Abstract
We used 2016–2018 outpatient claims data to calculate direct outpatient medical costs per case of trichomoniasis in 2019 US dollars. The outpatient, drug, and total costs per treated case of trichomoniasis were $174, $39, and $213, respectively. Total costs were higher for female patients ($220) than for male patients ($158).
Tichomoniasis is a sexually transmitted infection with estimated US national prevalence rates of 2.1% and 0.5% among female and male individuals aged 14 to 59 years, respectively.1 However, reliable data on the prevalence of trichomoniasis are limited, for 3 main reasons. First, not all facilities use nucleic amplification assay testing for trichomoniasis and instead use wet mount microscopy of vaginal swabs, which is not as sensitive.2–4 Second, screening for trichomoniasis, particularly in men, is not routinely recommended; routine screening is currently recommended only for sexually active women with HIV.3,5 Third, trichomoniasis is not a nationally notifiable infection.6 Many people with trichomoniasis are asymptomatic; therefore, infections may be missed if they are not tested, or are tested via a less sensitive method.2–5,7 Trichomoniasis can increase the risk for HIV acquisition and can lead to sequelae such as vaginitis, pelvic inflammatory disease, and adverse outcomes of pregnancy in women and urethritis and epididymitis in men.2–5
Diagnosed trichomonas infections impose a cost burden attributable to the direct medical cost of diagnosing and treating infections. Trichomoniasis has been estimated to cost the United States almost $24 million in direct medical costs annually.8 The direct medical cost per infection treated among women has previously been estimated to be $101 in 2005 dollars.9 However, this estimate is more than a decade old, limited to women, and primarily based on diagnosis via wet mount. Although wet mount is the lowest-cost testing option for trichomoniasis,10 current sexually transmitted disease (STD) treatment guidelines now recommend nucleic amplification assay testing, which is associated with greater sensitivity and higher cost than wet mount.4,5,8,10 The objective of this analysis was to develop updated estimates of the direct medical cost per treated case of trichomoniasis among men and women.
METHODS
We used IBM Watson Health MarketScan Outpatient and Outpatient Pharmaceutical Commercial Claims Databases for patients between 2016 and 2018.11 These databases captured person-specific enrollment and medical service information related to outpatient visits, dates of service, diagnosis codes, prescription drug use, and other billing information associated with each service. MarketScan databases consisted of deidentified patients with distinct enrollee IDs, making all databases linkable. Because these data do not contain personally identifying information, this study was determined to be exempt from the Centers for Disease Control and Prevention Institutional Review Board.
Patients with trichomoniasis were identified from the MarketScan outpatient services claims database using International Classification of Diseases, Tenth Revision (ICD-10) codes for trichomoniasis (“A59.0,” “A59.8,” and “A59.9”).12 Patients could receive up to 4 different diagnosis codes per medical visit (e.g., a trichomoniasis diagnosis code and a diagnosis code for another condition). To ensure that costs unrelated to trichomoniasis were not included, we included only costs of outpatient claims where a patient received a single diagnosis (i.e., trichomoniasis diagnosis with no other ICD-10 codes). We assumed that visits occurring within 30 days of the initial visit were part of the same case. This 30-day window was based on the previous study of the cost of trichomoniasis and was applied so that our analysis would include costs associated with persistent infection and follow-up.9 Costs occurring outside the 30-day window were assumed to be a subsequent case.
Because providers may assign a diagnosis code presumptively and MarketScan claims lacked laboratory data, we assumed that patients with a diagnosis code for trichomoniasis and with prescription drug treatment for trichomoniasis reflected actual trichomoniasis cases. Using the Centers for Disease Control and Prevention STD treatment guidelines, we identified recommended drugs for treatment of trichomoniasis, listed by generic name. The 2 generic drug names listed for trichomoniasis treatment in the guidelines were metronidazole and tinidazole.5 We linked claims from outpatient visits to prescription drug claims data using the patient’s enrollee ID and used National Drug Codes to identify metronidazole and tinidazole claims for patients diagnosed with trichomoniasis.13 We included prescription drugs received 7 days before to 30 days after the first case-related visit. Including pharmacy claims up to 7 days before the physician visit is a common practice in medical claims studies9,14 and allows for factors such as minor inconsistencies in the data regarding dates of service. Although our primary analysis focused on the costs associated with first trichomoniasis cases, we also calculated costs for subsequent cases (i.e., costs incurred more than 30 days after the first case-related visit).
Costs were calculated per case for outpatient visit costs (which included all services at each outpatient visit associated with the case, including laboratory costs) and drug costs. Total cost was the sum of these 2 components; we further stratified costs by sex. Our cost estimates included only those visits for which a claim for treatment was found. All costs were adjusted to 2019 dollars using the medical care component of the Consumer Price Index for All Urban Consumers.15 All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
In 2016 to 2018, there were 49,749 patients in the MarketScan Outpatient Commercial Database with a trichomoniasis diagnosis (Fig. 1). Of those, 13,744 patients (27.7%) had claims that included only trichomoniasis diagnoses; these patients had a total of 15,625 visits, yielding an average of 1.13 visits per case (1.14 for female patients and 1.06 for male patients). Most patients (92.1%) had prescription drug coverage; however, only 9871 (71.7%) of the 13,744 patients we identified with sole trichomoniasis diagnoses had prescription drug claims for treatment of trichomoniasis. Of these 9871 patients, 6124 (62.0%) were classified as first cases of trichomoniasis and with treatment for trichomoniasis within 7 days before and 30 days after an initial trichomoniasis diagnosis. Most first cases (88.1%) occurred in women.
Figure 1.
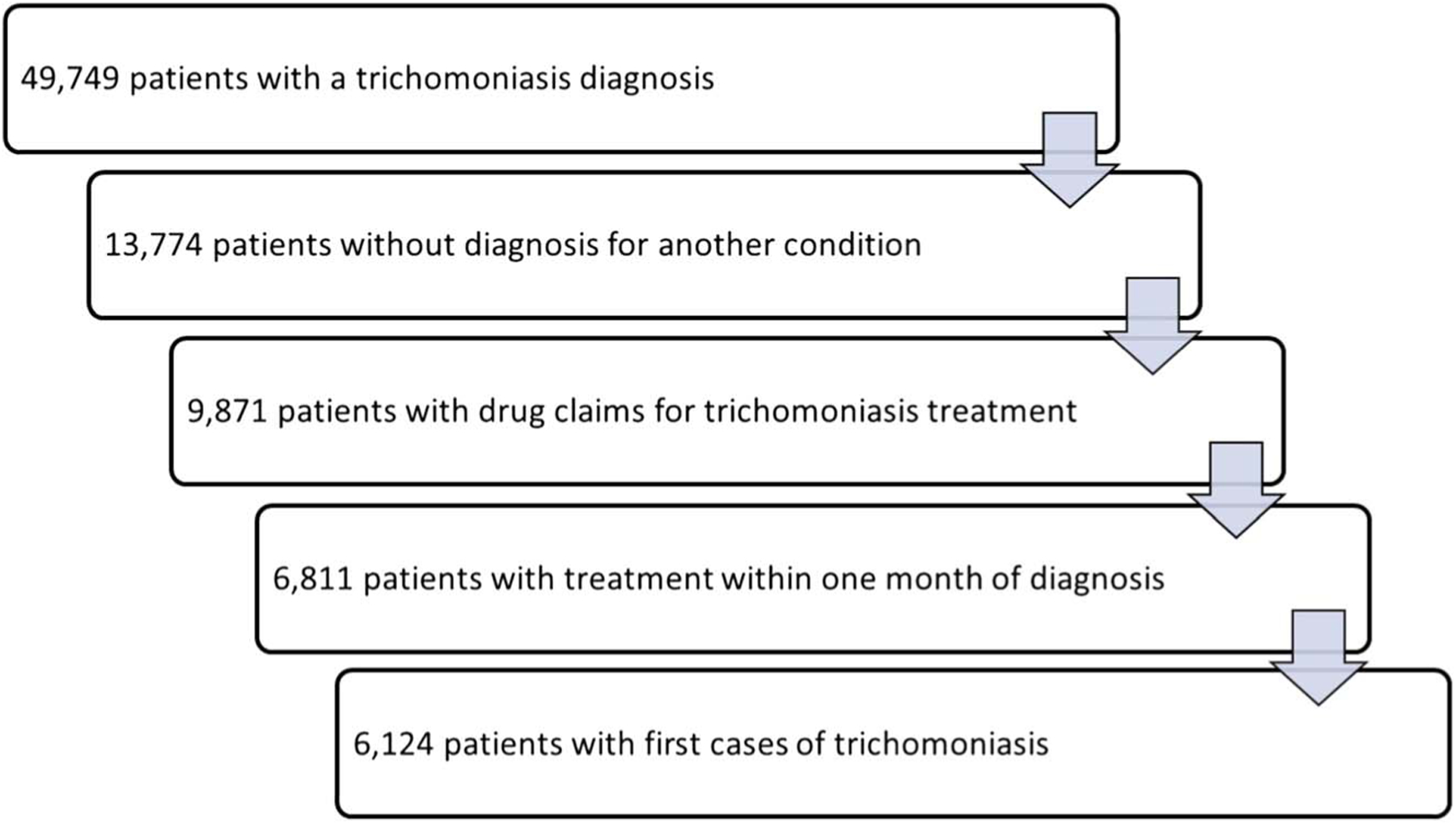
In the first step, patients with trichomoniasis were identified from the MarketScan outpatient services claims database using the ICD-10 codes for trichomoniasis (A59.00–.09, urogenital trichomoniasis; A59.8, trichomoniasis of other sites; and A59.9, trichomoniasis, unspecified). In the second step, we limited the analysis to outpatient claims where a patient received a single diagnosis (i.e., trichomoniasis diagnosis with no other ICD-10 codes), to ensure that costs unrelated to trichomoniasis were not included. In the third step, because of the lack of data on laboratory results, we limited the analysis to those with prescription drug treatment for trichomoniasis. In the fourth step, we limited the analysis to those patients with prescription drugs received within the same general time frame as the diagnosis. In the fifth step, we assumed that visits occurring within 30 days of the index visit were part of the same case. Costs occurring outside the 30-day window were assumed to be a subsequent case. In the final step, we limited the analysis to patients with no previous cases of trichomoniasis in the time frame we examined (2016–2018), although we performed a subanalysis on the costs associated with subsequent cases.
The outpatient, drug, and total costs per case of trichomoniasis were $174, $39, and $213, respectively (n = 6124; Table 1). When stratified by sex, the direct medical cost was higher for female patients. The outpatient, drug, and total costs were $178, $42, and $220, respectively, for female patients (n = 5394) and $144, $13, and $158, respectively, for male patients (n = 730). Few patients (9.2%) had subsequent cases with both outpatient and drug claims. The average outpatient, drug, and total costs of a subsequent case of trichomoniasis were $186, $75, and $261, respectively (n = 566).
TABLE 1.
Number of Cases Treated and Direct Medical Costs Per Treated Case of Trichomoniasis (2019 US Dollars)
| Item Estimated | Male | Female | Total |
|---|---|---|---|
| No. first cases of trichomonas treated* | 730 | 5394 | 6124 |
| Outpatient cost (95% CI), $ | 144.47 (132.93–156.00) | 177.80 (167.08–185.52) | 173.82 (166.02–181.63) |
| Drug cost (95% CI), $ | 13.27 (9.67–16.87) | 42.23 (38.82–46.64) | 38.78 (34.86–42.69) |
| Total cost (95% CI), $ | 157.74 (144.93–170.55) | 220.03 (209.10–230.95) | 212.60 (202.85–222.36) |
| Subsequent cases treated* | 23 | 543 | 566 |
| Outpatient cost (95% CI), $ | 106.36 (45.72–167.00) | 189.12 (164.10–214.14) | 185.75 (161.61–209.90) |
| Drug cost (95% CI), $ | 11.10 (2.53–19.67) | 77.88 (62.56–93.20) | 75.17 (60.43–89.91) |
| Total cost (95% CI), $ | 117.46 (51.68–183.24) | 267.00 (234.15–299.84) | 260.92 (229.22–292.62) |
“First cases” refers to cases in patients with no previous cases of trichomoniasis in the time frame we examined (2016–2018). Costs incurred more than 30 days after a first case were assumed to be attributable to a subsequent case.
CI indicates confidence interval.
DISCUSSION
Our study makes 3 key contributions to the literature on the costs of trichomoniasis. First, we updated the estimated treatment cost in female patients. Our estimate of the direct medical treatment cost of trichomoniasis in female patients ($220) was approximately 70% higher than the previous estimate obtained from the 2001–2005 data of $101 ($156 when updated to 2019 dollars), an estimate that, like ours, did not include costs of sequelae or partner services.9 Second, we provided estimates for treatment costs in male patients ($158). To our knowledge, no previous cost studies of trichomoniasis in male patients exist. Third, in addition to estimating the average cost per case, we provide data on the range of values we observed so that future users of our data can account for the uncertainty in our findings. Higher costs in women than in men may be attributable to women having pelvic examinations during their visits and more visits per case, as current STD treatment guidelines recommend follow-up visits for women but not men.5
This analysis was subject to limitations associated with using medical claims data to estimate the cost of STIs as discussed in the previous study.9 Because of data coding and entry errors and other factors, our analysis may have excluded some trichomoniasis cases and included some patients without trichomoniasis. In addition, most patients with a trichomoniasis diagnosis had additional diagnosis codes. Our attempt to avoid biases in our cost estimate by excluding patients with multiple diagnosis codes may have introduced unintended bias in our estimates, to the extent that costs attributable to trichomoniasis in these excluded patients differ from that of the included patients. Similarly, biases may have arisen by our exclusion of patients without pharmacy claims, if indeed these patients were treated for trichomoniasis and if their average costs per case differed from patients with pharmacy claims. The results of this study are also limited to the costs associated with treatment of infection among patients receiving recommended therapies and excluded costs associated with sequelae, expedited partner therapy, or other partner services. We included costs within 30 days of the index date to include costs associated with persistent infection and follow-up. However, it is possible that the use of this 30-day window led to the inadvertent inclusion of costs associated with a new infection incurred within 30 days or the inadvertent exclusion of costs associated with the initial infection incurred beyond 30 days. Finally, the commercially insured patients in our study are not nationally representative; costs may differ among similarly insured patients or among publicly insured or uninsured populations. Trichomoniasis is associated with lower socioeconomic status16; therefore, many trichomonas infections might be treated in public settings with lower costs than in settings where commercially insured patients are treated.
Our estimates represent costs of treatment of trichomoniasis but do not include potential costs of treatment of sequelae and other comorbidities associated with trichomoniasis such as pelvic inflammatory disease, adverse outcomes of pregnancy, and HIV.2–5,17,18 Despite limitations, our study presents updated estimates of costs per episode of trichomoniasis under current STD treatment guidelines. The cost estimates presented can be used in cost-effectiveness studies of STI prevention interventions and can inform studies of expected lifetime costs per infection, which can account not only for the possibility of treatment of infection but also for the possibility of sequelae among those with untreated or inadequately treated infections.
Acknowledgment:
This research was supported, in part, by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention (CDC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC.
Conflict of Interest and Sources of Funding:
None declared.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
REFERENCES
- 1.Flagg EW, Meites E, Phillips C, et al. Prevalence of Trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis 2019; 46:e93–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200: 188.e181–188.e187. [DOI] [PubMed] [Google Scholar]
- 3.Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TVassay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56:e01091–e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis A, Gaynor A. Testing for sexually transmitted diseases in US public health laboratories, 2016. Sex Transm Dis 2020; 47:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2018. Atlanta, GA: US Department of Health and Human Services, 2019. [Google Scholar]
- 7.Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: A case for rescreening. Ann Intern Med 2006; 145:564–572. [DOI] [PubMed] [Google Scholar]
- 8.Owusu-Edusei K Jr., Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
- 9.Owusu-Edusei K Jr., Tejani MN, Gift TL, et al. Estimates of the direct cost per case and overall burden of trichomoniasis for the employer-sponsored privately insured women population in the United States, 2001 to 2005. Sex Transm Dis 2009; 36:395–399. [DOI] [PubMed] [Google Scholar]
- 10.Owusu-Edusei K Jr., Nguyen HT, Gift TL. Utilization and cost of diagnostic methods for sexually transmitted infection screening among insured American youth, 2008. Sex Transm Dis 2013; 40:354–361. [DOI] [PubMed] [Google Scholar]
- 11.Hansen L. The Truven Health MarketScan Databases for Life Sciences Researcher. Truven Health Analytics. Cambridge, MA: IBM Watson Health, 2017. [Google Scholar]
- 12.Odysseus Data Services, Inc. Athena-OHDSI Vocabularies Repository (2015–2020). Available at: athena.ohdsi.org. Accessed January 31, 2020.
- 13.Micromedex Solutions. RED BOOK Online. Ann Arobor, MI: Truven Health Analytics, Inc. Available at: http://www.micromedexsolutions.com. Accessed October 29, 2019. [Google Scholar]
- 14.Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Department of Labor. Consumer Price Indexes—All Urban Consumers. Washington, DC: United States Department of Labor. Available at: https://www.bls.gov/cpi/home.htm. Accessed February 6, 2020. [Google Scholar]
- 16.Korich F, Reddy NG, Trent M. Mycoplasma genitalium and Trichomonas vaginalis: Addressing disparities and promoting public health control of two emerging sexually transmitted infections. Curr Opin Pediatr 2020; 32:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rein DB, Kassler WJ, Irwin KL, et al. Direct medical cost of pelvic inflammatory disease and its sequelae: Decreasing, but still substantial. Obstet Gynecol 2000; 95:397–402. [DOI] [PubMed] [Google Scholar]
- 18.Muzny CA, Kissinger P. Trichomonas vaginalis infections. In: Bachmann L, ed. Sexually Transmitted Infections in HIV-Infected Adults and Special Populations. Cham: Springer, 2017:125–140. [Google Scholar]


