Abstract
iLLUMINATE is a randomized, open-label phase III study of ibrutinib plus obinutuzumab (n=113) versus chlorambucil plus obinutuzumab (n=116) as first-line therapy for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma. Eligible patients were aged ≥65 years, or <65 years with coexisting conditions. Patients received oral ibrutinib 420 mg once daily until disease progression or unacceptable toxicity or six cycles of oral chlorambucil, each in combination with six cycles of intravenous obinutuzumab. After a median follow-up of 45 months (range, 0.2-52), median progression-free survival continued to be significantly longer in the ibrutinib plus obinutuzumab arm than in the chlorambucil plus obinutuzumab arm (median not reached versus 22 months; hazard ratio=0.25; 95% confidence interval: 0.16-0.39; P<0.0001). The best overall rate of undetectable minimal residual disease (<0.01% by flow cytometry) remained higher with ibrutinib plus obinutuzumab (38%) than with chlorambucil plus obinutuzumab (25%). With a median treatment duration of 42 months, 13 months longer than the primary analysis, no new safety signals were identified for ibrutinib. As is typical for ibrutinib-based regimens, common grade ≥3 adverse events were most prevalent in the first 6 months of ibrutinib plus obinutuzumab treatment and generally decreased over time, except for hypertension. In this final analysis with up to 52 months of follow-up (median 45 months), ibrutinib plus obinutuzumab showed sustained clinical benefit, in terms of progression-free survival, in first-line treatment of chronic lymphocytic leukemia, including in patients with high-risk features. ClinicalTrials.gov identifier: NCT02264574.
Introduction
Over the past decade, the introduction of novel therapies targeting B-cell receptor signaling has shifted the treatment landscape for patients with chronic lymphocytic leukemia (CLL), including for patients with high-risk genomic features who previously had limited treatment options.1 Ibrutinib is an oral, once-daily inhibitor of Bruton tyrosine kinase (BTK) approved for the treatment of patients with CLL, including in combination with the anti-CD20 monoclonal antibody obinutuzumab based on the primary results of the iLLUMINATE study.2,3 In two first-line studies ibrutinib or ibrutinib-based combination therapy was associated with superior progression-free survival (PFS) and overall survival (OS) compared with chlorambu-cil4 or fludarabine, cyclophosphamide, and rituximab (FCR)5 regimens in patients with CLL/small lymphocytic lymphoma (SLL). Ibrutinib (with or without rituximab) was also associated with superior PFS compared to benda-mustine plus rituximab.6 With up to 7 years of follow-up (median: 74.9 months; range, 0.1‒86.8) in the phase III RESONATE-2 study in patients with CLL aged ≥65 years, single-agent ibrutinib provided sustained PFS with extended follow-up, with a PFS of 61% at 6.5 years.4,7 Notably, in patients with high-risk genomic features such as del(17p)/TP53 mutation, del(11q), and unmutated immuno-globulin heavy-chain variable region gene (IGHV), which are known to be associated with inferior outcomes with chemoimmunotherapy,8 ibrutinib-based regimens have consistently achieved superior PFS versus established chemotherapy/chemoimmunotherapies and appear to confer comparable outcomes to those of ibrutinib-treated patients without these high-risk features.4,5,9,10
The multicenter, randomized, phase III iLLUMINATE study was initiated to compare the efficacy of ibrutinib combined with obinutuzumab versus chlorambucil combined with obinutuzumab as first-line therapy for patients with CLL/SLL. Results from the primary analysis with a median follow-up of 31 months demonstrated that ibrutinib plus obinutuzumab significantly prolonged PFS compared with chlorambucil plus obinutuzumab as assessed by an independent review committee (median not reached vs. 19 months) as well as by the study investigators (median not reached vs. 22 months) in both the intention-to-treat population and in patients with high-risk genomic features.3
Here we report the final analysis of iLLUMINATE with a median follow-up of 45 months.
Methods
Study design
iLLUMINATE was a multicenter, randomized, open-label, phase III study that enrolled patients at 71 sites in Australia, New Zealand, Canada, Israel, Turkey, Russia, the European Union, and the USA (ClinicalTrials.gov identifier: NCT02264574). The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and local regulations. The protocol was approved by the institutional review boards, research ethics boards, or independent ethics committees of participating institutions. All patients provided written informed consent. Full details of the study methodology have been published previously3 and are briefly described below.
Participants
Eligible patients had previously untreated CLL/SLL requiring treatment according to International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria11 and were aged ≥65 years or <65 years with at least one of the following co-existing conditions: a cumulative illness rating score >6, creatinine clearance <70 mL/min, del(17p) confirmed by fluorescence in situ hybridization analysis, or TP53 mutation confirmed by next-generation sequencing. Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status 0-2, measurable lymph node disease (longest diameter >1.5 cm), adequate hematologic function (absolute neutrophil count ≥1×109 cells/L; platelet count >50×109 cells/L), and adequate hepatic and renal function.
Treatments
Patients were randomized 1:1 to receive ibrutinib plus obinutuzumab or chlorambucil plus obinutuzumab, as stratified by geographic region (North America vs. rest of world), ECOG performance status (0-1 vs. 2), and cytogenetic status (del[17p] vs. del[11q] without del[17p] vs. others [neither del11q nor del[17p]). Patients received oral ibrutinib 420 mg once daily until disease progression or unacceptable toxicity or oral chlorambucil 0.5 mg/kg body weight on days 1 and 15 of each 28-day cycle for six cycles. Intravenous obinutuzumab (100 mg on day 1 and 900 mg on day 2, and 1,000 mg on days 8 and 15 of cycle 1, followed by 1,000 mg on day 1 of each subsequent 28-day cycle) was administered for up to six cycles.
Outcomes
The primary endpoint for the final analysis was PFS by investigator assessment based on iwCLL 2008 criteria with subsequent clarifications to account for treatment-related lymphocytosis.11 Secondary endpoints included overall response rate (ORR; defined as complete response [CR], CR with incomplete bone marrow (BM) recovery [CRi], nodular partial response [nPR], or partial response [PR]), rate of undetectable minimal residual disease (MRD) (<1 CLL cell per 10,000 leukocytes in peripheral blood (PB) and/or BM aspirate, as measured by central laboratory flow cytometry), OS, sustained hematologic improvement (continuous ≥2 g/dL increase in hemoglobin levels from baseline, or ≥50% increase in platelet counts from baseline for ≥56 days without blood transfusion or growth factors), and safety. Responses were assessed by the investigator for the final analysis. Non-hematologic adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03; hematologic adverse events were assessed using iwCLL criteria.11 Because the additional follow-up since the primary analysis extends beyond the adverse event reporting period for the chlorambucil plus obinutuzumab arm, safety findings are presented only for the ibrutinib plus obinutuzumab arm.
Results
Patient demographics and characteristics
Two hundred twenty-nine patients were randomized to receive ibrutinib plus obinutuzumab (n=113) or chlorambucil plus obinutuzumab (n=116). Patient baseline demographics and disease characteristics were generally comparable between treatment arms (Online Supplementary Table S1). The median age was 71 years (range, 40-87), with most patients (n=183; 80%) aged ≥65 years. Most patients (148 [65%]) had high-risk disease features of del(17p), TP53 mutation, del(11q), or unmutated IGHV.
Patient disposition and treatment
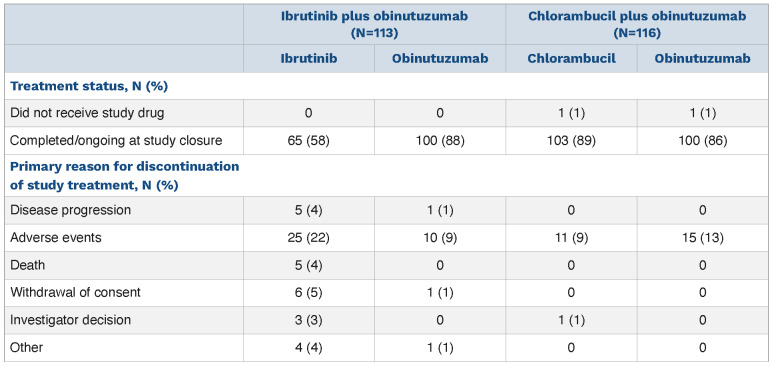
The final analysis was performed upon study completion. The median time on study was 45 months (range, 0.2-52 months) for the total study population, 45.5 months (range, 0.2-52 months) for the ibrutinib plus obinutuzumab arm, and 43 months (range, 1-52 months) for the chlorambucil plus obinutuzumab arm. With study completion, discontinuations across both arms were due to sponsor termination of the study (74%; n=170), death (18%; n=42), withdrawal of consent (6%; n=14), and other (1%; n=1 each: high dose of steroids, obinutuzumab allergy, and infusion reaction). Of patients treated with ibrutinib and obinutuzumab, 26 (23%) and 10 (9%) patients, respectively, discontinued these agents due to adverse events; notably, treatment duration in the ibrutinib plus obinutuzumab arm was longer than that in the chlorambucil plus obinutuzumab arm (median: 42 vs. 5 months). In the ibrutinib plus obinutuzumab arm, discontinuation of ibrutinib therapy due to disease progression was infrequent (n=5; 4%); 65 (58%) patients remained on ibrutinib until study completion (Table 1) and 100 (88%) patients completed six cycles of obinutuzumab (Online Supplementary Figure S1). In the chlorambucil plus obinutuzumab arm, 103 (89%) patients and 100 (86%) patients completed the planned six cycles of chlorambucil and obinutuzumab, respectively.
Of the 116 patients randomized to chlorambucil plus obinutuzumab, 50 (43%) patients subsequently received ibrutinib with study crossover (n=45; 39%) or with commercial ibrutinib (n=6; 5%); one patient received both commercial and crossover ibrutinib. At study completion, 35 of the 45 crossover patients (78%) were still receiving single-agent ibrutinib.
All patients received concomitant medications during study treatment; in the ibrutinib plus obinutuzumab arm, 63/113 (56%) patients received any anticoagulant or antiplatelet agent; of these, 36/113 (32%) patients received anticoagulants and 48/113 (42%) received antiplatelet agents. Ninety-three of 113 patients (82%) received systemic antibacterial drugs. Of the 113 patients in the ibrutinib plus obinutuzumab arm, 78 (69%) received acid-reducing medications; 71/113 (63%) received proton pump inhibitors, 14/113 (12%) received H2-receptor antagonists, and 8/113 (7%) received other acid-reducing agents.
Progression-free survival
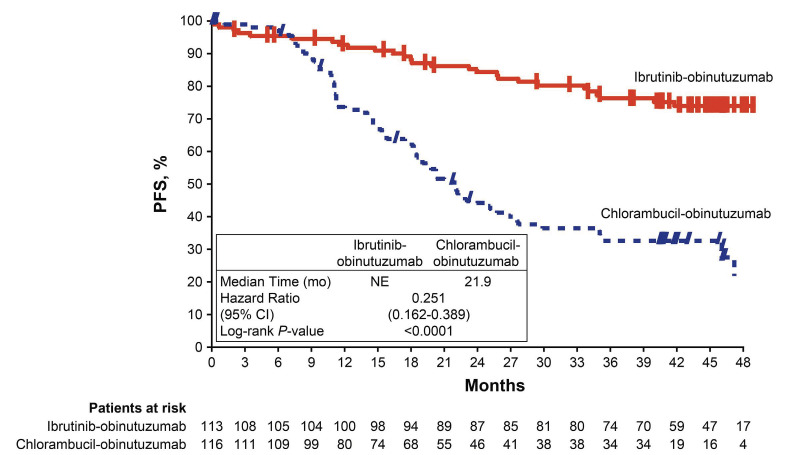
At the final analysis, with a median follow-up of 45 months, PFS remained significantly longer in the ibrutinib plus obinutuzumab arm (median not reached; 95% confidence interval [95% CI], 49 months to not estimable [NE]) than in the chlorambucil plus obinutuzumab arm (22 months; 95% CI: 18-27 months), resulting in a 75% reduction in the risk of disease progression or death with ibrutinib plus obinutuzumab (hazard ratio [HR]=0.25; 95% CI: 0.16-0.39; P<0.0001) (Figure 1). The estimated PFS at 42 months was 74% in the ibrutinib plus obinutuzumab arm and 33% in the chlorambucil plus obinutuzumab arm.
Table 1.
Summary of treatment disposition.
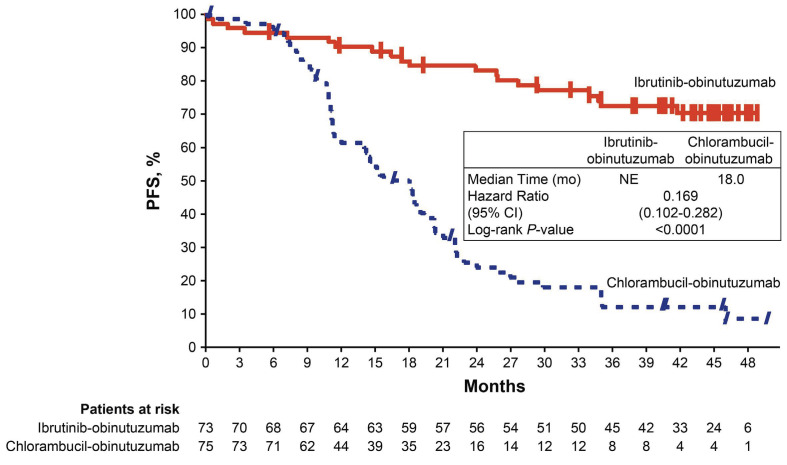
Similar to the overall population, there was a significant PFS benefit for patients with high-risk features (del[17p], del[17p]/TP53 mutation, del[11q] and/or unmutated IGHV) treated with ibrutinib plus obinutuzumab versus chloram-bucil plus obinutuzumab (HR=0.17; 95% CI: 0.10-0.28; P<0.0001). Within the high-risk population, PFS estimates at 42 months were significantly higher in the ibrutinib plus obinutuzumab arm than in the chlorambucil plus obinutuzumab arm (70% vs. 12%). At 48 months, the PFS benefit was maintained since the time of the primary analysis at 30 months (70% vs. 77%, respectively) (Figure 2). The exclusion of patients with del(17p) did not affect 42-month PFS estimates for the rest of the high-risk population, which were 71% and 14%, respectively, for the two treatment arms (Online Supplementary Figure S2).
Figure 1.
Progression-free survival per investigator assessment in the intention-to-treat population. CI: confidence interval; mo: months; NE, not estimable; PFS, progression-free survival.
Figure 2.
Progression-free survival per investigator assessment in the high-risk population of patients with del(17p), del(11q), TP53 mutations, and/or unmutated IGHV. CI: confidence interval; mo: months; NE: not estimable; PFS: progression-free survival.
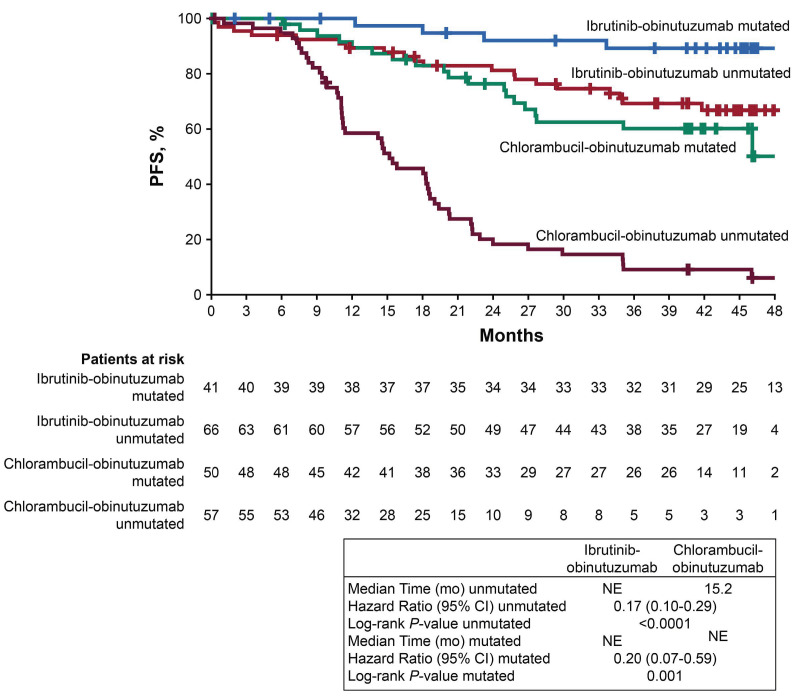
Likewise, a PFS benefit with ibrutinib plus obinutuzumab was observed irrespective of IGHV mutation status (Figure 3). Among patients with unmutated IGHV, up to 48 months median PFS was not reached with ibrutinib plus obinutuzumab versus 15 months with chlorambucil plus obinutuzumab (HR=0.17; 95% CI: 0.10-0.29). Whereas the median PFS was not reached in either treatment arm for patients with mutated IGHV, ibrutinib plus obinutuzumab significantly reduced the risk of progression or death in this subgroup (HR=0.20; 95% CI: 0.07-0.59). Within the ibrutinib plus obinutuzumab arm, the estimated PFS at 48 months was 67% and 89% for patients with unmutated and mutated IGHV, respectively. The PFS benefit from ibrutinib plus obinutuzumab among both subgroups of unmutated and mutated IGHV patients persisted after excluding patients with del(17p) (unmutated: HR=0.17; 95% CI: 0.09-0.30; mutated: HR=0.25; 95% CI: 0.08-0.74) (Online Supplementary Figure S3). The hazard ratios of 0.20 and 0.17 observed in the mutated and unmutated IGHV subgroups of the ibrutinib plus obinutuzumab arm are consistent with the hazard ratio for the intention-to-treat population (HR=0.25) which includes other non-high-risk subgroups (Figure 1). Online Supplementary Figure S4 depicts consistently superior PFS associated with ibrutinib plus obinutuzumab across various subgroups of patients defined by age, Rai stage, ECOG performance status, and bulky disease, in addition to specified genomic risk factors mentioned above.
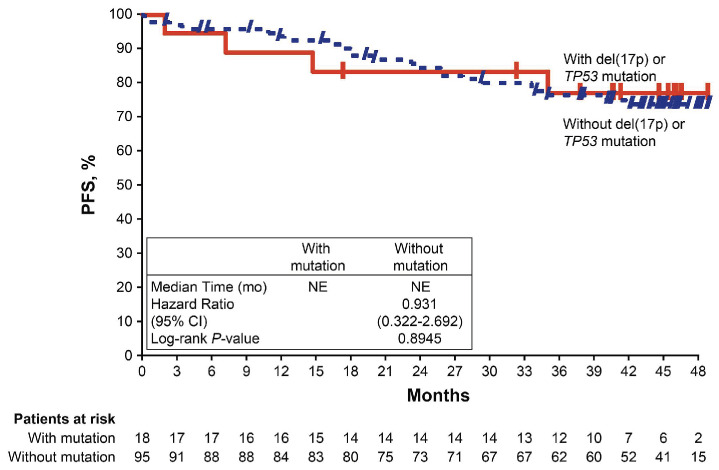
In patients randomized to ibrutinib plus obinutuzumab, no significant difference in PFS was shown between patients with or without del(17p)/TP53 mutation (HR=0.93; 95% CI: 0.32-2.69; P=0.895) (Figure 4). The median PFS was not reached in either subgroup, with estimated 48-month PFS rates of 77% and 74% in patients wih and without del(17p)/TP53 mutation, respectively.
Figure 3.
Progression-free survival per investigator assessment according to IGHV mutation status. CI: confidence interval; mo: months; NE, not estimable; PFS, progression-free survival.
Figure 4.
Progression-free survival per investigator assessment according to TP53 aberration status (del[17p] or TP53 mutation) in the ibrutinib plus obinutuzumab arm. CI: confidence interval; mo: months; NE, not estimable; PFS, progression-free survival.
Time to next treatment
Over a median follow-up of 45 months, fewer patients receiving ibrutinib plus obinutuzumab initiated subsequent treatment for CLL/SLL compared with those receiving chlorambucil plus obinutuzumab (3/113 [3%] vs. 55/116 [47%] patients, respectively). The median time to next treatment was not reached in the ibrutinib plus obinutuzumab arm compared with 33 months (95% CI: 24 months-NE) in the chlorambucil plus obinutuzumab arm, corresponding to a 96% reduction in the risk of needing next-line therapy (HR=0.04; 95% CI: 0.01-0.13; P<0.0001).
Response rates
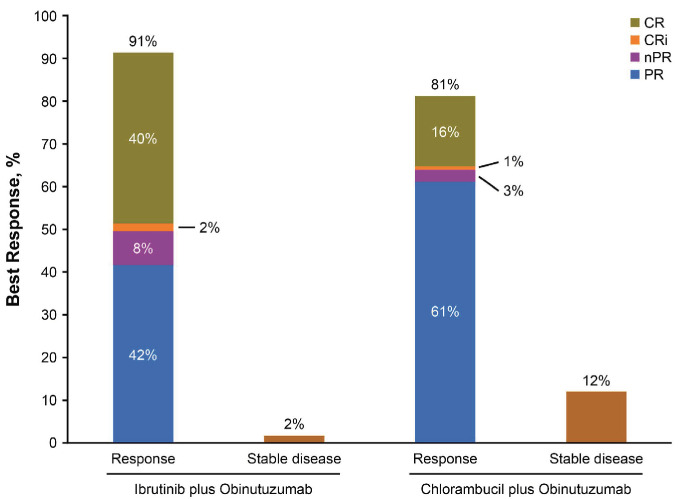
Consistent with the primary analysis, the ORR by investigator assessment was higher in the ibrutinib plus obinutuzumab arm (n=103/113; 91%) than in the chlorambucil plus obinutuzumab arm (n=94/116; 81%) (Figure 5). CR rates, including CRi, in the ibrutinib plus obinutuzumab arm (47 [42%]) were slightly increased relative to rates reported at the primary analysis (46 [41%]); CR/CRi rates in the chlorambucil plus obinutuzumab arm were unchanged compared to those reported at the primary analysis (n=20 [17%]; P<0.0001 for the difference between arms). The median duration of response was not reached in the ibrutinib plus obinutuzumab arm (95% CI: NE-NE) and was 19 months (95% CI: 16-31) in the chlorambucil plus obinutuzumab arm.
Minimal residual disease
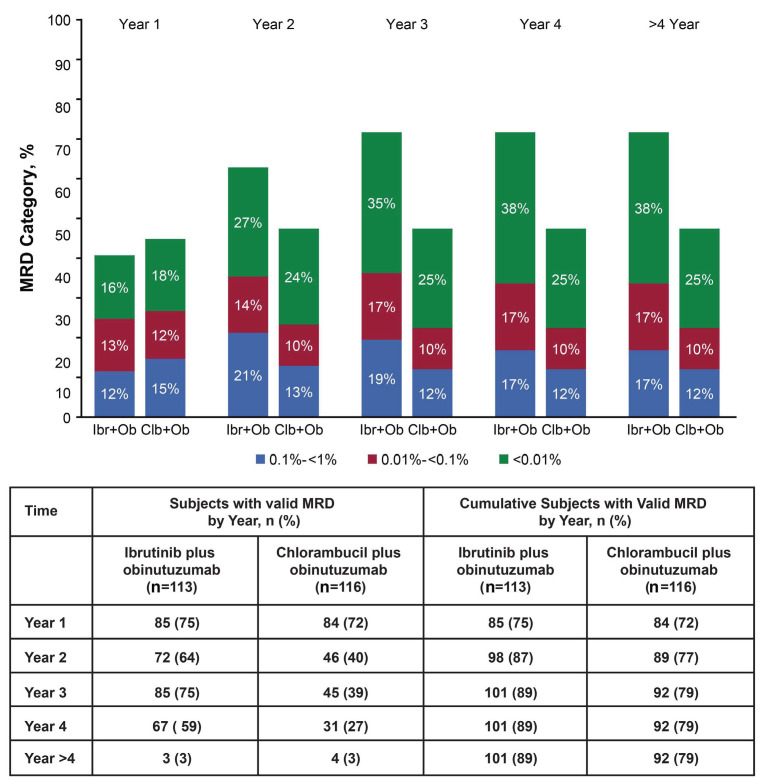
Initially MRD information was obtained from BM from all patients at cycle 9 and upon achieving CR, but subsequent protocol amendments enabled data collection from both BM and PB, and from all responders on a scheduled basis thereafter. Overall, MRD assessments were obtained from 101/113 patients on ibrutinib plus obinutuzumab (93 BM and 90 PB) and from 92/116 patients in the chlorambucil plus obinutuzumab arm (84 BM and 60 PB). Most patients with undetectable MRD (<0.01%) were subsequently followed with at least one repeat MRD assessment (91% for ibrutinib plus obinutuzumab; 86% for chlorambucil plus obinutuzumab), with a median follow-up of 23 and 30 months, respectively, following initial attainment of undetectable MRD. Overall, 38% (43/113) of patients in the ibrutinib plus obinutuzumab arm achieved undetectable MRD in BM or PB compared with 25% (29/116) in the chlorambucil plus obinutuzumab arm (P=0.033) (Online Supplementary Table S2). Rates of undetectable MRD for the ibrutinib plus obinutuzumab arm were 25% for BM (28/113) and 33% for PB (37/113); rates for the chlorambucil plus obinutuzumab arm were 17% (20/116) in BM and 20% (23/116) in PB. In these patients, the cumulative rate of undetectable MRD (<0.01%) increased over the first 3 years with continuous ibrutinib therapy and then remained stable through final analysis (Figure 6). As expected, given the differences in PFS for the two arms following initial attainment of undetectable MRD, 60% of patients receiving ibrutinib plus obinutuzumab maintained undetectable MRD status through last PB or BM testing compared to 31% of patients receiving chlorambucil plus obinutuzumab (Online Supplementary Figure S5A). The cumulative MRD relapse rate (defined as MRD ≥1% after previously achieving undetectable MRD) was lower in the ibrutinib plus obinutuzumab group than in the chlorambucil plus obinutuzumab group (12% vs. 24%) and the median time to MRD relapse was not estimable (range, 0.03-44.2 months) in the ibrutinib plus obinutuzumab arm versus 37.5 months (range, 0.03-44.2 months) in the chlorambucil plus obinutuzumab arm.
Figure 5.
Best overall response per investigator assessment with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab. CR: complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; PR, partial response.
Examined among patients who achieved a best response of CR/CRi with ibrutinib plus obinutuzumab, 28/47 patients (60%) had undetectable MRD while among those in the chlorambucil plus obinutuzumab arm achieving CR, 15/20 patients (75%) had undetectable MRD in PB or BM. By contrast, among patients who achieved a best response of PR/nPR, 14/56 patients had undetectable MRD levels in PB or BM in the ibrutinib plus obinutuzumab arm (25%) and 14/74 (19%) in the chlorambucil plus obinutuzumab arms. Although limited by the small number of evaluable patients, the median PFS was similar in patients with CR/CRi, regardless of MRD status (Online Supplementary Figure S5B, C); undetectable MRD trended to correlate positively with longer PFS in patients with PR/nPR, especially in the chlorambucil plus obinutuzumab arm.
Likewise, in the high-risk population, more than double the percentage of patients in the ibrutinib plus obinutuzumab arm maintained undetectable MRD than in the chlorambucil plus obinutuzumab group ([n=15/23]; 65% vs. [n=3/11]; 27%). In high-risk patients with >24 months of MRD follow-up, five of six patients (83%) in the ibrutinib plus obinutuzumab arm remain MRD negative compared with two of five patients (40%) in the chlorambucil plus obinutuzumab arm. As in the overall population, the cumulative MRD relapse rate in the high-risk population after achieving undetectable MRD was lower in the ibrutinib plus obinutuzumab group than in the chlorambucil plus obinutuzumab group (0%; [n=0/23] vs. 27%; [n=3/11]). The median time to MRD relapse was not estimable in either the ibrutinib plus obinutuzumab arm (range, 0.03-38.6 months) or the chlorambucil plus obinutuzumab arm (range, 0.03-33.4 months).
Overall survival
At the time of final analysis, the median OS was not reached in either treatment arm (HR=1.08; 95% CI: 0.60-1.97; P=0.793) (Online Supplementary Figure S6). Among the 45 patients who crossed-over to single-agent ibrutinib, the 24-month OS was 88% (95% CI: 74.0-94.9) and six deaths were reported (13%). Overall, 22 (19%) patients in the ibrutinib plus obinutuzumab arm and 21 (18%) patients in the chlorambucil plus obinutuzumab died during the study.
Adverse events in the ibrutinib plus obinutuzumab arm
At final analysis, the median duration of exposure to ibrutinib was 42 months (range, 0.1-52) in the ibrutinib plus obinutuzumab group, which was 13 months longer than ibrutinib exposure at the primary analysis (median 29 months3); 73 patients (65%) received ≥3 years of ibrutinib and 18 (16%) received ≥4 years of ibrutinib therapy. All patients had completed or discontinued obinutuzumab (on both arms) and chlorambucil before the primary analysis, thus the median durations of treatment for obinutuzumab did not change since the primary analysis (4.6 months [range, 4.6-4.9] for the chlorambucil plus obinutuzumab group; 4.6 months [range, 4.6-4.7] for the ibrutinib plus obinutuzumab group).3 The median duration of treatment with chlorambucil was 5.1 months (range, 5.1-5.3) in the chlorambucil plus obinutuzumab group.3
Figure 6.
Cumulative rates of minimal residual disease over time (in bone marrow or peripheral blood). Clb: chlorambucil; Ibr: ibrutinib; MRD: minimal residual disease; Ob: obinutuzumab.
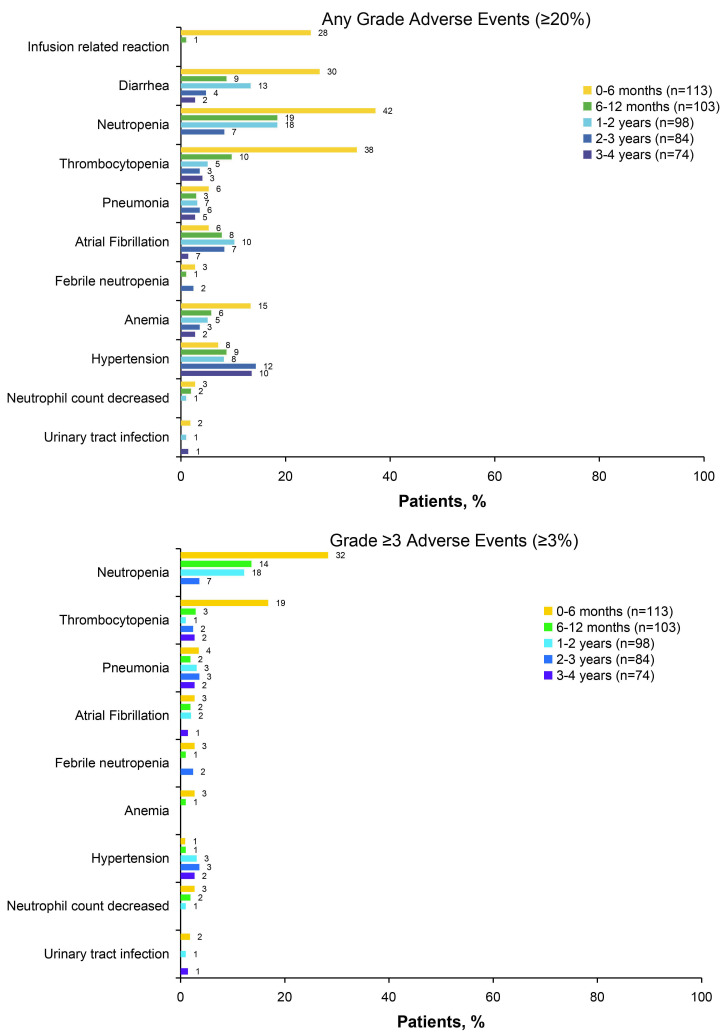
With ongoing treatment in the ibrutinib plus obinutuzumab arm, no new safety signals were noted in this analysis relative to the primary analysis.3 With a median treatment exposure of 42 months in the ibrutinib plus obinutuzumab arm, the most common adverse events (occurring in ≥10% of patients in either arm) were neutropenia (44%), throm-bocytopenia (35%), diarrhea (35%), cough (29%), infusion-related reaction (25%), and arthralgia (24%) (Online Supplementary Table S3). The most common grade ≥3 adverse events in the ibrutinib plus obinutuzumab arm were neutropenia (36%), thrombocytopenia (19%), pneumonia (9%), atrial fibrillation (6%), and febrile neutropenia (5%). The prevalence of these grade ≥3 adverse events was highest during the first 6 months of treatment with ibrutinib plus obinutuzumab and generally decreased over time, with the exception of hypertension (Figure 7). The prevalence of grade ≥3 hypertension was 2% (n=2/113), 3% (n=3/98), 4% (n=3/84), and 3% (n=2/74) in years 0-1, 1-2, 2-3, and 3-4, respectively. No patient discontinued ibrutinib therapy due to hypertension of any grade. The prevalence of any grade atrial fibrillation decreased, being 10% (n=11/113), 10% (n=10/98), 8% (n=7/84), and 9% (n=7/74) in years 0-1, 1-2, 2-3, and 3-4, respectively; the prevalence of grade ≥3 atrial fibrillation was 4% (n=5/113), 2% (n=2/98), 0%, and 1% (n=1/74), in years 0-1, 1-2, 2-3, and 3-4, respectively. Among 17 patients with atrial fibrillation of any grade, atrial fibrillation led to discontinuation of ibrutinib in one patient (a grade 3 event).
Figure 7.
Prevalence of most common adverse events of any grade (≥20%) and grade ≥3 adverse events (≥3%) over time in patients treated with ibrutinib plus obinutuzumab.
Seven major hemorrhagic adverse events (grade 1/2 for 2 events, grade 3 for 5 events, and no grade 4/5 events) occurred in five (4%) patients in the ibrutinib plus obinutuzumab arm: catheter site hematoma, ecchymosis, hematemesis, hemoptysis, post-procedural hematoma, subdural hematoma, and traumatic hematoma. Major hemorrhage led to discontinuation of ibrutinib in one patient (grade 2 hemoptysis).
Over a median duration of 42 months of ibrutinib therapy, the only adverse event leading to discontinuation of ibrutinib in more than one patient was thrombocytopenia (n=2); all other adverse events leading to discontinuation of ibrutinib occurred in only one patient each. Of these, seven and six patients discontinued during the first 6 and 12 months of treatment, respectively. Four patients each discontinued at time intervals of >12‒≤24 months, >24‒≤36 months, and >36‒≤48 months.
In the ibrutinib plus obinutuzumab arm, 17 (15%) patients experienced a total of 32 adverse events (including 18 grade ≥3 events) that led to ibrutinib dose reduction, most commonly due to neutropenia (6 [5%] patients) (Online Supplementary Figure S7). Most (28/32 [88%]) of these adverse events resolved or recovered with dose reduction, including 94% (17/18) of grade ≥3 events.
At the time of final analysis, 14 and three patients had died from treatment-emergent adverse events in the ibrutinib plus obinutuzumab and chlorambucil plus obinutuzumab arms, respectively (Online Supplementary Table S4). Of the five deaths that occurred in the ibrutinib plus obinutuzumab arm with the additional follow-up, four were due to adverse events (1 each due to respiratory tract infection, pneumonia, septic shock and one death was due to an unknown cause after the adverse event reporting period). In the chlorambucil plus obinutuzumab arm, an additional two deaths occurred since the primary analysis, after crossover to the ibrutinib plus obinutuzumab arm. One was due to sepsis, the other was due to progressive disease.
Outcomes afer discontinuation of ibrutinib
Thirty-eight patients discontinued ibrutinib after a median of 15.5 months (range, 0.1-43.7) of treatment for reasons other than progression or death at any time on study, 25 patients discontinued due to adverse events (Table 1). Among patients who discontinued ibrutinib for reasons other than progressive disease or death, eight patients had achieved a CR or CRi, 22 patients had PR or nPR, and one patient had stable disease; seven patients had no opportunity for response assessment. With a median follow-up of 17 months (range, 0.03-42) after ibrutinib discontinuation, 18-month PFS in these 38 patients who discontinued for reasons other than progression or death was 73.8% (95% CI: 55.4-85.5).
Discussion
Results from the final analysis of the iLLUMINATE study with up to 52 months of follow-up (median: 45 months) confirmed the durable efficacy of ibrutinib plus obinutu-zumab in previously untreated patients with CLL. Ibrutinib plus obinutuzumab resulted in sustained PFS compared to chlorambucil plus obinutuzumab, with median PFS not reached after a median follow-up of 45 months and the risk of disease progression or death reduced by 75%. Notably, durable PFS was also seen in the high-risk patient population (i.e., with del[17p], TP53 mutation, del[11q], or unmutated IGHV) treated with ibrutinib plus obinutuzumab, confirming earlier observations from the primary analysis of iLLUMINATE.3 At 42 months, the PFS estimates among patients with del(17p), TP53, unmutated IGHV or del(11q) mutations were significantly higher in the ibrutinib plus obinutuzumab arm than in the chlorambucil plus obinutuzumab arm. Importantly, the benefit in PFS was observed regardless of high-risk features.3 A similar benefit was observed in the CLL14 trial for the venetoclax plus obinutuzumab regimen in patients with unmutated and mutated IGHV.12 These results support current global consensus guidelines,13,14 noting ibrutinib as a preferred therapeutic regimen for older patients and/or those with comorbidities regardless of the presence of del(17p)/TP53 mutations and unmutated CLL. The follow-up for other, similarly recommended novel treatment options is more limited.
With this extended follow-up, as has been observed consistently across several large, randomized phase III studies of ibrutinib in previously untreated15 and relapsed/refractory patients with CLL/SLL,16,17 highly durable PFS and OS have been confirmed even in patients with high-risk disease characteristics.
In line with the results of the iLLUMINATE trial, other phase III studies have also reported the superiority of ibrutinib-based regimens over chemoimmunotherapy in the first-line treatment of CLL. Concurrently, in the phase III ALLIANCE 041202 study in patients with CLL aged ≥65 years (median follow-up: 38 months), first-line singleagent ibrutinib or ibrutinib plus rituximab significantly prolonged PFS compared with bendamustine plus rituximab; estimated 2-year PFS rates were 87% and 88% versus 74%, respectively.6 In patients aged ≤70 years, the phase III ECOG1912 study (median follow-up: 34 months) demonstrated that first-line treatment with ibrutinib plus rituximab resulted in significantly longer PFS (3-year rate 89% vs. 73%) and OS (3-year rate 99% vs. 92%) compared with FCR.5 With additional follow-up (median: 45 months), PFS favored ibrutinib plus rituximab over FCR (HR=0.39; 95% CI: 0.26-0.57; P<0.0001).18
With additional follow-up since the primary analysis of iLLUMINATE, the percentage of patients treated with ibrutinib plus obinutuzumab achieving undetectable MRD increased (from 34.5% at primary analysis to 38.1% at final analysis), demonstrating deepening of remission with continued ibrutinib treatment. Furthermore, fewer patients in the ibrutinib arm experienced MRD relapse, with approximately 60% of patients maintaining undetectable MRD at a median follow-up of 2 years after first attaining undetectable MRD status. Due to the small numbers of patients in the subgroups, the association between MRD status and PFS was not further studied.
Few patients discontinued treatment during the additional 13 months of follow-up after the primary analysis (n=14), and no new safety signals were reported with ongoing ibrutinib treatment. This is consistent with prior reports that rates of most adverse events are highest during the first year of ibrutinib-based treatment and decrease in frequency thereafter.4,9,19,20 Most patients had improvement or resolution of adverse events following dose reduction, suggesting that many such events are managed effectively by dose modification, allowing patients to stay on therapy and continue to maintain disease control. The discontinuation rate due to adverse events (22%) is comparable to that for first-line, single-agent ibrutinib after 4 years of follow-up in the RESONATE-2 study (19%)4 and is supported by real-world studies showing that treatment discontinuation is similar in patients with CLL/SLL receiving single-agent ibrutinib or ibrutinib-based combination therapy.21
Overall, first-line ibrutinib plus obinutuzumab was well tolerated, which is important given that the median age at diagnosis of CLL is over 70 years,22 a demographic that often presents with comorbidities that preclude the use of chemotherapy-containing regimens. In patients receiving concomitant medications, ibrutinib was well tolerated. Notably, ibrutinib can be co-administered with acid-reducing medications, offering an advantage over other kinase inhibitors known to have drug‒drug interactions with acid-reducing agents.23 Overall, these results strengthen and build upon the broad experience with ibrutinib.
Several studies have assessed whether adding anti-CD20 therapy to ibrutinib results in a clinically relevant improvement in efficacy in CLL.5,6,24 Evidence suggests that addition of anti-CD20 therapy to ibrutinib may increase depth of response and decrease the time to absolute lymphocyte count normalization relative to single-agent ibrutinib therapy, although survival outcomes have not been significantly improved over the impressive results with single-agent ibrutinib.6,24,25 The lack of data showing quality of life or survival outcome benefits notwithstanding, the addition of an anti-CD20 agent may nonetheless provide reassurance for patients concerned with increased absolute lymphocyte count. In a cross-trial comparison of RESONATE-2 and iLLUMINATE, patients treated with ibrutinib plus obinutuzumab combination therapy had higher CR/CRi rates (44% vs. 27%; P=0.006) and shorter time to absolute lymphocyte count normalization (8 vs. 55 weeks) than patients treated with single-agent ibrutinib.25 Compared to single-agent ibrutinib, the combination of ibrutinib plus rituximab was demonstrated by Burger et al. to improve CR/CRi rates (particularly in the first-line setting: 50% vs. 20%) and shortened time to absolute lymphocyte count normalization (24 vs. 48 weeks) in a phase II study with a median follow-up of 36 months.24 In the phase III ALLIANCE 041202 study, the CR rates (12% and 7%) and rates of undetectable MRD (4% and 1%) were slightly higher with ibrutinib plus rituximab than with single-agent ibrutinib.6 The difference in response rates tended to be greater in patients with high-risk disease, suggesting that combination therapy may be most useful for patients with high-risk or bulky disease.24 In the relapsed/refractory high-risk setting, the GENUINE study demonstrated significantly higher ORR with ublituximab plus ibrutinib compared to single-agent ibrutinib after a median follow-up of 41.6 months (83% vs. 65%; P=0.02).26 In the setting of other BTK inhibitors, the ELEVATE-TN study reported investigator-assessed CR/CRi rates of 24% with acalabrutinib plus obinutuzumab compared to 8% for acalabrutinib monotherapy and 13% for obinutuzumab plus chlorambucil.27
In terms of PFS, 48-month estimates between RESONATE-2 and the current study were similar across ibrutinib-based treatment arms (76% vs. 74%), although it is worth noting that the current study included patients with high-risk genomic features whereas RESONATE-2 excluded those with del17p.4,10 Regardless, these broad clinical data clearly refute preclinical hypotheses of ibrutinib negating anti-CD20 efficacy, although anti-CD20 use may be important to optimize efficacy of other BTK inhibitors.27
In conclusion, ibrutinib plus obinutuzumab remains an effective chemotherapy-free regimen for patients with CLL/SLL that provides sustained efficacy and significantly reduces the risk of disease progression or death compared with chlorambucil plus obinutuzumab, including in patients with high-risk genomic features. With ongoing once-daily dosing, long-term ibrutinib therapy was well tolerated with no new safety signals observed.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this trial and their families. The authors also thank Cindi A. Hoover, PhD, for medical writing, which was supported by Pharmacyclics LLC, an AbbVie Company.
Funding Statement
Funding: Pharmacyclics LLC, an AbbVie Company, sponsored and designed the study. Study investigators and their research teams collected the data. The sponsor confirmed data accuracy and analyzed the data. Medical writing was funded by the sponsor.
References
- 1.Burger JA, O'Brien S. Evolution of CLL treatment - from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;15(8):510-527. [DOI] [PubMed] [Google Scholar]
- 2.IMBRUVICA (ibrutinib) [prescribing information]. Sunnyvale, CA: Pharmacyclics LLC, an AbbVie Company; 2020. [Google Scholar]
- 3.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43-56. [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr PM, Owen C, Robak T, et al. Up to seven years of follow-up in the RESONATE-2 study of first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. J Clin Oncol. 2021;39(15_suppl):7253. [Google Scholar]
- 8.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24(3):437-443. [DOI] [PubMed] [Google Scholar]
- 9.Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to 6 years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger JA, Robak T, Demirkan F, et al. Outcomes of first-line ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and high-risk genomic features with up to 6.5 years follow-up: integrated analysis of two phase 3 studies (RESONATE-2 and iLLUMINATE). Blood. 2020;136(Suppl 1):25-26. [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188-1200. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. Chronic lymphocytic leukemia/small cell lymphocytic Lymphoma. Version 3.2021. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1478 [Google Scholar]
- 14.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23-33. [DOI] [PubMed] [Google Scholar]
- 15.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200-211. [DOI] [PubMed] [Google Scholar]
- 18.Shanafelt TD, Wang V, Kay NE, et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 trial. Blood. 2019;134(Suppl 1):33. [Google Scholar]
- 19.Byrd JC, Furman RR, Coutre SE, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uminski K, Brown K, Bucher O, et al. Descriptive analysis of dosing and outcomes for patients with ibrutinib-treated relapsed or refractory chronic lymphocytic leukemia in a Canadian centre. Curr Oncol. 2019;26(5):e610-e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241. [DOI] [PubMed] [Google Scholar]
- 23.CALQUENCE (acalabrutinib) capsules for oral use [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019. [Google Scholar]
- 24.Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedeschi A, Greil R, Demirkan F, et al. A cross-trial comparison of single-agent ibrutinib versus chlorambucil-obinutuzumab in previously untreated patients with chronic lymphocytic leukemia or small lymphocytic lymphoma. Haematologica. 2020;105(4):e164-e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharman JP, Brander DM, Mato AR, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021;8(4):e254-e266. [DOI] [PubMed] [Google Scholar]
- 27.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.