Over the past decade there has been a remarkable progress in the treatment of multiple myeloma (MM) and non-Hodgkin lymphoma (NHL). Nevertheless, autologous stem cell transplant (ASCT) remains an integral part of the care for patients with MM, and one of few curative options for patients with relapsed/refractory (R/R) NHL. Historically, an arbitrary age of 65 has been used to determine patient’s eligibility for ASCT. This stems from the fact that the majority of prospective studies evaluating efficacy of ASCT in MM have excluded patients >65 years old.1 Currently, nearly half of new diagnoses of MM and NHL are >75 years old.2,3 However, the data on safety and efficacy of ASCT patients >75 years remains limited.
Racial minorities remain severely underrepresented in cancer clinical trials, thus limiting the generalizability of clinical cancer research to these populations.4 Data on ASCT in elderly minority patients has to our knowledge not yet been reported. In this letter we present results of a retrospective study showing comparable transplant related mortality in minority patients >75 years old as compared to those aged 55-66 years old.
We conducted a retrospective cohort study comparing ASCT outcomes in patients >75 years old and 55-65 years old for the diagnosis of MM or NHL, who were conditioned with either melphalan or BEAM (carmustine, etoposide, cytarabine, melphalan) respectively. Patients were selected from an internal database which has all ASCT performed at our center between 2005-2021. The study group included patients >75 years old. The control group included patients 55-65 years old that were matched to the study group patients by sex and date of transplant. Electronic medical records were reviewed to gather data. The primary outcomes were admission mortality, length of stay, time to white blood cell (WBC) and platelet engraftment, incidence of neutropenic fever, positive blood culture, intensive care unit (ICU) admission, and 30-day rehospitalization rate. Secondary outcome were 1- and 5-year mortality rates. Patients with no follow-up post ASCT and ASCT prior to 1- and 5-year follow-up were excluded from analysis. Admission mortality and long-term survival probability were calculated using log rank test. Continuous data was reported as medians and interquartile ratios (IQR) and analyzed using Wilcoxon rank sum test. Significance was denoted by α=0.05.
Between 12/2005 and 3/2021, there were 43 patients >75 years old who underwent ASCT for MM or NHL. Data collection was censored on 4/2/22. Table 1 summarizes patient characteristics at index ASCT. Twenty-four (55.8%) patients were female. The median age in the study group was 77.1 (range, 76.2-77.9) years old and 61.9 (range, 57.4,-63.0) years old in the control group. Both groups predominantly included minority patients: 55.8% and 46.5% were Spanish/Hispanic/Latino and 25.6% and 14.0% were African American, in the study and control groups, respectively. MM was the most common indication for auto-HSCT comprising 34 (79.1%) and 33 (76.1%) patients in the study and control groups, respectively.
Table 1.
Patient characteristics.
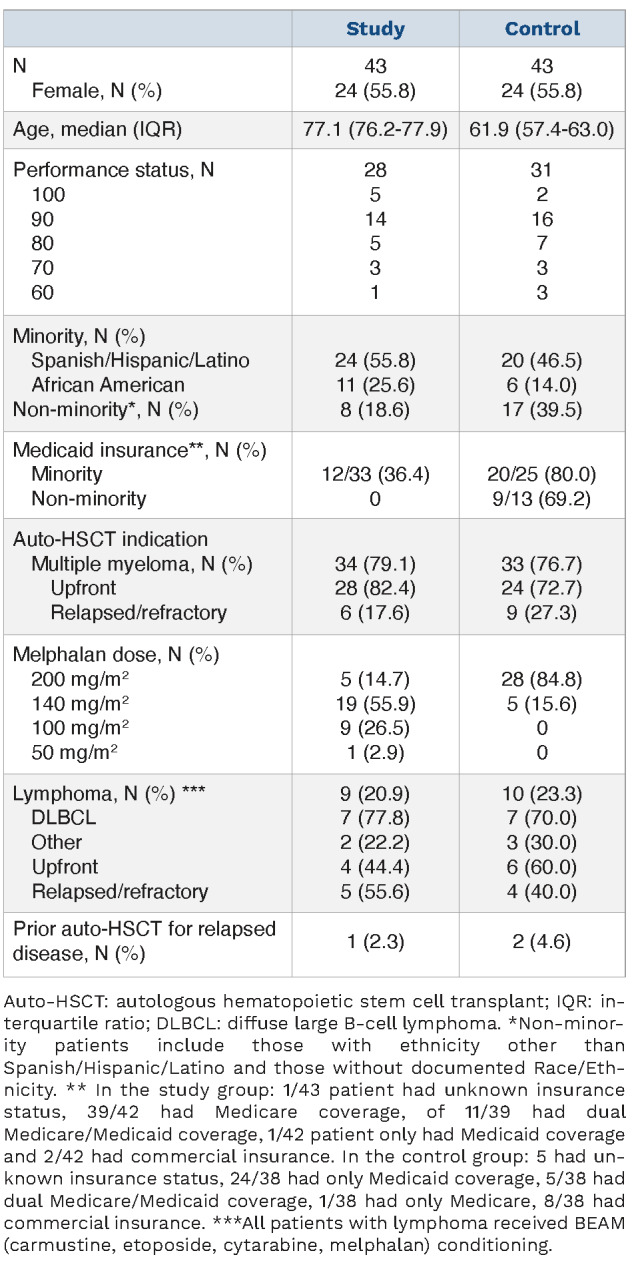
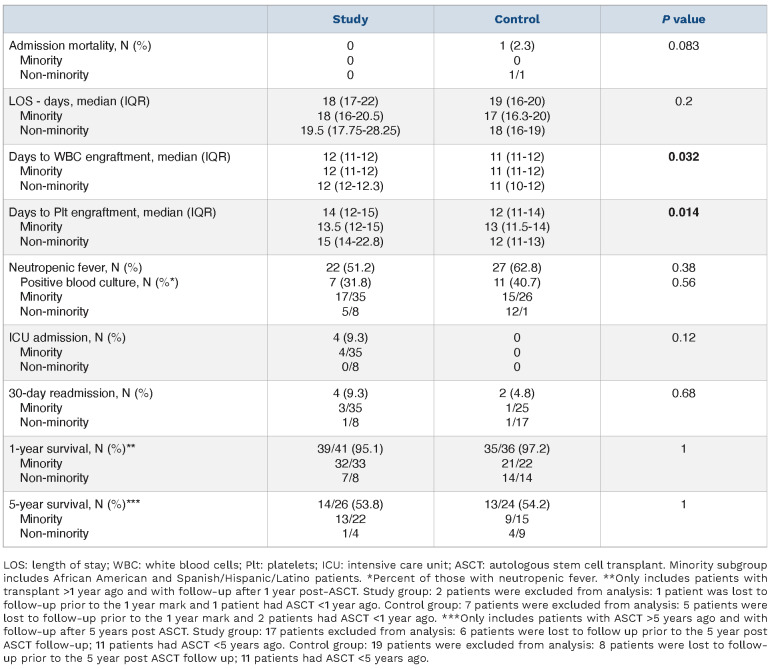
Table 2 outlines patient outcomes. Admission mortality did not differ significantly between the groups, with only one death in the control group (P=0.083). The length of stay was comparable at 18 (range, 17-22) days and 19 (range, 16-20) days (P=0.2) for study and control groups, respectively. Time to WBC engraftment in the study group was 12 (range, 11-12) days and 11 (range, 11-12) days in the control group (P=0.032). Time to platelet engraftment in the study group was 14 (range, 12-15) days and 12 (range, 11-14) days in the control group (P=0.014). Although time to both WBC and platelet engraftment was significantly longer in the study group, the clinical significance of this finding is questionable, especially as it did not significantly prolong length of stay. There was no significant difference between incidence of neutropenic fever, or between incidence of positive blood cultures in patients with neutropenic fever. There was a non-statistically significant increase in the rate of ICU admissions in the study (4/43) versus control group (0/43) (P=0.12). The 30-day re-hospitalization rate was comparable between the two groups (P=0.68).
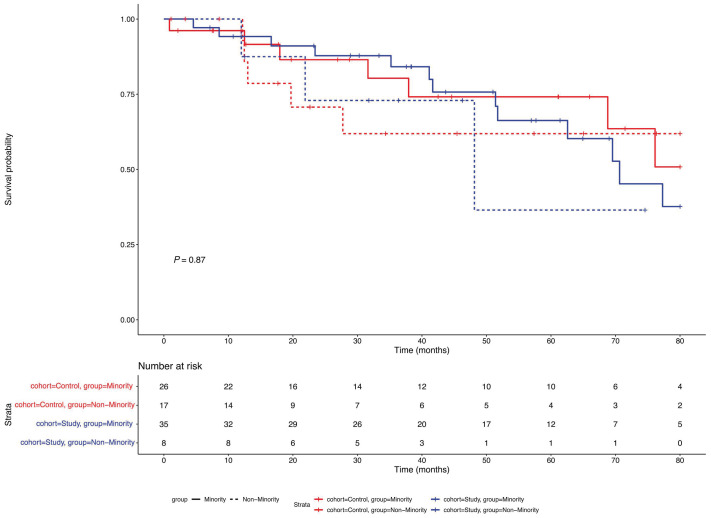
In the study group, two patients died within 1 year of ASCT (day +360 and +133) translating into 1-year mortality of 4.9% (2/41). Five-year survival was 53.8% (14/26). In the control group, one patient died on day +26 of ASCT translating into 1-year mortality of 2.8% (1/36). Five-year survival was 54.2% (13/24) (Figure 1).
Table 2.
Outcomes.
The treatment landscape for elderly patients with both MM and NHL has dramatically improved in recent years. However, ASCT remains a significant contributor to improved outcomes in the elderly population with MM, and one of the few options for long term disease control for patients with R/R NHL.5-10 The main concern related to ASCT in the elderly has historically been transplant-related mortality (TRM). With the improvement of supportive care, TRM rates in elderly patients have been declining. Our finding of 0% TRM is similar to that of prior reports in this age category.7,1 1 Additionally, in our study we observed a comparable 5-year mortality rate for elderly patients and their younger counterparts, despite a 15-year age difference.
In contrast to above-mentioned reports, our elderly cohort includes 80% patients from racial minority groups, predominantly Hispanic or African American. Studies have shown that Hispanics and African Americans are far less likely to undergo ASCT, despite an abundance of data showing similar or better outcomes.12,13 Our study is the first to our knowledge to address the outcomes for elderly minority patients undergoing ASCT. It is worth noting that in the two large reports comparing outcomes of ASCT in minorities and Caucasians with MM, the upper age limit in minority cohorts was 75 years old, while Caucasian cohorts included patients up to age of 80.12,14 Similar to patients with MM, favorable outcomes for elderly patients with NHL receiving ASCT with BEAM conditioning have been reported, with no reports in the literature on minority elderly patients.15 This is of clinical importance as various studies have shown that minority patients with NHL have worse outcomes compared to Caucasians.16,17 This data suggests that elderly minority patients have two separate variables contributing to a decreased chance of being offered ASCT. 26.4% of the Bronx’s population lives below the poverty line.18 Montefiore Medical Center is one of the largest providers of Medicaid and Medicare in New York State. Analysis of National Cancer Database (NCDB) showed that ASCT improved survival for MM patients from all economic backgrounds, but uninsured patients or those with Medicaid had significantly lower overall survival.19 Similar trends have been observed with in patients with NHL.20
Figure 1.
Overall survival. Survival probablity of individual patient cohorts was plotted again time with control groups marked in blue and study groups marked in red.
This study has limitations such as retrospective design, small sample size, variability in minority proportion, and melphalan dosing discrepancy between the groups.
Our study is one of the few to demonstrate the safety of ASCT in patients >75 years old and the only study to evaluate ASCT in a predominantly minority elderly population. We did not find a statistically significant increase in 100-day transplant related mortality in patients > 75 years old compared to patients 55-65 years old. Older patients and those younger than 65 years old appear to have comparable 5-year mortality.
Acknowledgments
We would like to acknowledge all the physician assistants and nurses on the bone marrow transplant and cellular therapy unit for their compassionate care for these patients.
Funding Statement
Funding: This work was supported by grants from Izzy Englander, Emanuel Chirico, the Jane and Myles Dempsey Family, and Bristol Myers Squibb Foundation Diversity in Clinical Trials Career Development Program.
References
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91-97. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Stat Facts: Myeloma: National Cancer Institute; https://seer.cancer.gov/statfacts/html/mulmy.html [Google Scholar]
- 3.Cancer Stat Facts: Non-Hodgkin Lymphoma: National Cancer Institute; https://seer.cancer.gov/statfacts/html/nhl.html [Google Scholar]
- 4.Guerrero S, López-Cortés A, Indacochea A, et al. Analysis of racial/ethnic representation in select basic and applied cancer research studies. Sci Rep. 2018;8(1):13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntanasis-Stathopoulos I, Gavriatopoulou M, Kastritis E, Terpos E, Dimopoulos MA. Multiple myeloma: role of autologous transplantation. Cancer Treat Rev. 2020;82:101929. [DOI] [PubMed] [Google Scholar]
- 6.Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaxman I, Visram A, Kumar S, et al. Autologous stem cell transplantation for multiple myeloma patients aged >/= 75 treated with novel agents. Bone Marrow Transplant. 2021;56(5):1144-1150. [DOI] [PubMed] [Google Scholar]
- 8.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. [DOI] [PubMed] [Google Scholar]
- 9.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096-2107. [DOI] [PubMed] [Google Scholar]
- 10.Usmani SZ, Nahi H, Plesner T, et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020;7(6):e447-e455. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Zhang MJ, Zhong X, et al. Older patients with myeloma derive similar benefit from autologous transplantation. Biol Blood Marrow Transplant. 2014;20(11):1796-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hari PN, Majhail NS, Zhang MJ, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2010;16(3):395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ailawadhi S, Parikh K, Abouzaid S, et al. Racial disparities in treatment patterns and outcomes among patients with multiple myeloma: a SEER-Medicare analysis. Blood Adv. 2019;3(20):2986-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma PS, Howard RS, Weiss BM. The impact of race on outcomes of autologous transplantation in patients with multiple myeloma. Am J Hematol. 2008;83(5):355-358. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Li S, El-Jawahri A, et al. Autologous stem cell transplantation in elderly lymphoma patients in their 70s: outcomes and analysis. Oncologist. 2018;23(5):624-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan THM. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. 2014;123(23):3553-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011;117(11):2530-2540. [DOI] [PubMed] [Google Scholar]
- 18.Neighborhood Profiles: The Bronx BX: NYU Furman Center; https://furmancenter.org/neighborhoods/view/the-bronx [Google Scholar]
- 19.Chamoun K, Firoozmand A, Caimi P, et al. Socioeconomic factors and survival of multiple myeloma patients. Cancers (Basel). 2021;13(4):590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118(17):4271-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]