Abstract
Multiple myeloma (MM) is an incurable hematologic neoplasm, whose poor prognosis is deeply affected by the propensity of tumor cells to localize in the bone marrow (BM) and induce the protumorigenic activity of normal BM cells, leading to events associated with tumor progression, including tumor angiogenesis, osteoclastogenesis, and the spread of osteolytic bone lesions. The interplay between MM cells and the BM niche does not only rely on direct cell-cell interaction, but a crucial role is also played by MM-derived extracellular vesicles (MM-EV). Here, we demonstrated that the oncogenic NOTCH receptors are part of MM-EV cargo and play a key role in EV protumorigenic ability. We used in vitro and in vivo models to investigate the role of EV-derived NOTCH2 in stimulating the protumorigenic behavior of endothelial cells and osteoclast progenitors. Importantly, MM-EV can transfer NOTCH2 between distant cells and increase NOTCH signaling in target cells. MM-EV stimulation increases endothelial cell angiogenic ability and osteoclast differentiation in a NOTCH2-dependent way. Indeed, interfering with NOTCH2 expression in MM cells may decrease the amount of NOTCH2 also in MM-EV and affect their angiogenic and osteoclastogenic potential. Finally, we demonstrated that the pharmacologic blockade of NOTCH activation by γ-secretase inhibitors may hamper the biological effect of EV derived by MM cell lines and by the BM of MM patients. These results provide the first evidence that targeting the NOTCH pathway may be a valid therapeutic strategy to hamper the protumorigenic role of EV in MM as well as other tumors.
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm representing alone 13% of all hematological malignancies.1 Despite the development of new therapies, MM still remains incurable,2 mainly due to MM cell ability to shape the bone marrow (BM) niche sustaining tumor progression. Upon the localization in the BM, MM cells establish anomalous signaling loops with the neighboring cells and "educate" BM-residing non-tumor cells to support different steps of MM progression, including tumor cell growth, survival, angiogenesis, and bone osteolysis.3
In this complex picture, extracellular vesicles (EV) are new key players recently come to light. EV include a heterogeneous group of cell-derived membranous structures classified into two main subtypes according to their origin. Exosomes, the smaller ones, originate from the endosomal system, while the larger vesicles are shed from the plasma cell membrane. Due to the difficulty to distinguish these subtypes based on their origin, a recent position statement of the International Society for Extracellular Vesicles has suggested a distinction based on their size: i.e., small EV <200 nm and large EV >200 nm.4
EV are key mediators in the communication between tumor and stroma due to their ability to transport proteins and RNA.5 Circulating EV from MM patients display characteristic size distribution and concentration,6,7 and their microRNA (miRNA) cargo is prognostic in MM.8-10 Recent evidence indicates that MM cell-derived EV (MM-EV) modulate the BM niche, promoting angiogenesis, immunosuppression,11 and bone disease.12 Additionally, several features of MM-EV may contribute to MM dissemination at distant sites, thereby favoring skeletal metastasis formation, progression and bone disease.13
This work elucidates how MM cells exploit the aberrantly expressed NOTCH2 oncogene to shape the BM niche via MM-EV, specifically focusing on tumor angiogenesis and osteoclastogenesis.
NOTCH is a family of transmembrane receptors (NOTCH1-4) activated by the interaction with five different membrane-bound ligands (JAGGED1-2 and DLL1-3-4) present on the adjacent cells. The consequence of this interaction is the activation of cleavage by γ-secretase, which releases the active form of NOTCH (NOTCH-IC) from the cell membrane and allows its translocation to the nucleus and the activation of the CSL transcription factor.14
NOTCH deregulation in MM cells is due to the aberrant expression of NOTCH receptors and/or ligands.15 High levels of NOTCH pathway activity are associated with increased myeloma cell infiltration in BM biopsies of MM patients.16 Other studies suggest that MM cell skeletal infiltration may be due to events promoted by NOTCH, including MM cell recruitment at the BM,17 mitogenic or anti-apoptotic effect17-19 or MM stem cell self-renewal.20 Additionally, MM infiltration of BM niche is also associated with the activation of NOTCH signaling in the tumor niche, which promotes angiogenesis,16,21 osteoclastogenesis,22-24 and bone marrow stromal cell (BMSC)-mediated release of cytokines involved in these events (IL-6, VEGF, IGF-1, SDF-1, RANKL, etc.).16,18,19,25
Up to now, the increased activation of NOTCH signaling in the tumor microenvironment has been attributed to the presence of high levels of MM cell-derived JAGGED ligands. Here, we demonstrate that MM cells may trigger tumor angiogenesis and osteoclastogenesis by transferring NOTCH2 receptor via EV. Moreover, we provide evidence that targeting the NOTCH pathway may represent a suitable strategy to hamper the MM-EV-mediated pathological communication with the BM niche.
Methods
Extracellular vesicles isolation from human multiple myeloma cell line and multiple myeloma patients’ bone marrow aspirates
EV were obtained from supernatants of RPMI8226 and OPM2 cells cultured for 48 hours (h) in RPMI1640 medium depleted of fetal bovine serum-derived bovine EV or from the plasma obtained by BM aspirates of monoclonal gammopathy of undetermined significance (MGUS) (MGUS-BM-EV) and MM patients (MM-BM-EV). The Institutional Review Board of Insubria Italy approved the design of this study (approval n. 1 on 27th February 2018). Written informed consent was obtained in accordance with the Declaration of Helsinki. Clinical information of patients is reported in the Online Supplementary Table S1.
EV pellets were resuspended in the appropriate buffer/medium for subsequent studies. Further details are reported in Online Supplementary Appendix.
Production of viral supernatants and NOTCH2 knockdown
Viral supernatants were generated by calcium phosphate-DNA transfection of HEK293T cells with the Dharmacon Trans-lentiviral packaging kit containing pTRIPZ vector carrying a doxycycline-inducible system (Tet-on) expressing short hairpin RNA (shRNA) against NOTCH2, or the corresponding scrambled shRNA (Horizon Discovery, United Kingdom). A pilot experiment on HEK293T cells was carried out by transient transfection of four shRNA for NOTCH2 to select the more effective NOTCH2 shRNA (Cat.ID RHS5087-EG4853 - mature antisense sequence: ATGTCACAAGAGACATTGG). Lentiviral supernatants were used to infect and generate stable clones of RPMI8226 and OPM2 cells. shRNA expression was induced by treatment with 1 µg/mL doxycycline (Sigma Aldrich, Italy).
In vivo experiments
In vivo experiments were carried out on transgenic zebra-fish (Danio rerio) embryos obtained by crossing Tg(T2KTp1bglob:hmgb1-mCherry) with Tg(fli1a:EGFP) obtained from the Wilson lab, University College London, UK. Zebrafish embryos were raised and maintained under standard conditions and national guidelines (Italian decree 4th March 2014, n. 26). All experiments have been conducted within 5 days post fertilization (dpf). EV were injected into the duct of Cuvier of embryos at 48 hours post fertilization (hpf) with a manual microinjector (Eppendorf, Germany) using glass microinjection needles. Further details on the above procedures and information concerning cell cultures, transmission electron microscopy, in vitro uptake, western blot, EV-derived NOTCH2 tracking system, viral particle production, luciferase reporter assay, in vivo experiments, osteoclastogenesis and angiogenesis assays, ex vivo experiments and statistical analyses are reported in the Online Supplementary Appendix.
Results
Multiple myeloma-derived extracellular vesicles are uptaken by bone marrow cell populations
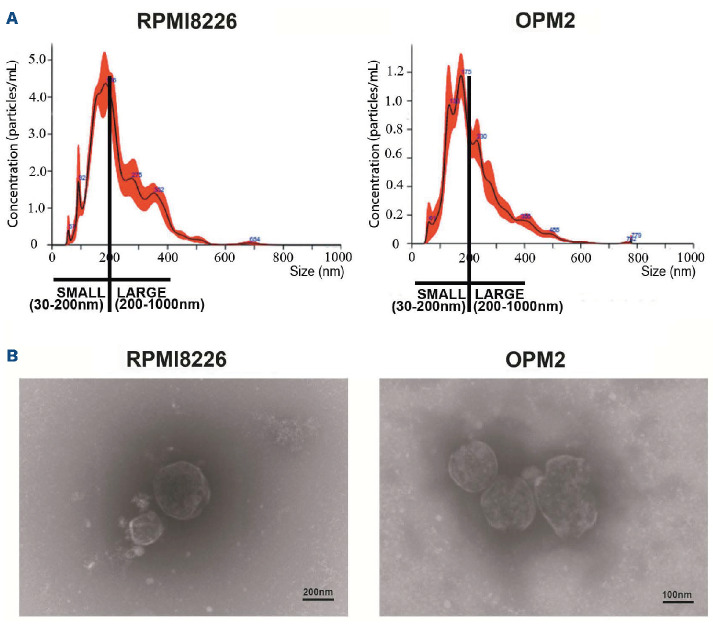
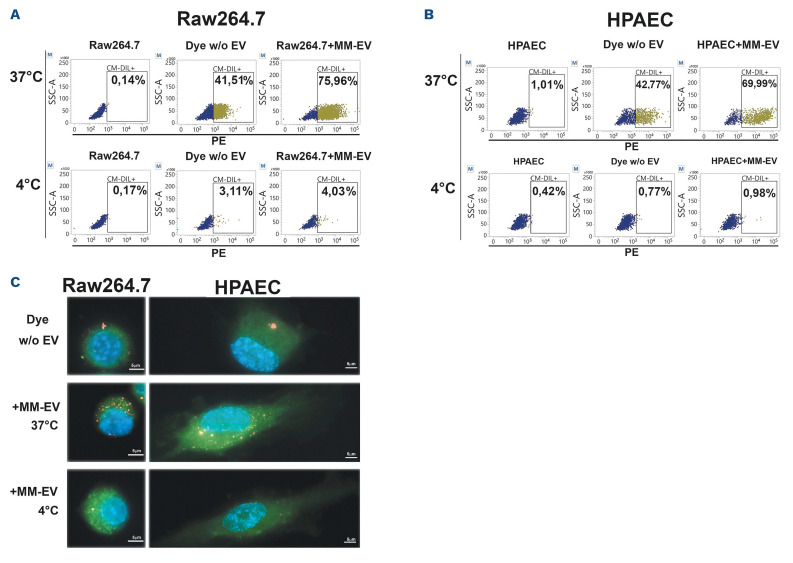
EV produced by two different human MM cell lines (HMCL), RPMI8226 and OPM2, were isolated by ultracentrifugation and fully characterized. Particle size distribution assessed by nanoparticle tracking analysis (NTA) revealed that the EV populations produced by the two HMCL were characterized by the presence of both small (30-200 nm) and large (200-1,000 nm) vesicles (Figure 1A). The shape and integrity of MM-EV was assessed by transmission electronic microscopy (TEM), showing the presence of whole, undamaged small and large EV (Figure 1B). We assessed the ability of MM-EV to transfer their content to two key BM cell populations crucial in supporting MM progression, osteoclasts (OCL) and endothelial cells (EC). The uptake was assessed in a quantitative (Figure 2A and 2) and qualitative way (Figure 2C). EV isolated by a 48 hours (h) culture of RPMI8226 cells were stained with the fluorescent lipophilic dye CM-DIL and put in contact with a monolayer of OCL progenitors (Raw264.7 cells) and EC (primary human pulmonary arterial cells [HPAEC]) for 4 h at 37°C. The negative control was maintained at 4°C to inhibit the uptake. In Figure 2A and B, MM-EV uptake was quantified through flow cytometry by measuring the CM-DIL fluorescent signal in receiving cells. The dot plot analysis clearly shows that the two different cell types take up MM-EV with a similar efficiency. The graph in the Online Supplementary Figure S1 summarizes the mean values and the statistical analysis of flow cytometry detection. These results were confirmed by fluorescence microscopy analysis. Stack projection images (Figure 2C) and z-stack videos (Online Supplementary Videos) show the presence of high levels of fluorescent signal in Raw264.7 cells (Online Supplementary Videos S1) and HPAEC (Online Supplementary Videos S2) treated with MM-EV at 37°C, indicating that MM-EV may be internalized by these cells. As expected, internalization is blocked when cells are kept at 4°C, suggesting that an active process is involved.
Multiple myeloma-derived extracellular vesicles carry NOTCH2
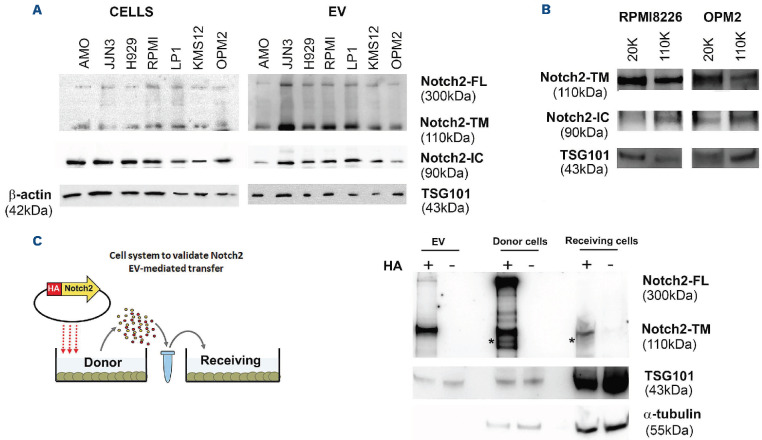
Due to the important role of NOTCH in the interplay between MM and the BM microenvironment, we wondered if MM-EV contribute to NOTCH activation in BM cells by carrying NOTCH receptors and, in particular the over-expressed receptor NOTCH2,26 as part of their cargo. By western blot analysis, we compared NOTCH2 expression in protein extracts from seven HMCL, namely AMO1, JJN3, H929, RPMI8226, LP1, KMS12, OPM2, and EV isolated from HMCL conditioned media (CM). Figure 3A shows that MM-EV are able to carry NOTCH2, whose relative amount reflects that expressed in the different protein extracts of the HMCL. The analysis of the different forms of NOTCH2 indicated that MM-EV could carry not only the transmembrane NOTCH2 (NOTCH2-TM) but also the full-length uncleaved NOTCH2 (NOTCH2-FL). Since the cleavage operated by γ-secretase on the intracellular portion of NOTCH takes place inside the endocytic bodies27 and exosomes arise from late endosomes,28 we investigated whether the active cleaved intracellular NOTCH2-IC may be included in MM-EV cargo by using a specific antibody. Results in Figure 3A indicate that MM-EV cargo also carries NOTCH2-IC. Online Supplementary Figure S2 shows that also NOTCH1 is widely represented in MM-EV, while the presence of other two isoforms in MM-EV is less noticeable.
Figure 1.
Characterization of multiple myeloma cell-released extracellular vesicles. Extracellular vesicles (EV) from multiple myeloma cell lines (HMCL) RPMI8226 and OPM2 cells (MM-EV), were isolated by ultracentrifugation and analyzed by (A) nanot-racking particle analysis (NTA) and (B) electron transmission microscopy (TEM). (A) NTA analysis reveals the presence of small (30-200 nm) and large (200-1,000 nm) vesicles. Size and concentration of EV were determined by NanoSight NS300 system (Malvern Panalytical Ltd, Malvern, UK). A camera level of 12 and 5 30-second recordings were used for the acquisition of each sample of 3 independent EV isolations and one representative image is shown. (B) TEM analysis confirms the isolation of intact small and large vesicles.
Figure 2.
Multiple myeloma cell-released extracellular vesicles can be taken up by osteoclasts and endothelial cells. Osteoclast (OCL) progenitor and endothelial cell (EC) uptake of multiple myeloma cell-released extracellular vesicles (MM-EV) from RPMI8226 cells. Raw264.7 cells and primary human pulmonary arterial cells (HPAEC) were treated with CM-DIL stained MM-EV or the negative control for 4 hours (h) at 37°C or 4°C. (A and B) Representative flow cytometry dot plots show the CM-DIL-labeled RPMI8226-EV uptake by Raw264.7 cells (A) and HPAEC (B) by measuring CM-DIL-positive cells in the PE channel. OPM2-EV provided similar results (not shown). Data are presented as the mean values of 3 independent experiments. (C) Maximum intensity projection of CM-DIL-positive RPMI8226-EV internalization in Raw264.7 cells and HPAEC after 4 h of incubation at 37°C and 4°C. Red fluorescence: RPMI8226-EV labeled with CM-DIL dye; green fluorescence: CFSE+ labeled cells; blue fluorescence: nuclei with DAPI (63x magnification).
In order to assess which EV fraction expresses NOTCH2, we performed a western blot analysis on large and small vesicles collected from the HMCL CM by sequential ultra-centrifugation at 20,000 g (20 K) and 110,000 g (110 K). We found that NOTCH2-TM and NOTCH2-IC were present both in large and small vesicles (Figure 3B). Interestingly, NOTCH2-IC level was increased in 110K MM-EV fraction.
EV allow distant cells to communicate between each other, thus modifying their behavior. In order to demonstrate that NOTCH2 may be involved in these processes and can be transferred to distant cells via EV, we developed a model system of HEK293 donor and receiving cells (Figure 3C). The first were forced to constitutively express NOTCH2 tagged with HA at the C-terminus (NOTCH2-HA)29 to distinguish it from the endogenous NOTCH2. In addition, the position of the HA-tag at the C-terminus of NOTCH2 enabled us to detect NOTCH2-FL, the NOTCH2-TM portion of the heterodimeric NOTCH2 form, matured in the trans-Golgi network upon the cleavage by a furin-like convertase,30 and the mature NOTCH2-IC, due to homotypic activation mediated by ADAM10 and the γ-secretase.29 EV-donor cells were added to the culture medium of receiving HEK293 cells. Figure 3C shows a western blot analysis performed with an anti-HA antibody, confirming the presence of the HA-signal in donor cells carrying NOTCH2-HA, isolated EV, and receiving cells. This demonstrates that EV can transfer NOTCH2 between distant cells. In this model system, the EV cargo include NOTCH2-TM and NOTCH2-FL, while both donor and receiving cells show also the presence of NOTCH2-IC. The absence of NOTCH2-IC in HEK293-derived EV might be due to a lower level of NOTCH activation in HEK293 cells in comparison to HMCL.
Figure 3.
NOTCH receptors and ligands in extracellular vesicles. (A) Western blot analysis for NOTCH2-FL (full length), NOTCH2-TM (transmembrane form), and NOTCH2-IC (active intracellular NOTCH2) expressed in 7 different human multiple myeloma cell lines (HMCL) and the respective produced extracellular vesicles (EV). β-actin and TSG101 were used as loading controls for cells and vesicle protein extracts, respectively. In order to perform all the hybridizations, two western blots were performed with cell and EV extracts loaded with an identical amount of protein. (B) Western blot analysis shows the expression of NOTCH2-TM and NOTCH2-IC in EV populations of different sizes. Large and small EV were isolated from RPMI8226 and OPM2 cells by sequential ultracentrifugation at 20,000 g (20K) and 110,000 g (110K), and the expression of the two NOTCH2 forms was separately assessed by western blot analyses using specific antibodies for NOTCH2 and NOTCH2-IC; TSG101 was used as control for vesicle protein extracts. (C) EV-mediated cell-to-cell transfer of NOTCH2: the donor HEK293 cell line was forced to express HA-tagged NOTCH2 carried by pCDNA3.1 or the corresponding empty vector (negative control); EV secreted by donor cells were collected by ultra-centrifugation and used to treat receiving HEK293 cells for 24 hours. Cell and EV protein extracts were analyzed by western blot using a specific primary antibody anti-HA. α-tubulin and TSG101 were used to normalize cellular and vesicular protein extract loading, respectively. NOTCH2-IC identified by rehybridization of the same membrane with anti-NOTCH-IC (see the Online Supplementary Figure S3) is indicated by an asterisk.
Multiple myeloma-derived extracellular vesicles activate NOTCH signaling in receiving cells
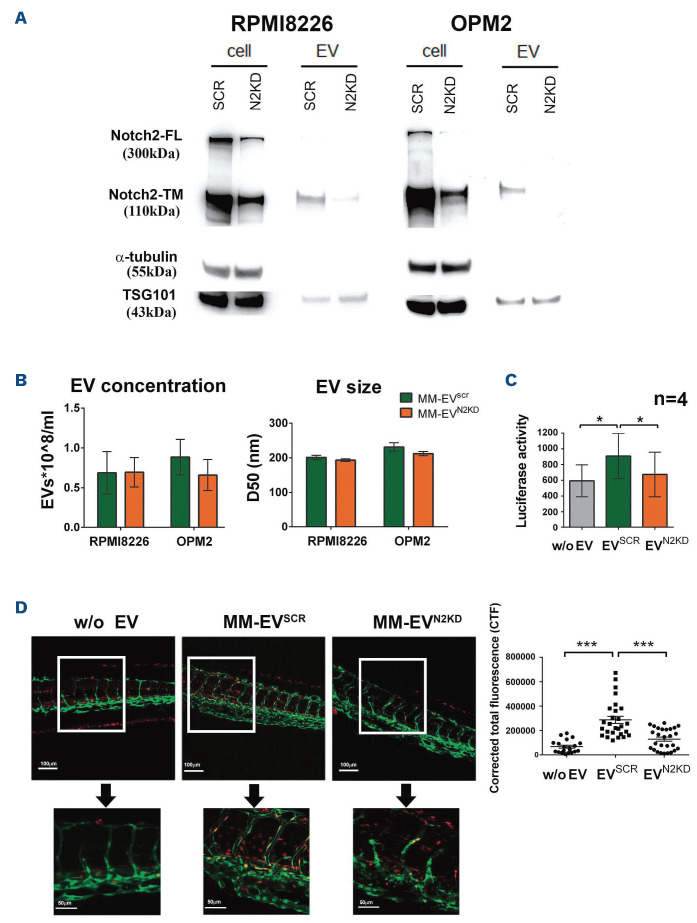
In order to address if the variation of NOTCH2 levels in MM cells may affect MM-EV-mediated communication, we studied the effect of NOTCH2 silencing in RPMI8226 and OPM2 cells. These cells were transduced with the pTRIPZ lentiviral vector conditionally expressing shRNA for NOTCH2 (HMCLN2KD) or the scrambled sequence (HMCLSCR) and single cell clones were isolated. Figure 4A confirms that RPMI8226 and OPM2 cells are knocked down (KD) for NOTCH2 and clearly shows that also the produced MM-EV displayed a reduced level of NOTCH2. Also NOTCH2-IC decreased in OPM2 cells with a corresponding decrease in the shed EV, while NOTCH2-IC decrease in EV release from RPMI8226 was less evident (Online Supplementary Figure S4). Through alignment search tool BlastN (USA National Center for Biotechnology Information) we excluded regions of local similarities between the used shRNA and the sequences of other NOTCH receptors and ligands (E values range between 1 and 15). However, the high sequence homology between the four NOTCH receptors prompted us to analyze by western blot the expression of the other NOTCH receptor isoforms. The Online Supplementary Figure S5 shows that NOTCH2 KD did not affect the expression of NOTCH1, 3 and 4 in protein extracts from cells and MM-EV. The outcome of NOTCH2 KD on EV size and concentration evaluated by NTA showed no significant effect on MM-EV size and concentration (Figure 4B).
In order to assess if the NOTCH2 protein carried by MM-EV is functionally active and is able to trigger NOTCH signaling in receiving cells, we tested the effect of EV isolated from HMCLN2KD (MM-EVN2KD) or HMCLSCR (MM-EVSCR) through a NOTCH responsive luciferase reporter assay. This assay was carried out in HeLa cells, which is a highly-transfectable cell line characterized by a low level of NOTCH signaling activation (not shown). Figure 4C shows that MM-EVSCR can activate the NOTCH signaling pathway in the receiving HeLa cells, while this ability is significantly reduced for MM-EVN2KD.
The ability of MM-EV to activate NOTCH signaling was also validated in an in vivo NOTCH reporter zebrafish embryo obtained by crossing Tg(T2KTp1bglob:hmgb1-mCherry) with Tg (fli1a:EGFP) that carry EGFP+ endothelial cells (green) and express the mCherry protein (red) under the control of a NOTCH-responsive element. EV isolated from RPMI8226 cells were injected in the duct of Cuvier of 48 hpf transgenic zebrafish embryos. Images were acquired 4 h postinjection. Figure 4D shows MM-EVSCR mediated NOTCH activation in the intersegmental vessels (Se), caudal artery (CA), and in the area of the caudal hematopoietic tissue (CHT), while MM-EVN2KD induces a barely visible stimulation.
Figure 4.
Molecular effects of NOTCH2 modulation on multiple myeloma cell-released extracellular vesicles. (A) Western blot analysis confirms the knockdown (KD) efficacy on NOTCH2-TM levels in human multiple myleoma cell lines (HMCL) and the produced multiple myeloma extracellular vesicles (MM-EV). α-tubulin and TSG101 were used as loading controls for cell and vesicle protein extracts, respectively. (B) Nanoparticle tracking analysis (NTA) on MM-EVSCR and MM-EVN2KD from HMCL does not show significant changes in concentration and size; D50=size point below which 50% of the extracellular vesicles (EV) are contained. Data are expressed as mean value ± standard error of the mean of at least 4 experiments (RPMI8226 n=4; OPM2 n=6). Statistics by two-tailed t-test did not show any significant difference. (C) MM-derived EV activate NOTCH signaling in receiving cells: a NOTCH reporter assay was carried out on HeLa cells stimulated with MM-EVSCR and MM-EVN2KD from HMCL (EV derived from OPM2 cells), or control fresh medium (w/o EV). Luciferase activity is expressed as the ratio between Nano/Firefly luciferase luminescence units. Data are expressed as mean value ± standard error of 4 experiments. Statistics by ANOVA and Tukey post-test: *P<0.05. (D) Activation of NOTCH signaling in the trunk of Tg(T2KTp1bglob:hmgb1-mCherry) zebrafish embryos (zf) 4 hours after the injection of MM-EVSCR and MM-EVN2KD (EV derived from RPMI8226 cells), or control fresh medium (w/o EV). Representative pictures of each condition are reported on the left (20x and 60x magnification, the upper and the lower respectively); a graph on the right represents the mean value +/- standard error of the mean of the corrected total fluorescence (CTF) measured in caudal hematopoietic tissue (CHT). In particular four in vivo experiments involved zf embryos injected with negative control (n=20) or MM-EVSCR (n=27) and MM-EVN2KD (n=27). Statistics by ANOVA and Tukey post-test excluding outliers identified through the ROUT method (Q=1%): *** P<0.001.
NOTCH2 carried by multiple myeloma-derived extracellular vesicles contributes to the education of bone marrow cell populations
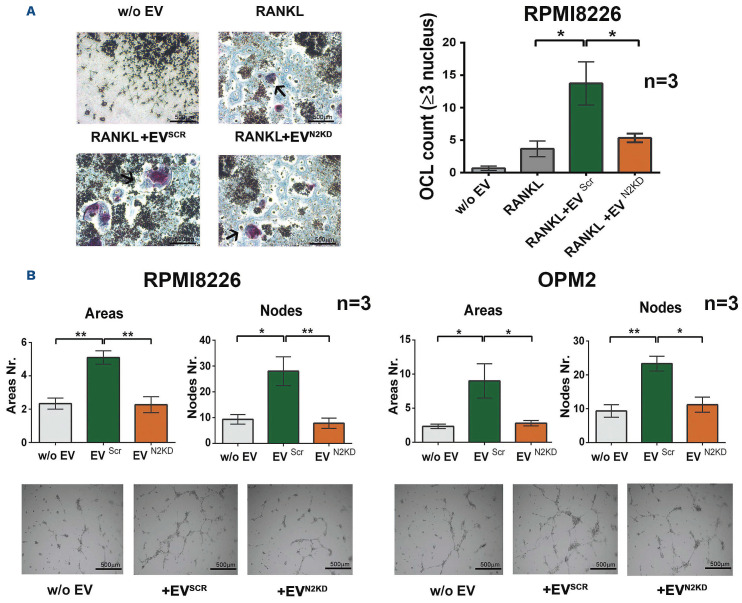
We and other groups previously reported that MM cells affect the surrounding BM microenvironment inducing osteo-clastogenesis22-24 and tumor angiogenesis16,21 in a NOTCH-dependent way. Moreover, recent evidence indicates that MM-EV stimulate osteoclastogenesis,13,31-33 angiogenesis and carry pro-angiogenic proteins.11,34 Therefore, we verified the osteoclastogenic potential of MM-EVSCR and MM-EVN2KD by treating the monocyte cell line Raw264.7 in the presence of the osteoclastogenic chemokine RANKL (30 ng/mL). We used MM-EV released by the RPMI8226 cell line due to the ability of these cells to induce osteoclastogenesis, differently from OPM2 cells.22 After 7 days of treatment with MM-EV, OCL count showed that MM-EVSCR induced Raw264.7 cell differentiation, while MM-EVN2KD lost this ability (Figure 5A).
We assessed MM-EVN2KD angiogenic potential using a tube formation assay with HPAEC seeded on a Matrigel layer. Results in Figure 5B show that MM-EVSCR promotes tube organization of HPAEC, while treatment with MM-EVN2KD reduces this effect. In conclusion, this specific RNA interference approach unequivocally demonstrated that NOTCH2 KD affects the MM-EV mediated osteoclastogenesis and angiogenesis.
Targeting NOTCH signaling blocks the pathological communication mediated by multiple myeloma-derived extracellular vesicles
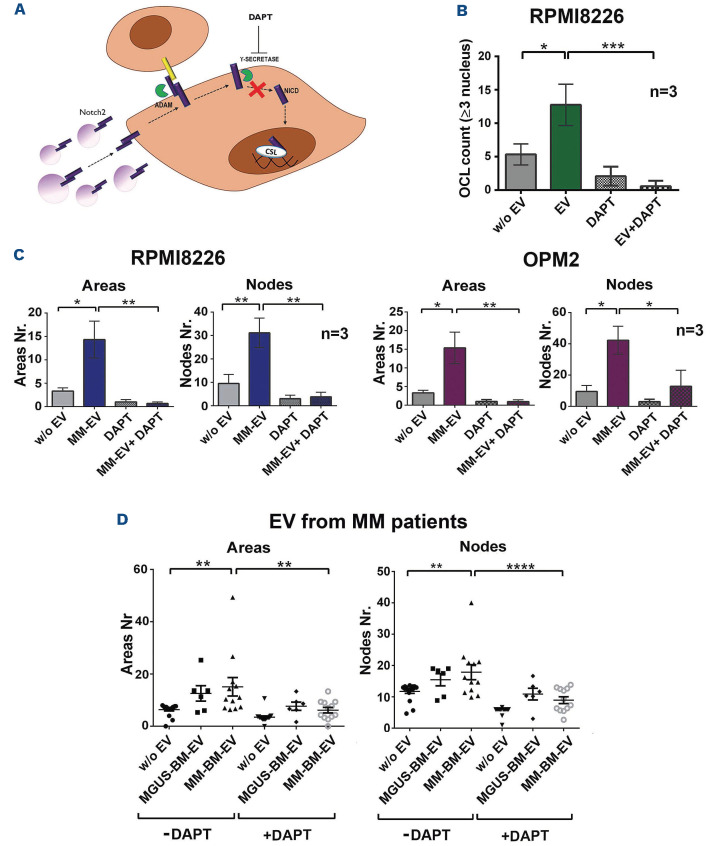
In order to provide a higher translational potential to our findings, we exploited the strategy illustrated in Figure 6A. We used γ-secretase inhibitors (50 µM DAPT), used in research works and clinics to inhibit pan-Notch signaling15 to block NOTCH activation in EC and OCL induced by MM-EV. Figure 6B and C clearly show that MM-EV induce angiogenesis and osteoclastogenesis in a NOTCH-dependent way. In consideration of the well known osteoclastogenic and angiogenic roles of NOTCH signaling,16,21-24 we planned our experiments to distinguish the effect of the endogenous and vesicular NOTCH. Indeed, if we compare the effect of DAPT on osteoclastogenesis (Figure 6B) in the absence of EV and suboptimal concentration of RANKL, we can see that it abrogates OCL differentiation with a non-statistically significant decrease, while DAPT abrogates much higher levels of osteoclastogenesis induced by MM-EV (+250%) in a statistically significant way, indicating that the greater effect of MM-EV is NOTCH dependent.
The effect of DAPT on MM-EV-induced angiogenesis was analogous. The high increase of HPAEC tube organization upon the administration of MM-EV from RPMI8226 and OPM2 cells was completely abrogated by DAPT, showing a statistically significant reduction in areas and nodes (ranging from 29,5% to 51,3%). This effect was clearly higher than the slight inhibitory trend observed on basal angiogenesis upon DAPT administration (Figure 6C). We also ruled out that the obtained results could be due to the toxic effect of DAPT on OCL and EC (Online Supplementary Figure S6).
Overall, these results confirm that the studied biological effects of EV are NOTCH mediated and can be blocked by treatment with γ-secretase inhibitors.
Finally, in order to strengthen the translational potential of our in vitro findings, we reasoned that the BM of MM patients does not contain only MM-derived EV, but EV derived from the whole MM-educated BM cell populations. Therefore, in order to confirm the NOTCH-dependent role of EV in the pathological communication occurring in the BM of MM patients, we got advantage of EV from BM aspirates of patients with the benign MGUS (MGUS-BM-EV) or MM (MM-BM-EV) (Online Supplementary Table S1), which may recapitulate the complexity of the BM microenvironment. We compared the angiogenic potential of HPAEC untreated or treated with MGUS-BM-EV or MM-BM-EV. Figure 6D shows that MM-BM-EV boost the angiogenic potential of HPAEC while MGUS-BM-EV showed a non-statistically significant increasing trend. Importantly, the inhibitory effect of DAPT is statistically significant when added to HPAEC treated with MM-BM-EV. These results confirm the increasing angiogenic potential of EV released in the BM during MM progression, the role played by NOTCH delivered via MM-BM-EV and strengthen the potential of a NOTCH-directed therapeutic approach to block the support of MM microenvironment to the disease progression.
Figure 5.
NOTCH2 contributes to the protumorigenic communication of multiple myeloma cell-released extracellular vesicles toward osteoclasts and endothelial cells. (A) The effect of MM-EVSCR and MM-EVN2KD collected from the osteoclastogenic cell line RPMI8226. The Raw264.7 cell line was treated with or without the osteoclastogenic RANKL (30 ng/mL), multiple myeloma cell-released extracellular vesicles (MM-EV) or control fresh medium (w/o EV). After 7 days TRAP-positive multinucleated cells (≥3 nuclei) were enumerated (TRAP-positive multinucleated cells are indicated by an arrow). Representative images are shown for each condition on the left (4x magnification); a graph on the right represents the mean value of the absolute number of TRAP+ multinucleated cells +/- standard error of the mean. Statistical analysis by a one-way ANOVA with Tukey post-test; *P<0.05. (B) Tumor angiogenesis induced by MM-EVSCR and MM-EVN2KD. Tube formation assay performed for 13 hours with primary human pulmonary arterial cells (HPAEC) laid on a matrigel-coated support stimulated with MM-EVSCR and MM-EVN2KD collected from RPMI8226 and OPM2 cells or control fresh medium (w/o EV). The graphs show the mean values of areas and nodes (branch points) enumerated in four quadrant of the well +/- standard error of the mean. Statistical analysis was performed by ANOVA and Tukey post-test; *P<0.05, **P<0.01. Representative images are shown below for each condition (4x magnification).
Discussion
The pathological interplay between malignant cells and the surrounding non-tumoral BM cells promotes neoplastic cell growth and survival as well as key events of tumor progression including bone disease and angiogenesis. These lines of evidence suggest that an effective therapeutic approach should not be focused merely on the MM cells, but it should target their interaction with the surrounding BM niche.
Figure 6.
γ-secretase blockade of NOTCH2 activation inhibits the effect of multiple myeloma cell-released extracellular vesicles on osteoclastogenesis and angiogenesis. (A) Experimental rationale. Multiple myeloma cell-released extracellular vesicles (MM-EV) derived NOTCH2 may increase NOTCH signaling activation on target cells (osteoclast [OCL] progenitors and endothelial cells [EC]) that may be blocked by DAPT administration. (B) Raw267.4 cells induced to differentiate into OCL in the presence of 30 ng/mL RANKL were treated with MM-EV collected from the osteoclastogenic cell line RPMI8226 or the control fresh medium (w/o EV) and in the presence or absence of DAPT to inhibit NOTCH signaling activation. For each condition a negative control cultured in the absence of RANKL was carried out. After 7 days TRAP+ multinucleated cells (≥3 nuclei) were enumerated. The graph shows the mean values of TRAP+ multinucleated cells obtained in the different conditions for RANKL treated cells. Given the large number of conditions, to make the graph simpler and easier to understand, each value was subtracted of the respective control without RANKL (+/- standard error of the mean [SEM]). Statistical analysis was performed by a one-way ANOVA with Tukey post-test; *P<0.05;***P<0.001. (C) Tube formation assay on primary human pulmonary arterial cells (HPAEC) was performed for 13 hours (h) with MM-EV collected from RPMI8226 and OPM2 cells or control fresh medium (w/o EV) in the presence or the absence of DAPT. The graphs show the mean values of areas and nodes enumerated in four quadrant of the well +/- SEM. Statistical analysis was performed by ANOVA and Tukey post-test; *P<0.05, **P<0.01. (D) Tube formation assay on HPAEC treated for 13 h with EV collected from the bone marrow of MGUS patients or MM patients in the presence or the absence of DAPT. The graphs show the mean values of areas and nodes enumerated in 4 quadrant of the well (+/- SEM). Statistical analysis was performed by ANOVA and Tukey post-test; **P<0.01, ****P<0.0001. The characteristics and number of MM patients are reported in the Online Supplementary Table S1.
Recently, EV have been reported as critical players in the communication between MM cells and the nearby BM cells and leading to MM progression. Indeed, MM-EV promote different events associated with MM progression, including angiogenesis11,13,34,35 and osteoclastogenesis.13,31-33
Here, we contribute to elucidate the molecular mechanisms involved in MM-EV pathological communication with the BM microenvironment, strengthening the role of the EV pathological communication as a promising therapeutic target in MM.
The evidence that NOTCH signaling activation, mediated by MM cell heterotypic interaction with the surrounding BM cells, plays a key role in tumor angiogenesis16,21 and osteoclastogenesis22-24 prompted us to investigate whether NOTCH signaling contributes to determine the impact of MM-EV on these processes.
Our analysis on a panel of HMCL and the respective shed EV indicate that NOTCH receptors were present in MM-EV cargo with high levels of NOTCH2 and slightly lower levels of NOTCH1, consistently with evidence from EV released by other cell types.36 We focused on NOTCH2 receptor, widely expressed in MM cell lines and in primary MM cells, particularly from high-risk patients.18,26 In details, we found that MM-EV carried the mature heterodimeric form of NOTCH2 (since we detected the NOTCH2-TM portion), the immature NOTCH2-FL and the activated NOTCH2-IC, that upon delivering to target cells might directly activate the transcription of the NOTCH target genes without requiring the interaction with ligands and the activation by ADAM protease and γ-secretase.
The expression of NOTCH receptors has been reported in exosomes37 but also in microvesicles.38 Thereby, we wondered which of the two subpopulations of EV hosted NOTCH2. We separated large and small vesicles and found the presence of NOTCH2-TM both in large and small particles, while NOTCH2-IC level was higher in 110K small EV fraction. Although it is impossible to distinguish exosome and microvesicles only on the basis of their dimension, we presume that small vesicles are enriched with exosomes respect to large vesicles. Therefore our results suggest the presence of NOTCH2-IC within exosomes consistently with its presence in the endosomal compartment from which exosomes take origin.
Before validating the hypothesis that vesicular NOTCH2 contributes to molecular and biological effects on relevant BM cell populations as OCL and EC, we monitored if these cells uptake MM-EV, using respectively Raw264.7 cells and HPAEC. Flow cytometry detection showed a quick (4 h) uptake of MM-EV by the majority of cells in both models, confirmed by a Z-stack analysis in confocal microscopy.
Additionally, we unequivocally assessed that NOTCH signaling members could be transferred via EV from one cell to another using an experimental system model based on HEK293 cells forced to express NOTCH2 tagged with HA. This model system allowed us to assess that NOTCH2-HA could be released within EV and be transferred to distant cells. In this system, we have confirmed that EV carried high levels of NOTCH2-TM form and NOTCH2-FL, even if at much lower level. On the contrary, although the NOTCH2-IC form was present in MM-EV shed by HMCL, we could not detect it in HEK293-derived EV, possibly due to a lower level of NOTCH2 activation in HEK293 cells in comparison to HMCL. Receiving cells clearly took up the transmembrane form of NOTCH2-HA, but also showed a very faint band corresponding to NOTCH2-IC, consistently with a slight NOTCH2 activation after EV uptake. This result provided a first indication that EV carrying NOTCH2 may activate NOTCH signaling in receiving cells.
Using a selective RNA interference of NOTCH2 in RPMI8226 and OPM2 cells, we confirmed that NOTCH2 KD in HMCL impacted the levels of vescicular NOTCH2-TM, NOTCH2-FL and NOTCH2-IC, although the decrease of the activated form was evident only in OPM2 cells. On the contrary, NOTCH2 KD did not significantly affect MM-EV size and concentration.
In order to confirm that MM-EV might activate the oncogenic NOTCH signaling in receiving cells, we tested MM-EVSCR or MM-EVN2KD in in vitro and in vivo NOTCH reporter systems. The first in vitro cellular model transfected with a Nanoluc-based NOTCH reporter vector, indicated that MM-EVSCR might activate a NOTCH-dependent gene reporter, while MM-EVN2KD induced a significantly lower activation. This result was confirmed by a second reporter in vivo system recapitulating the complexity of a whole organism. The injection of MM-EVSCR or MM-EVN2KD in transgenic zebrafish embryo reporter for NOTCH not only confirmed the observed MM-EV-mediated NOTCH signaling activation but also provided evidence of MM-EV ability to induce NOTCH signaling activation at distant sites through the circulation. Indeed, MM-EV injected in the duct of Cuvier may be transported through the circulation and activate NOTCH signaling in the caudal hematopoietic tissue which represents the main hematopoietic organ in zebrafish embryo, analogous to the human BM.39 In contrast, NOTCH signaling activation mediated by MM-EVN2KD was significantly lower. MM-EV effectiveness in inducing NOTCH signaling activation at distant sites in zebrafish embryos carried by the blood stream suggests that MM-EV could also play an important role in the metastatic process, as reported for pancreatic cancer.40
For instance they may help preparing the premetastatic niche through the formation of new permeable vessels for the extravasation of tumor cells, and the destruction of the bone matrix to make space for metastatic cells.
Taken as a whole, the in vitro and in vivo NOTCH reporter assays confirmed that NOTCH activity in target cells was due to NOTCH2 delivered by the injected MM-EV.
This evidence and the acknowledged effect of NOTCH signaling on OCL and EC, prompted us to verify if NOTCH2 delivery by MM-EV could affect the biology of these cells. Through the same specific RNA interference approach on two different HMCL, we provided an unequivocal demonstration that vesicular NOTCH2 participates in MM-in-duced OCL differentiation and angiogenesis, assessed by a tube formation assay. The dependency of these processes on NOTCH signaling was clearly demonstrated by the fact that MM-EVN2KD impact was significantly lower. The presence of other NOTCH receptors in MM-EV cargo, even if at a lower level, (i.e. NOTCH1) suggests that they may also provide a contribution.
In order to strengthen the translational potential of our results we used a dual approach: i) the outcome of an anti-NOTCH therapeutic approach already tested in clinics was assessed in vitro and ex vivo; ii) ex vivo experiments were carried out with EV released in the BM of MM patients, taking into account that a systemic treatment is expected to affect the communication of MM-EV, as well as that of EV from all the BM cell populations. In the first case, we showed that γ-secretase inhibitors (GSI), already used in clinics,15 greatly affected MM-EV ability to inhibit angiogenesis and osteoclastogenesis in vitro. Concerning the second point, EV collected from the BM of MM patients, but not MGUS patients, displayed a clear pro-angiogenic effect that could be hampered by GSI.
In conclusion, the RNA interfering approach specific for NOTCH2 on HMCL, complemented by a pan-NOTCH chemical inhibition on HMCL- and MM patients’ BM-derived EV, provides a new important evidence of the effect of NOTCH signaling pathway on EV-mediated pathological communication in myelomatous BM. The important inhibitory effect of GSI suggests that the form of the NOTCH2 oncogene which mostly contributes to MM-EV mediated education is NOTCH2-TM and not NOTCH2-IC whose activity is GSI resistant. Although, the presence of NOTCH2-IC in MM-EV cargo is much intriguing since it may deliver an active oncogenic signal, we believe that vesicular NOTCH-IC might be more relevant in tumor that expresses the constitutively active form of NOTCH, such as T-cell acute lymphoblastic leukemia.
In conclusion, our results strengthen the rationale for therapeutic approaches directed to inhibit NOTCH activation mediated by MM-EV, suggesting that they have the potential of interfering with the pathological communication of the MM cells mediated by EV in the short and potentially in the long range and, thereby, they may influence the cross-talk with the surrounding microenvironment and the dissemination of the disease at distant skeletal sites.
Supplementary Material
Funding Statement
Funding: This study was supported by grants from Associazione Italiana Ricerca sul Cancro, Investigator Grant to RC (20614), My First Grant to AP (18714); Fondazione Italiana per la Ricerca sul Cancro to MC (post-doctoral fellowship 18013); Università degli Studi di Milano to RC (Linea 2B-2017 -Dept. Health Sciences), to NP (postdoctoral fellowship type A) and DG (PhD fellowship in experimental medicine).
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4(9):1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J iomed Biotechnol. 2012;2012:157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webber J, Yeung V, Clayton A. Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell Dev Biol. 2015;40:27-34. [DOI] [PubMed] [Google Scholar]
- 6.Caivano A, Laurenzana I, De Luca L, et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015;36(12):9739-9752. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan SR, Luk F, Brown RD, et al. Isolation of human CD138(+) microparticles from the plasma of patients with multiple myeloma. Neoplasia. 2016;18(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manier S, Liu CJ, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129(17):2429-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caivano A, La Rocca F, Simeon V, et al. MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies - a short report. Cell Oncol (Dordr). 2017;40(1):97-103. [DOI] [PubMed] [Google Scholar]
- 10.Rajeev Krishnan S, De Rubis G, Suen H, et al. A liquid biopsy to detect multidrug resistance and disease burden in multiple myeloma. Blood Cancer J. 2020;10(3):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, De Veirman K, Faict S, et al. Multiple myeloma exo-somes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239(2):162-173. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Lei Q, Wang H, et al. Tumor-derived extracellular vesicles inhibit osteogenesis and exacerbate myeloma bone disease. Theranostics. 2019;9(1):196-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M, Giannandrea D, Lesma E, Basile A, Chiaramonte R. Extracellular vesicles enhance multiple myeloma metastatic dissemination. Int J Mol Sci. 2019;20(13):3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo M, Mirandola L, Platonova N, et al. Notch-directed microenvironment reprogramming in myeloma: a single path to multiple outcomes. Leukemia. 2013;27(5):1009-1018. [DOI] [PubMed] [Google Scholar]
- 15.Colombo M, Galletti S, Garavelli S, et al. Notch signaling deregulation in multiple myeloma: a rational molecular target. On-cotarget. 2015;6(29):26826-26840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palano MT, Giannandrea D, Platonova N, et al. Jagged ligands enhance the pro-angiogenic activity of multiple myeloma cells. Cancers (Basel). 2020;12(9):2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirandola L, Apicella L, Colombo M, et al. Anti-Notch treatment prevents multiple myeloma cells localization to the bone marrow via the chemokine system CXCR4/SDF-1. Leukemia. 2013;27(7):1558-1566. [DOI] [PubMed] [Google Scholar]
- 18.Colombo M, Galletti S, Bulfamante G, et al. Multiple myeloma-derived Jagged ligands increases autocrine and paracrine inter-leukin-6 expression in bone marrow niche. Oncotarget. 2016;7(35):56013-56029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo M, Garavelli S, Mazzola M, et al. Multiple myeloma exploits Jagged1 and Jagged2 to promote intrinsic and bone marrow-dependent drug resistance. Haematologica. 2020;105(7):1925-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiron D, Maiga S, Descamps G, et al. Critical role of the NOTCH ligand JAG2 in self-renewal of myeloma cells. Blood Cells Mol Dis. 2012;48(4):247-253. [DOI] [PubMed] [Google Scholar]
- 21.Saltarella I, Frassanito MA, Lamanuzzi A, et al. Homotypic and heterotypic activation of the Notch pathway in multiple myeloma-enhanced angiogenesis: a novel therapeutic target? Neo-plasia. 2019;21(1):93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo M, Thummler K, Mirandola L, et al. Notch signaling drives multiple myeloma induced osteoclastogenesis. Oncotarget. 2014;5(21):10393-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzer R, Kaiser M, Acikgoez O, et al. Notch inhibition blocks multiple myeloma cell-induced osteoclast activation. Leukemia. 2008;22(12):2273-2277. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzer R, Nickel N, Godau J, et al. Notch pathway inhibition controls myeloma bone disease in the murine MOPC315.BM model. Blood Cancer J. 2014;4:e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houde C, Li Y, Song L, et al. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood. 2004;104(12):3697-3704. [DOI] [PubMed] [Google Scholar]
- 26.van Stralen E, van de Wetering M, Agnelli L, et al. Identification of primary MAFB target genes in multiple myeloma. Exp Hema-tol. 2009;37(1):78-86. [DOI] [PubMed] [Google Scholar]
- 27.Chastagner P, Brou C. Tracking trafficking of Notch and its ligands in mammalian cells. Methods Mol Biol. 2014;1187:87-100. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopal C, Harikumar KB. The origin and functions of exo-somes in cancer. Front Oncol. 2018;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groot AJ, Habets R, Yahyanejad S, et al. Regulated proteolysis of NOTCH2 and NOTCH3 receptors by ADAM10 and presenilins. Mol Cell Biol. 2014;34(15):2822-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intra-cellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90(2):281-291. [DOI] [PubMed] [Google Scholar]
- 31.Raimondi L, De Luca A, Fontana S, et al. Multiple myeloma-derived extracellular vesicles induce osteoclastogenesis through the activation of the XBP1/IRE1α axis. Cancers (Basel). 2020;12(8):2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raimondo S, Saieva L, Vicario E, et al. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J Hematol Oncol. 2019;12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faict S, Muller J, De Veirman K, et al. Exosomes play a role in multiple myeloma bone disease and tumor development by targeting osteoclasts and osteoblasts. Blood Cancer J. 2018;8(11):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Zhu XJ, Zeng C, et al. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol Sin. 2014;35(2):230-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Lu Q. Plasma membrane-derived extracellular microve-sicles mediate non-canonical intercellular NOTCH signaling. Nat Commun. 2017;8(1):709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel B, Patel J, Cho JH, et al. Exosomes mediate the acquisition of the disease phenotypes by cells with normal genome in tuberous sclerosis complex. Oncogene. 2016;35(23):3027-3036. [DOI] [PubMed] [Google Scholar]
- 38.Suwakulsiri W, Rai A, Xu R, et al. Proteomic profiling reveals key cancer progression modulators in shed microvesicles released from isogenic human primary and metastatic colorectal cancer cell lines. Biochim Biophys Acta Proteins Proteom. 2019;1867(12):140171. [DOI] [PubMed] [Google Scholar]
- 39.Sacco A, Roccaro AM, Ma D, et al. Cancer cell dissemination and homing to the bone marrow in a Zebrafish model. Cancer Res. 2016;76(2):463-471. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa K, Lin Q, Li L, et al. Prometastatic secretome trafficking via exosomes initiates pancreatic cancer pulmonary metastasis. Cancer Lett. 2020;481:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.