.
Posttest Probabilities for Severe Developmental Delay Based on the Results of ASQ-2SD
Fagan nomogram (eFigure 11 in the Supplement) illustrates that if the baseline prevalence of severe developmental delay is 5% and a child from that population fails ASQ-2SD, the posttest probability of having severe developmental delay increases to 16% for that child. On the other hand, if a child from that population passes ASQ-2SD, the posttest probability of having severe developmental delay decreases from 5% to 1%. eTable 4 in the Supplement gives posttest probabilities for various baseline prevalence rates.
Secondary Outcomes
The pooled sensitivity, specificity, PLR, NLR, and DOR were as follows, respectively; ASQ-2SD motor domain to predict motor delay (n = 7), 0.41 (95% CI, 0.26-0.57), 0.94 (95% CI, 0.87-0.97), 6.5 (95% CI, 3.8-11.1), 0.63 (95% CI, 0.50-0.81), and 10 (95% CI, 6.0-18.0) and ASQ-2SD cognitive domain to predict cognitive delay (n = 2), 0.44 (95% CI, 0.24-0.65), 0.93 (95% CI, 0.81-0.95), 6.4 (95% CI, 2.4-16.8), 0.61 (95% CI, 0.43-0.86), and 11 (95% CI, 4.0-31.0). The Table provides results of all secondary outcomes.
Table. Results of Primary and Secondary Outcomes.
| ASQ details | No. of studies | No. of comparisons | Sample size | Sources | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| ASQ-2SD vs any developmental delay | 16 | 18 | 6089 | Squires et al,3 1997; Flamant et al,4 2011; Gollenberg et al,40 2010;,Kapci et al,46 2010; Kerstjens et al,45 2015; Lépine et al,52 2022; Limbos and Joyce,53 2011; Lindsay et al,542008; Mackin et al,552017; O’Connor,57 2020; Romero Otalvaro,582018;Schonhaut et al,59 2013; Skellern et al,62 2001; Steenis et al,64 2015; Woodward et al,672011 | 0.77 (0.64-0.86); I2 = 94.9% | 0.81 (0.75-0.86); I2 = 96.6% | 4.10 (3.17-5.30) | 0.28 (0.18-0.44) | 14.62 (8.32-25.68) | 0.86 (0.83-0.89) |
| ASQ-2SD vs severe developmental delay | 15 | 17 | 3942 | Simpson et al,18 2021; Mezawa et al,19 2019; Agarwal et al,33 2017; Bian et al,34 2021; Ga and Kwon,39 2011; Gollenberg et al,40 2010; Jang et al,43 2019; Juneja et al,44 2021; Kerstjens et al,46 2015, Lindsay et al,54 2008; O’Connor et al,57 2020; Steenis et al,64 2015; Veldhuizen et al,66 2015; Woodward et al,67 2011; Yu et al,68 2007 | 0.84 (0.75-0.90); I2 = 85.3% | 0.77 (0.71-0.82); I2 = 95.8% | 3.72 (2.98-4.64) | 0.20 (0.13-0.32) | 18.24 (11.09-30.00) | 0.87 (0.84-0.90) |
| ASQ-2SD motor domain vs any motor delay | 7 | 11 | 1417 | Carmichael et al,35 2022; Fauls et al,37 2020; King-Dowling et al,49 2016; Noeder et al,56 2017; Simard et al,61 2012; Vanvuchelen et al,65 2017 | 0.41 (0.26-0.57) | 0.94 (0.87-0.97) | 6.5 (3.8-11.1) | 0.63 (0.50-0.81) | 10 (6-18) | 0.80 (0.76-0.83) |
| ASQ-2SD motor domain vs severe motor delay | 4 | 7 | 2410 | Carmichael et al,35 2022; , Vanvuchelen et al,65 2017; Veldhuizen et al,66 2015; Yue et al,69 2019 | 0.43 (0.23-0.66) | 0.87 (0.80-0.92) | 3.4 (2.2-5.5) | 0.65 (0.45-0.94) | 5 (2-11) | 0.77 (0.73-0.80) |
| ASQ-2SD C/L domain vs any C/L delay | 2 | 4 | 697 | Noeder et al,56 2017; Simard et al,61 2012 | 0.44 (0.24-0.65) | 0.93 (0.81-0.98) | 6.4 (2.4-16.8) | 0.61 (0.43-0.86) | 11 (4-31) | 0.78 (0.75-0.82) |
| ASQ-2SD C/L vs severe C/L delay | 3 | 7 | 3625 | Kim et al,47 2016; Veldhuizen et al,66 2015; Yue et al,69 2019 | 0.32 (0.17-0.51) | 0.93 (0.89-0.95) | 4.5 (3.1-6.6) | 0.73 (0.58-0.93) | 6 (3-11) | 0.86 (0.83-0.89) |
| ASQ-1SD vs any developmental delay | 4 | 5 | 798 | Kim et al,48 2010; Lépine et al,52 2022; Lindsay et al,54 2008; Steenis et al,64 2015 | 0.79 (0.63-0.90) | 0.67 (0.42-0.85) | 2.4 (1.31-4.39) | 0.31 (0.18-0.54) | 7.73 (3.1-19.26) | 0.81 (0.77-0.84) |
| ASQ-1SD vs severe developmental delay | 3 | 3 | 773 | Kim et al,48 2010; Steenis et al,64 2015; Woodward et al,67 2011 | 0.88 (0.73-0.95) | 0.53 (0.35-0.71) | 1.9 (1.0-3.6) | 0.22 (0.08-0.58) | 8.7 (2.7-27.7) | 0.75 (0.62-0.84) |
| ASQ-1SD motor domain vs any motor delay | 6 | 10 | 2322 | Hwarng et al,20 2021; Fauls et al,37 2020; King-Dowling et al,49 2016; Noeder et al,56 2017; Simard et al,61 2012; Yue et al,69 2019 | 0.64 (0.53-0.73) | 0.79 (0.70-0.85) | 3.0 (2.1-4.1) | 0.46 (0.36-0.60) | 6 (4-11) | 0.77 (0.74-0.81) |
| ASQ-1SD motor domain vs severe motor delay | No data | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ASQ-1SD C/L vs any C/L delay | 4 | 9 | 2954 | Hwarng et al,20 2012; Noeder et al,56 2017; Simard et al,61 2021; Yue et al,69 2019 | 0.58 (0.39-0.75) | 0.79 (0.71-0.85) | 2.8 (2.0-3.9) | 0.53 (0.35-0.80) | 5 (3-10) | 0.78 (0.74-0.82) |
| ASQ-1SD C/L domain vs severe C/L delay | No data | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: AUC, area under the ROC Curve; ASQ, Ages and Stages Questionnaire; ASQ-1SD, Ages and Stages Questionnaire score more than 1 SD below the mean; ASQ-2SD, Ages and Stages Questionnaire score more than 2 SDs below the mean; DOR, diagnostic odds ratio; NLR, negative likelihood ratio; NA, not applicable; PLR, positive likelihood ratio.
Studies That Could Not Be Pooled by Meta-analysis
Eight studies could not be pooled due to lack of data in a suitable format.36,38,39,41,42,50,51,70 Of them, 538,39,41,42,50 concluded that ASQ is a useful tool (eTable 5 in the Supplement). The data for these outcomes were limited (Table).
Summary of Findings and COE
The COE was low for primary outcomes and very low for secondary outcomes due to unclear/high risk of bias (ROB), statistical heterogeneity, and inability to assess publication bias for many secondary outcomes since the number of studies were less than 10 (eTables 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17 in the Supplement).
Sensitivity Analyses to Explore Statistical Heterogeneity
For the 2 primary outcomes, the study team conducted the following sensitivity analyses by (1) excluding studies with a high/unclear ROB, (2) excluding studies with a sample size less than the median (ie, <145), (3) conducting separate analyses based on ASQ versions 2 and 3, (4) age at assessment younger than 24 months and 24 months or older, (5) type of reference test (BSID, Second Edition [BSID-II] and BSID-III), and (6) design (prospective/retrospective). The pooled estimates and statistical heterogeneity for most of these analyses were similar to the primary analyses. However, ASQ-2SD had better diagnostic indices for severe delay for (1) children 24 months or older than younger than 24 months and (2) BSID-II than BSID-III (eTable 18 in the Supplement).
Discussion
This systematic review and meta-analysis found that ASQ-2SD had a pooled sensitivity of 0.77 (95% CI, 0.64-0.86) and specificity of 0.81 (95% CI, 0.75-0.86) to diagnose any developmental delay, and a sensitivity of 0.84 (95% CI, 0.75-0.90) and specificity of 0.77 (95% CI, 0.71-0.82) to diagnose severe developmental delay in children aged 12 to 60 months. Sensitivity and specificity are broadly categorized as less than 69 as low, 70 to 89 as moderate, and 90 or more as high 1,71 for developmental screening tools. Hence, based on our results, ASQ has moderate sensitivity and specificity.
A drawback of sensitivity/specificity is that they answer the less important question, what is the chance of a positive or negative test in the presence or absence of the clinical condition? Whereas, for clinicians/patients, the important question is what is the chance that the clinical condition will be present or absent in the context of a positive or negative test result?72 Positive and negative predictive values can answer the latter question, but they are dependent on the prevalence, hence why calculated positive and negative predictive values from 1 study cannot be applied to populations with a different disease prevalence.72
LRs are considered more informative26,73,74 because they (1) take into consideration sensitivity and specificity, (2) are not affected by the disease prevalence, and (3) enable calculation of the posttest probabilities based on the baseline prevalence. Hence, they can be used in settings with different prevalence rates.
Our systematic review and meta-analysis found that ASQ-2SD had a PLR of 4.1, and an NLR of 0.28 to diagnose “any developmental delay.” Since these values are outside required values (PLR > 5 and NLR ≤ 0.2), ASQ-2SD may not be useful to rule in or rule out any developmental delay. On the other hand, ASQ-2SD had a PLR of 3.7 and an NLR of 0.2 for severe developmental delay. Hence, based on the generally accepted interpretation of LR values,27,28,29 if a child passes ASQ-2SD, there is a moderate probability that the child does not have severe delay.
ASQ-2SD motor domain had a specificity of 0.94 and PLR of 6.4. Hence, if a child fails ASQ motor domain at 2-SD level, there is a moderate probability that the child has some motor delay. Similarly, ASQ-2SD cognitive domain had a specificity of 0.93 and PLR of 6.4 to predict cognitive delay. Hence, if a child fails ASQ cognitive domain at 2-SD level, there is a moderate probability that the child has some cognitive delay.
There was significant statistical heterogeneity, which remained substantial even on sensitivity analyses. Heterogeneity is usually high in meta-analyses of diagnostic studies,75 an important source being the threshold effect.15 If the included studies use different threshold scores to define pass/fail on ASQ, it could contribute to heterogeneity. Hence, we analyzed the data separately for ASQ-1SD and ASQ-2SD. Similarly, we analyzed separately for any delay and severe delay on reference tests. Despite this, statistical heterogeneity remained high, which was one of the reasons for downgrading the COE. In addition, we conducted various exploratory/hypothesis-generating sensitivity analyses to evaluate the influence of (1) age of children, (2) type of reference standard, (3) ASQ version, (4) study design, (5) ROB, and (6) sample size. For most of these analyses, results were similar to the primary analyses with slight variations. However, 2 results need attention; (1) ASQ-2SD had better NLR for severe delay for children 24 months or older than younger than 24 months, which suggests that ASQ may be more accurate in older children and (2) its performance on BSID-II was better than BSID-III, possibly because BSID-III is known to underestimate severity of developmental delay.76
Even though the conventional threshold on ASQ is 2 SDs, we wanted to know if decreasing the threshold to 1 SD would increase its sensitivity and NLR. However, on meta-analyses, this was not evident (Table), but the sample size was small.
Comparisons With Other Systematic Reviews on Developmental Screening Tools
Schonhaut et al13 evaluated the ASQ and other tools for predicting future cognitive achievement or school performance. In contrast, our focus was to compare ASQ vs concurrently performed (plus or minus 2 months) developmental assessments. Van Dokkum et al11 included 11 studies, of which only 1 was about ASQ.49 Cairney et al6 included 13 studies on screening tools, of which only 1 was on ASQ77 (not included in our systematic review because it was about future prediction). Kjølbye et al9 included 7 articles on ASQ of which 3 were diagnostic accuracy studies.3,40,50 Velikonja et al12 included all types of studies on ASQ (diagnostic accuracy, interrater agreement, feasibility to implement, validity, and reliability). Of the 18 studies, only 3 were diagnostic accuracy studies.34,59,66 They concluded that the evidence was heterogeneous. Mendonça et al10 included 23 studies of which 2 used ASQ70,78 of which only 1 was a diagnostic accuracy study.70 Warren et al,8 included 4 studies40,53,64,79 (1001 children) and reported a median sensitivity of 55.0%, specificity 86.0%, PLR 4.2, and NLR 0.61. Of them, we excluded 179 because only screen-positive children underwent the reference test. Sheldrick7 included 11 studies of which 1 was on ASQ80 (excluded in our systematic review because only screen-positive children underwent the reference test).
Overall, the above 8 systematic reviews identified 10 studies evaluating the diagnostic accuracy of ASQ compared with simultaneously performed reference tests.3,34,40,49,50,53,54,59,66,70 In comparison, our review identified 43 studies and used advanced statistical methods. Another strength was the sensitivity analyses and generation of COE using GRADE guidelines.
Limitations
The limitations of our systematic review and meta-analysis were the low COE for primary outcomes and very low COE and small sample size for the comparison of individual domains. Hence the results need to be interpreted cautiously by clinicians. Another issue is that the efficacy and test-retest reliability of different versions of ASQ and reference tests are known to vary over different ages and settings. Given these issues and multiplicity of comparisons, these results should be considered as exploratory in nature and facilitate well-conducted prospective studies addressing such specific questions. The current results cannot be extrapolated to infants younger than 12 months because studies done exclusively in that population were excluded.
Directions for Future Research
Future studies should (1) evaluate ASQ separately for different age groups (eg, <12 months, 12-23 months, and ≥24 months), (2) be prospective in design, (3) achieve low ROB, (4) follow STARD guidelines,81 and (5) report raw numbers for TP, FP, FN, and TN. Even if they use their own cutoff ASQ scores, they should also give results for the conventional cutoff scores to enable comparison with other studies.
Conclusions
Based on generally accepted interpretation of LRs, if a child aged 12 to 60 months passes ASQ-2SD in all domains, there is a moderate probability that they do not have severe developmental delay (low COE). If a child fails in the motor domain of ASQ, there is a moderate probability that they have some motor delay (very low COE). If a child fails in the cognitive domain of ASQ, there is a moderate probability that they have some cognitive delay (very low COE).
eFigure 1. PRISMA Flow Diagram of Study Selection
eFigure 2. Risk of Bias of Included Studies-Summary Graph
eFigure 3. Methodological Quality of Included Studies
eFigure 4. SROC ASQ-2SD to Predict “Any Delay”
eFigure 5. Diagnostic Odds Ratio of ASQ-2SD to Predict “Any Delay”
eFigure 6. Funnel Plot for Publication Bias (ASQ-2SD to predict “any delay”)
eFigure 7. Fagan Nomogram if Baseline Prevalence of “Any Delay” Is 5% (ASQ-2SD to predict “any delay”)
eFigure 8. SROC ASQ-2SD to Predict “Severe Delay”
eFigure 9. Diagnostic Odds Ratio of ASQ-2SD to Predict “Severe Delay”
eFigure 10. Funnel Plot for Publication Bias (ASQ-2SD to predict “severe delay”)
eFigure 11. Fagan Nomogram if Baseline Prevalence of “Severe Delay” Is 5% (ASQ-2SD to predict “severe delay”)
eTable4. Post-test probabilities for various pre-test prevalence of “severe delay” based on the results of ASQ-2SD
eTable5. Results of studies included in the systematic review, but could not be pooled in the meta-analysis
eTables 6-17. Summary of findings tables and Certainty of Evidence
eTable18. Results of sensitivity analyses
References
- 1.Lipkin PH, Macias MM; Council on Children With Disabilities, Section on Developmental and Behavioral Pediatrics . Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1):e20193449. doi: 10.1542/peds.2019-3449 [DOI] [PubMed] [Google Scholar]
- 2.Squires J, Bricker DD, Twombly E. Ages & Stages Questionnaires. 3rd ed. Brooks Publishing Company; 2009. [Google Scholar]
- 3.Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: ages and stages questionnaires. J Pediatr Psychol. 1997;22(3):313-328. doi: 10.1093/jpepsy/22.3.313 [DOI] [PubMed] [Google Scholar]
- 4.Flamant C, Branger B, Nguyen The Tich S, et al. Parent-completed developmental screening in premature children: a valid tool for follow-up programs. PLoS One. 2011;6(5):e20004. doi: 10.1371/journal.pone.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Yeh CJ, Boone Blanchard S. Ages and Stages Questionnaire: a global screening scale. Bol Med Hosp Infant Mex. 2017;74(1):5-12. doi: 10.1016/j.bmhimx.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 6.Cairney DG, Kazmi A, Delahunty L, Marryat L, Wood R. The predictive value of universal preschool developmental assessment in identifying children with later educational difficulties: a systematic review. PLoS One. 2021;16(3):e0247299. doi: 10.1371/journal.pone.0247299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldrick RC, Merchant S, Perrin EC. Identification of developmental-behavioral problems in primary care: a systematic review. Pediatrics. 2011;128(2):356-363. doi: 10.1542/peds.2010-3261 [DOI] [PubMed] [Google Scholar]
- 8.Warren R, Kenny M, Bennett T, et al. Screening for developmental delay among children aged 1-4 years: a systematic review. CMAJ Open. 2016;4(1):E20-E27. doi: 10.9778/cmajo.20140121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjølbye CB, Drivsholm TB, Ertmann RK, Lykke K, Rasmussen RK. Motor function tests for 0-2-year-old children—a systematic review. Dan Med J. 2018;65(6):A5484. [PubMed] [Google Scholar]
- 10.Mendonça B, Sargent B, Fetters L. Cross-cultural validity of standardized motor development screening and assessment tools: a systematic review. Dev Med Child Neurol. 2016;58(12):1213-1222. doi: 10.1111/dmcn.13263 [DOI] [PubMed] [Google Scholar]
- 11.van Dokkum NH, Reijneveld SA, de Best JTBW, et al. Criterion validity and applicability of motor screening instruments in children aged 5-6 years: a systematic review. Int J Environ Res Public Health. 2022;19(2):781. doi: 10.3390/ijerph19020781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velikonja T, Edbrooke-Childs J, Calderon A, Sleed M, Brown A, Deighton J. The psychometric properties of the ages & stages questionnaires for ages 2-2.5: a systematic review. Child Care Health Dev. 2017;43(1):1-17. doi: 10.1111/cch.12397 [DOI] [PubMed] [Google Scholar]
- 13.Schonhaut L, Maturana A, Cepeda O, Serón P. Predictive validity of developmental screening questionnaires for identifying children with later cognitive or educational difficulties: a systematic review. Front Pediatr. 2021;9:698549. doi: 10.3389/fped.2021.698549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane handbook for systematic reviews of diagnostic test accuracy. Accessed February 2022, https://methods.cochrane.org/sdt/handbook-dta-reviews
- 15.Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part i. general guidance and tips. Korean J Radiol. 2015;16(6):1175-1187. doi: 10.3348/kjr.2015.16.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin P, Kelly AM, Altaee D, Foerster B, Petrou M, Dwamena BA. How to perform a systematic review and meta-analysis of diagnostic imaging studies. Acad Radiol. 2018;25(5):573-593. doi: 10.1016/j.acra.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 17.McInnes MDF, Moher D, Thombs BD, et al. ; and the PRISMA-DTA Group . Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA. 2018;319(4):388-396. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 18.Simpson S, Eadie T, Khoo ST, et al. The ASQ-TRAK: validating a culturally adapted developmental screening tool for Australian Aboriginal children. Early Hum Dev. 2021;163:105481. doi: 10.1016/j.earlhumdev.2021.105481 [DOI] [PubMed] [Google Scholar]
- 19.Mezawa H, Aoki S, Nakayama SF, et al. Psychometric profile of the Ages and Stages Questionnaires, Japanese translation. Pediatr Int. 2019;61(11):1086-1095. doi: 10.1111/ped.13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwarng GYH, Ereno IL, Ho SKY, Allen JC, Moorakonda RB, Yeo CL. Accuracy of parent-reported Ages and Stages Questionnaire in assessing the motor and language skills of preterm infants. J Neonatal Perinatal Med. 2021;14(2):193-202. doi: 10.3233/NPM-200449 [DOI] [PubMed] [Google Scholar]
- 21.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 22.Dwamena BA. Midas: a program for meta-analytical integration of diagnostic accuracy studies in Stata. Accessed July 27, 2022.https://ideas.repec.org/c/boc/bocode/s456880.html
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med. 1975;293(5):257. doi: 10.1056/NEJM197507312930513 [DOI] [PubMed] [Google Scholar]
- 25.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882-893. doi: 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 26.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329(7458):168-169. doi: 10.1136/bmj.329.7458.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. what are the results and will they help me in caring for my patients? the Evidence-Based Medicine Working Group. JAMA. 1994;271(9):703-707. doi: 10.1001/jama.1994.03510330081039 [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein ML, Kraft CS, Parrott JS. Determining qualitative effect size ratings using a likelihood ratio scatter matrix in diagnostic test accuracy systematic reviews. Diagnosis (Berl). 2018;5(4):205-214. doi: 10.1515/dx-2018-0061 [DOI] [PubMed] [Google Scholar]
- 29.Bhandari M, Montori VM, Swiontkowski MF, Guyatt GH. User’s guide to the surgical literature: how to use an article about a diagnostic test. J Bone Joint Surg Am. 2003;85(6):1133-1140. doi: 10.2106/00004623-200306000-00027 [DOI] [PubMed] [Google Scholar]
- 30.Schünemann HJ, Mustafa RA, Brozek J, et al. ; GRADE Working Group . GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol. 2020;122:142-152. doi: 10.1016/j.jclinepi.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 31.Schünemann HJ, Mustafa RA, Brozek J, et al. ; GRADE Working Group . GRADE guidelines: 22. The GRADE approach for tests and strategies-from test accuracy to patient-important outcomes and recommendations. J Clin Epidemiol. 2019;111:69-82. doi: 10.1016/j.jclinepi.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Bian XY, Yao GY, Squires J, Wei M, Chen CI, Fang BH. Studies of the norm and psychometric properties of Ages and Stages Questionnaires in Shanghai children. Zhonghua Er Ke Za Zhi. 2010;48(7):492-496. [PubMed] [Google Scholar]
- 33.Agarwal PK, Shi L, Daniel LM, et al. Prospective evaluation of the Ages and Stages Questionnaire 3rd Edition in very-low-birthweight infants. Dev Med Child Neurol. 2017;59(5):484-489. doi: 10.1111/dmcn.13307 [DOI] [PubMed] [Google Scholar]
- 34.Bian X, Yao G, Squires J, et al. Translation and use of parent-completed developmental screening test in Shanghai. J Early Child Res. 2012;10(2):162-175. doi: 10.1177/1476718X11430071 [DOI] [Google Scholar]
- 35.Carmichael CMW. Willison EA, Zhang Q. Ability of Ages and stages questionaire 3rd edition to identify children in need of comprehensive motor evaluation. Accessed July 26, 2022. https://digitalscholarship.unlv.edu/cgi/viewcontent.cgi?article=3457&context=thesesdissertations
- 36.Colbert AM, Connery AK, Lamb MM, et al. Caregiver rating of early childhood development: reliability and validity of the ASQ-3 in rural Guatemala. Early Hum Dev. 2021;161:105453. doi: 10.1016/j.earlhumdev.2021.105453 [DOI] [PubMed] [Google Scholar]
- 37.Fauls JR, Thompson BL, Johnston LM. Validity of the Ages and Stages Questionnaire to identify young children with gross motor difficulties who require physiotherapy assessment. Dev Med Child Neurol. 2020;62(7):837-844. doi: 10.1111/dmcn.14480 [DOI] [PubMed] [Google Scholar]
- 38.Fuengfoo AS, Sakulnoom K, Owjinda S, Piromkit A. The feasibility of the Ages & Stages Questionnaires, third edition (ASQ-3, Thai Version) for the assessment of child development in Thailand. J Med Assoc Thai. 2020;103(12):1247-1254. doi: 10.35755/jmedassocthai.2020.12.10302 [DOI] [Google Scholar]
- 39.Ga HY, Kwon JY. A comparison of the Korean-Ages and Stages Questionnaires and Denver developmental delay screening test. Ann Rehabil Med. 2011;35(3):369-374. doi: 10.5535/arm.2011.35.3.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gollenberg AL, Lynch CD, Jackson LW, McGuinness BM, Msall ME. Concurrent validity of the parent-completed Ages and Stages Questionnaires, 2nd Ed. with the Bayley Scales of Infant Development II in a low-risk sample. Child Care Health Dev. 2010;36(4):485-490. doi: 10.1111/j.1365-2214.2009.01041.x [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez-Cruz N, Torres-Mohedas J, Carrasco-Marina ML, Olabarrieta-Arnal I, Martin-Del Valle F, Garcia-Garcia ML. Psychomotor development in late preterms at two years of age: a comparison with full-term newborn infants using two different instruments. Rev Neurol. 2019;68(12):503-509. doi: 10.33588/rn.6812.2018360 [DOI] [PubMed] [Google Scholar]
- 42.Halbwachs M, Muller JB, Nguyen The Tich S, et al. Usefulness of parent-completed ASQ for neurodevelopmental screening of preterm children at five years of age. PLoS One. 2013;8(8):e71925. doi: 10.1371/journal.pone.0071925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang CH, Kim SW, Jeon HR, et al. Clinical Usefulness of the Korean Developmental Screening Test (K-DST) for developmental delays. Ann Rehabil Med. 2019;43(4):490-496. doi: 10.5535/arm.2019.43.4.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juneja M, Mohanty M, Jain R, Ramji S. Ages and Stages Questionnaire as a screening tool for developmental delay in Indian children. Indian Pediatr. 2012;49(6):457-461. doi: 10.1007/s13312-012-0074-9 [DOI] [PubMed] [Google Scholar]
- 45.Kapci EG, Kucuker S, Uslu RI. How applicable are ages and stages questionnaires for use with Turkish children? Top in Early Childhood Spec Educ. 2010;30(3):176-188. doi: 10.1177/0271121410373149 [DOI] [Google Scholar]
- 46.Kerstjens JM, Nijhuis A, Hulzebos CV, et al. The Ages and Stages Questionnaire and neurodevelopmental impairment in two-year-old preterm-born children. PLoS One. 2015;10(7):e0133087. doi: 10.1371/journal.pone.0133087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SW, Kim JY, Lee SY, Jeon HR. The Comparison of M-B CDI-K short form and K-ASQ as screening test for language development. Ann Rehabil Med. 2016;40(6):1108-1113. doi: 10.5535/arm.2016.40.6.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YJL. Sohn JY, Lee JA, et al. A validity study of the Korean ages and stages questionnaires: screening for developmental delay in preterm infant. J Korean Soc Neonatol. 2010;17:217-223. doi: 10.5385/jksn.2010.17.2.217 [DOI] [Google Scholar]
- 49.King-Dowling S, Rodriguez MC, Missiuna C, Cairney J. Validity of the Ages and Stages Questionnaire to detect risk of Developmental Coordination Disorder in preschoolers. Child Care Health Dev. 2016;42(2):188-194. doi: 10.1111/cch.12314 [DOI] [PubMed] [Google Scholar]
- 50.Klamer A, Lando A, Pinborg A, Greisen G. Ages and Stages Questionnaire used to measure cognitive deficit in children born extremely preterm. Acta Paediatr. 2005;94(9):1327-1329. doi: 10.1111/j.1651-2227.2005.tb02095.x [DOI] [PubMed] [Google Scholar]
- 51.Kwun Y, Park HW, Kim MJ, Lee BS, Kim EA. Validity of the Ages and Stages Questionnaires in Korean compared to Bayley Scales of infant development-II for screening preterm infants at corrected age of 18-24 months for neurodevelopmental delay. J Korean Med Sci. 2015;30(4):450-455. doi: 10.3346/jkms.2015.30.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lépine J, Gagnon K, Prud’homme J, et al. ; Clinique d’Investigation Neuro-Cardiaque team . Utility of the Ages and Stages Questionnaires 3rd Edition for developmental screening in children with surgically repaired congenital heart disease. Dev Neurorehabil. 2022;25(2):125-132. doi: 10.1080/17518423.2021.1960918 [DOI] [PubMed] [Google Scholar]
- 53.Limbos MM, Joyce DP. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J Dev Behav Pediatr. 2011;32(7):499-511. doi: 10.1097/DBP.0b013e31822552e9 [DOI] [PubMed] [Google Scholar]
- 54.Lindsay NM, Healy GN, Colditz PB, Lingwood BE. Use of the Ages and Stages Questionnaire to predict outcome after hypoxic-ischaemic encephalopathy in the neonate. J Paediatr Child Health. 2008;44(10):590-595. doi: 10.1111/j.1440-1754.2008.01388.x [DOI] [PubMed] [Google Scholar]
- 55.Mackin R, Ben Fadel N, Feberova J, et al. ASQ3 and/or the Bayley-III to support clinicians’ decision making. PLoS One. 2017;12(2):e0170171. doi: 10.1371/journal.pone.0170171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noeder MM, Logan BA, Struemph KL, et al. Developmental screening in children with CHD: Ages and Stages Questionnaires. Cardiol Young. 2017;27(8):1447-1454. doi: 10.1017/S1047951117000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Connor A, Seeber C, Harris E, Hamilton D, Sachmann M, Fisher C. Developmental outcomes following prenatal exposure to methamphetamine: a Western Australian perspective. J Paediatr Child Health. 2020;56(3):372-378. doi: 10.1111/jpc.14618 [DOI] [PubMed] [Google Scholar]
- 58.Romero Otalvaro AM, Grañana N, Gaeto N, et al. ASQ-3: Validation of the Ages and Stages Questionnaire for the detection of neurodevelopmental disorders in Argentine children. Arch Argent Pediatr. 2018;116(1):7-13. doi: 10.5546/aap.2018.eng.7 [DOI] [PubMed] [Google Scholar]
- 59.Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M. Validity of the Ages and Stages Questionnaires in term and preterm infants. Pediatrics. 2013;131(5):e1468-e1474. doi: 10.1542/peds.2012-3313 [DOI] [PubMed] [Google Scholar]
- 60.Sheldrick RC, Marakovitz S, Garfinkel D, Carter AS, Perrin EC. Comparative accuracy of developmental screening questionnaires. JAMA Pediatr. 2020;174(4):366-374. doi: 10.1001/jamapediatrics.2019.6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simard MN, Luu TM, Gosselin J. Concurrent validity of Ages and Stages Questionnaires in preterm infants. Pediatrics. 2012;130(1):e108-e114. doi: 10.1542/peds.2011-3532 [DOI] [PubMed] [Google Scholar]
- 62.Skellern CY, Rogers Y, O’Callaghan MJ. A parent-completed developmental questionnaire: follow up of ex-premature infants. J Paediatr Child Health. 2001;37(2):125-129. doi: 10.1046/j.1440-1754.2001.00604.x [DOI] [PubMed] [Google Scholar]
- 63.Smith C, Wallen M, Walker K, Bundy A, Rolinson R, Badawi N. Validity of the fine motor area of the 12-month ages and stages questionnaire in infants following major surgery. Phys Occup Ther Pediatr. 2012;32(3):260-271. doi: 10.3109/01942638.2011.606261 [DOI] [PubMed] [Google Scholar]
- 64.Steenis LJ, Verhoeven M, Hessen DJ, van Baar AL. Parental and professional assessment of early child development: the ASQ-3 and the Bayley-III-NL. Early Hum Dev. 2015;91(3):217-225. doi: 10.1016/j.earlhumdev.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 65.Vanvuchelen M, Van Schuerbeeck L, Braeken MA. Screening accuracy of the parent-completed Ages and Stages Questionnaires—second edition as a broadband screener for motor problems in preschoolers with autism spectrum disorders. Autism. 2017;21(1):29-36. doi: 10.1177/1362361315621703 [DOI] [PubMed] [Google Scholar]
- 66.Veldhuizen S, Clinton J, Rodriguez C, Wade TJ, Cairney J. Concurrent validity of the Ages And Stages Questionnaires and Bayley Developmental Scales in a general population sample. Acad Pediatr. 2015;15(2):231-237. doi: 10.1016/j.acap.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 67.Woodward BJ, Papile LA, Lowe JR, et al. Use of the Ages and Stages Questionnaire and Bayley Scales of Infant Development-II in neurodevelopmental follow-up of extremely low birth weight infants. J Perinatol. 2011;31(10):641-646. doi: 10.1038/jp.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu LM, Hey E, Doyle LW, et al. ; Magpie Trial Follow-Up Study Collaborative Group . Evaluation of the Ages and Stages Questionnaires in identifying children with neurosensory disability in the Magpie Trial follow-up study. Acta Paediatr. 2007;96(12):1803-1808. doi: 10.1111/j.1651-2227.2007.00517.x [DOI] [PubMed] [Google Scholar]
- 69.Yue A, Jiang Q, Wang B, et al. Concurrent validity of the Ages and Stages Questionnaire and the Bayley Scales of Infant Development III in China. PLoS One. 2019;14(9):e0221675. doi: 10.1371/journal.pone.0221675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srinithiwat B, Ularntinon S. Concurrent validity of the Ages & Stages Questionnaires, Third Edition, Thai-version (ASQ-3 Thai) with the Denver Developmental Screening Test II (DDST-II) in developmental screening of 18, 24, and 30 months old children at Queen Sirikit National Institute of Child Health. J Med Assoc Thai. 2014;97 (Suppl 6):S6-13. [PubMed] [Google Scholar]
- 71.Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee . Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118(1):405-420. doi: 10.1542/peds.2006-1231 [DOI] [PubMed] [Google Scholar]
- 72.Baeyens JP, Serrien B, Goossens M, Clijsen R. Questioning the “SPIN and SNOUT” rule in clinical testing. Arch Physiother. 2019;9:4. doi: 10.1186/s40945-019-0056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan KS. Systematic reviews of diagnostic tests: a guide to methods and application. Best Pract Res Clin Obstet Gynaecol. 2005;19(1):37-46. doi: 10.1016/j.bpobgyn.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 74.Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. A likelihood ratio approach to meta-analysis of diagnostic studies. J Med Screen. 2003;10(1):47-51. doi: 10.1258/096914103321610806 [DOI] [PubMed] [Google Scholar]
- 75.Plana MN, Pérez T, Zamora J. New measures improved the reporting of heterogeneity in diagnostic test accuracy reviews: a metaepidemiological study. J Clin Epidemiol. 2021;131:101-112. doi: 10.1016/j.jclinepi.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 76.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW; Victorian Infant Collaborative Group . Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352-356. doi: 10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 77.Charkaluk ML, Rousseau J, Calderon J, et al. ; EDEN Mother–Child Cohort Study Group . Ages and Stages Questionnaire at 3 Years for Predicting IQ at 5-6 Years. Pediatrics. 2017;139(4):e20162798. doi: 10.1542/peds.2016-2798 [DOI] [PubMed] [Google Scholar]
- 78.D’Aprano A, Silburn S, Johnston V, Robinson G, Oberklaid F, Squires J. Adaptation of the Ages and Stages Questionnaire for remote Aboriginal Australia. Qual Health Res. 2016;26(5):613-625. doi: 10.1177/1049732314562891 [DOI] [PubMed] [Google Scholar]
- 79.Rydz D, Srour M, Oskoui M, et al. Screening for developmental delay in the setting of a community pediatric clinic: a prospective assessment of parent-report questionnaires. Pediatrics. 2006;118(4):e1178-e1186. doi: 10.1542/peds.2006-0466 [DOI] [PubMed] [Google Scholar]
- 80.Hix-Small H, Marks K, Squires J, Nickel R. Impact of implementing developmental screening at 12 and 24 months in a pediatric practice. Pediatrics. 2007;120(2):381-389. doi: 10.1542/peds.2006-3583 [DOI] [PubMed] [Google Scholar]
- 81.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; Standards for Reporting of Diagnostic Accuracy . Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326(7379):41-44. doi: 10.1136/bmj.326.7379.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram of Study Selection
eFigure 2. Risk of Bias of Included Studies-Summary Graph
eFigure 3. Methodological Quality of Included Studies
eFigure 4. SROC ASQ-2SD to Predict “Any Delay”
eFigure 5. Diagnostic Odds Ratio of ASQ-2SD to Predict “Any Delay”
eFigure 6. Funnel Plot for Publication Bias (ASQ-2SD to predict “any delay”)
eFigure 7. Fagan Nomogram if Baseline Prevalence of “Any Delay” Is 5% (ASQ-2SD to predict “any delay”)
eFigure 8. SROC ASQ-2SD to Predict “Severe Delay”
eFigure 9. Diagnostic Odds Ratio of ASQ-2SD to Predict “Severe Delay”
eFigure 10. Funnel Plot for Publication Bias (ASQ-2SD to predict “severe delay”)
eFigure 11. Fagan Nomogram if Baseline Prevalence of “Severe Delay” Is 5% (ASQ-2SD to predict “severe delay”)
eTable4. Post-test probabilities for various pre-test prevalence of “severe delay” based on the results of ASQ-2SD
eTable5. Results of studies included in the systematic review, but could not be pooled in the meta-analysis
eTables 6-17. Summary of findings tables and Certainty of Evidence
eTable18. Results of sensitivity analyses


