Abstract
Brazil is a non-endemic country for hepatitis E virus (HEV) infection with seroprevalence from 1% to 4% in blood donors and the general population. However, data on seroprevalence of HEV in the country are still limited. This study evaluated the prevalence of past or present HEV infection in a group of blood donors representative of the general population of the city of Sao Paulo, Southeastern Brazil. Serum samples from 500 blood donors were tested from July to September 2014 by serological and molecular methods. Anti-HEV IgG antibodies were detected in 49 (9.8%) subjects and categorized age groups revealed an age-dependent increase of HEV seroprevalence. Among the anti-HEV IgG positive subjects, only 1 had anti-HEV IgM while none tested positive for HEV-RNA. The present data demonstrate a higher seroprevalence of anti-HEV IgG than previously reported in the region.
Keywords: Hepatitis E virus, Blood donors, Seroprevalence, Brazil
Introduction
Hepatitis E virus (HEV) infection is among the most frequent causes of acute hepatitis worldwide. It is estimated that 2.3 billion people have already been infected with HEV with 70,000 deaths attributed to this virus every year.1, 2
Hepatitis E presents as large epidemics and sporadic cases in endemic areas, including genotype 1 in Asia and Africa, genotype 2 in Mexico and Africa, and genotype 4 in Asia. Sporadic cases of genotype 3 occur worldwide. HEV detected from Argentina and Brazil are more related to viruses from industrialized countries (North America and Europe), whereas the HEV in the Caribbean and Mexico include viral genotypes more closely related to outbreaks in Africa and Asia.3, 4
The total prevalence of antibodies against HEV in endemic countries is variable (3%–27%).5 In non-endemic areas with proper sanitary conditions and a well-controlled water supply, the prevalence of antibodies against HEV in the general population is relatively high (up to 7%–10%).5
Data on HEV seroprevalence in Brazil are still scarce. HEV is not routinely investigated in the country, even in cases of unexplained liver enzyme elevation or acute hepatitis, and only a few laboratories perform anti-HEV tests. Brazil is a non-endemic country for HEV, with rare cases confirmed by molecular methods. HEV seroprevalence in the country ranges from 1% to 4% in blood donors or the general population, 13% in individuals from an agricultural settlement in the Amazon Basin and 15% in renal transplant recipients.6, 7, 8, 9 Nonetheless, most available studies are limited, outdated and cannot be compared properly because of their small sample sizes and/or diverse methodology. Therefore, the occurrence and characteristics of hepatitis E in Brazil are poorly understood.
The aim of this study was to assess the prevalence of hepatitis E in Sao Paulo, Southeastern Brazil, through the analysis of anti-HEV antibodies in a group of blood donors representative of the general population.
Patients and methods
Study area
Sao Paulo, the most populous city in Brazil, is also considered the most multicultural city in the country, with approximately 12 million inhabitants and immigrants from all parts of the world.10 The city is divided in eight zones, with distinct socioeconomic characteristics.
Study design
A sampling plan accounting for demographic zone, gender and age group was created to gather a sample representative of the general adult population in the city.
A prospective, cross-sectional study was carried out involving 500 blood donors who consecutively underwent blood donation from July to September 2014 in the Beneficent Association for Blood Collection (COLSAN) in Sao Paulo, Southeastern Brazil, strictly following the sampling plan.
This sample was calculated to be sufficient to successfully estimate HEV seroprevalence of approximately 10% in the city population at a 95% confidence interval (CI).
Sample and data collection
Five hundred milliliter aliquots of the serum samples routinely collected for serological testing were separated and stored at –80 °C until laboratory analysis. Data regarding date of birth and gender were collected during enrollment.
Anti-HEV IgG and IgM antibodies detection
The presence of anti-HEV IgG antibodies was investigated through enzyme immunoassay using the WANTAI HEV-IgG ELISA kit (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China), strictly according to the manufacturer recommendations. Specimens with positive results were tested for anti-HEV IgM antibodies using a specific kit from the same manufacturer.
RNA extraction and quantitative RT-PCR
HEV RNA was extracted from the serum samples using QIAamp viral RNA mini kit (QIAGEN, Hilden, Germany), strictly according to the manufacturer's instructions.
Quantitative RT-PCR was performed according to a modified 1-step triplex real time protocol previously described11 with a set of primers and probe targeting a highly conserved 70 nt long sequence within overlapping parts of ORF2 and ORF312 and a set specific for a 113 nt sequence of ORF2.13 A third set of primers and probe targeting the human RNAseP gene was used as endogenous internal amplification control to certify specimen quality and RNA extraction.14
A plasmid clone from a Brazilian human HEV strain previously characterized (GenBank accession number KF152884)15 was constructed with TOPO® TA cloning® kit (Invitrogen, Carlsbad, CA, USA) and the primers described. Plasmid DNA was purified using QIAprep spin miniprep kit (QIAgen, Hilden, Germany), linearized and quantified with the Nanodrop ND-1000 instrument (Wilmington, DE, USA) following transcription to RNA with T7 RNA polymerase (Promega, Madison, WI, USA). Standard curves were generated using 100 to 1010 copies of plasmid RNA. HEV viral loads were determined based on the standard curves. The limit of detection of the real-time RT-PCR was 5 copies of RNA per reaction, while the limit of quantification was set at 50 copies of RNA per reaction. All screening reactions were run in duplicates using with proper controls.
Statistical analysis
All data were entered and analyzed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics consisted of the characterization of the studied population and anti-HEV IgG seroprevalence through the respective percentages and 95% confidence intervals (CI) or mean/median values and standard deviation (SD) for continuous variables. The bivariate analysis consisted of Pearson's Chi-square test to compare categorical values. Non-conditional logistic regression was used to identify associations between dependent and independent variable reported as odds ratio (OR). Mann–Whitney U test was used to compare means of non-normally distributed variables. Linear regression analysis was used to evaluate the trends of anti-HEV positivity with respect to age group. Statistical significance level was p < 0.05. All reported values are two-tailed.
Ethical aspects
The study protocol was approved by the Institutional Ethics Committee (162/09 and 86730/12).
Results
A total of 49 samples of 500 subjects tested positive for anti-HEV IgG antibodies, which relates to a seroprevalence of 9.8% (95% CI 7.5–12.7). From these, only 1 presented with anti-HEV IgM. None tested positive for HEV-RNA.
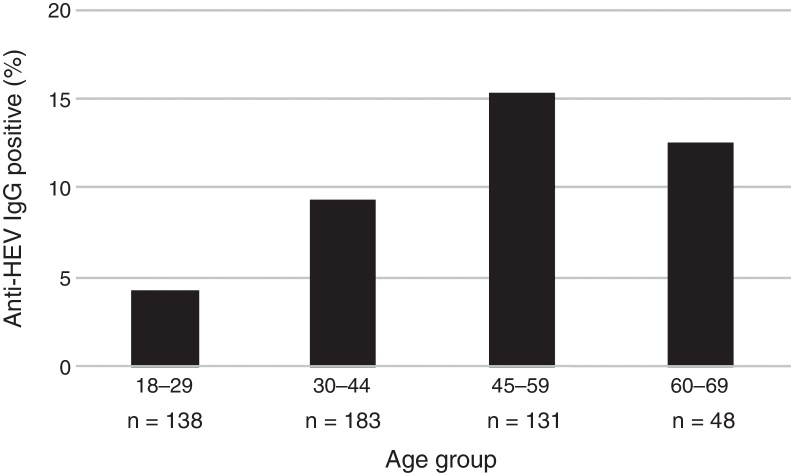
The mean age of the enrolled subjects was 38.8 years with a SD of 13, within a range of 18–67 and a median age of 36 years old. Mean age of subjects tested positive for anti-HEV IgG was 43.7 years with a SD of 11.7, range 24–67 years, significantly higher than that of those who tested negative for anti-HEV IgG (38.3 ± 13.0; p = 0.006). Categorized age groups revealed an age-dependent increase of HEV seroprevalence with R2 of 0.99 for up to 59 years and R2 of 0.70 for up to 69 years (p = 0.023) as shown in Fig. 1. Only 4.3% (95% CI 2.0–9.2) of the subjects showed positivity for anti-HEV IgG in the age group of 18–29, whereas 15.3% (95% CI 10.1–22.4) were positive for anti-HEV IgG in the age group of 45–59 years. These results show a 4-fold increase of the risk to undergo a HEV infection or present with positive anti-HEV IgG antibodies at an advanced age (45–59) than at an early age.
Fig. 1.
Estimated prevalence of anti-HEV IgG by age group in Sao Paulo, Brazil.
In all, 245 (49%) patients were male (as observed for the general population in Sao Paulo). There was no difference in distribution of anti-HEV positive cases between male and female subjects. Table 1 presents the characteristics of the studied individuals.
Table 1.
Characteristics of the studied individuals according to anti-HEV IgG status, Sao Paulo, Southeastern Brazil.
| Characteristic | Anti-HEV IgG positive | p | OR (95% CI) | Total |
|---|---|---|---|---|
| Gender | ||||
| Male | 24 (9.8) | 0.998 | – | 245 (49.0) |
| Female | 25 (9.8) | – | 255 (51.0) | |
| Age group | ||||
| 18–29 | 6 (4.3) | 0.023a | 1 (Ref) | 138 (27.6) |
| 30–44 | 17 (9.3) | 2.25 (0.86–5.88) | 183 (36.6) | |
| 45–59 | 20 (15.3) | 3.96 (1.54–10.22) | 131 (26.2) | |
| 60–69 | 6 (12.5) | 3.14 (0.96–10.23) | 48 (9.6) | |
Results are presented as number and percentage.
–: not applicable.
Significant at p < 0.05.
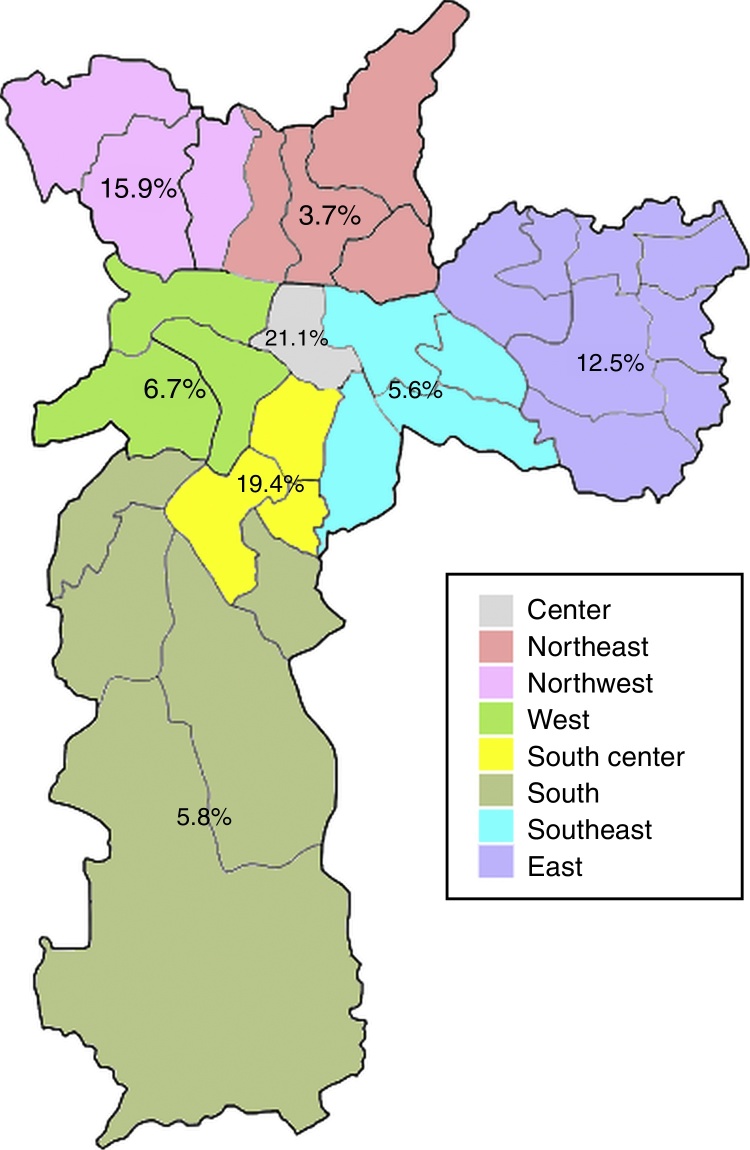
Anti-HEV IgG prevalence varied significantly among the demographic zones in the city of Sao Paulo (Fig. 2). Highest rates were observed in the Central (21.1%) and South-Central (19.4%) zones, while the Northeast (3.7%), Southeast (5.6%) and South (5.8%) presented with the lowest prevalences (p = 0.031). The Central and South-Central zones presented with a 7- and 6-fold increased risk of HEV past infection, respectively, when compared to the Northeast (Table 2).
Fig. 2.
City map of the city of Sao Paulo, Southeastern Brazil, and anti-HEV IgG seroprevalence according to city zone.
Table 2.
Seroprevalence of anti-HEV IgG according to demographic zone, Sao Paulo, Southeastern Brazil.
| City zone | Anti-HEV IgG positive | p | OR (95% CI) | Total |
|---|---|---|---|---|
| Central | 4 (21.1) | 0.031a | 6.93 (1.15–41.61) | 19 (3.8) |
| South-Central | 7 (19.4) | 6.28 (1.22–32.22) | 36 (7.2) | |
| Northwest | 7 (15.9) | 4.92 (0.97–25.03) | 44 (8.8) | |
| East | 16 (12.5) | 3.71 (0.82–16.75) | 128 (25.6) | |
| West | 3 (6.7) | 1.86 (0.30–11.63) | 45 (9.0) | |
| South | 6 (5.8) | 1.61 (0.31–8.25) | 103 (20.6) | |
| Southeast | 4 (5.6) | 1.55 (0.27–8.81) | 71 (14.2) | |
| Northeast | 2 (3.7) | 1 (Ref) | 54 (10.8) |
Results are presented as number and percentage.
Significant at p < 0.05.
Discussion
The present data demonstrate a higher seroprevalence of anti-HEV IgG in Sao Paulo, Southeastern Brazil, than ever reported for the general population (or blood donors) in the region, with a cumulative increase with age. In studies from 1997 to 2006, anti-HEV IgG prevalence varied from 2.0% to 4.3% in blood donors from Southeastern and Northeastern Brazil and from 0% to 4.3% in individuals from rural and urban areas and those living in low socioeconomic communities.6 A study on 699 patients from a low socioeconomic group living in Rio de Janeiro in 2002 reported a 2.4% HEV seroprevalence.16
The higher prevalence observed in the present study may be due to an increase in HEV seroprevalence in recent years. A recent study from 15 years of laboratory results of patients clinically suspected of HEV infection in the same region, described an overall anti-HEV positivity rate of 2.1% (47/2271), ranging from 0% (0/183) to 8.6% (12/139) with year. The highest frequencies were observed from 2011 to 2013: 5.9% (2/34), 8.6% (12/139), and 6.1% (9/148), respectively.9
The performance of anti-HEV IgG assays may also have played a role in the higher seroprevalence of anti-HEV antibodies found in the present study. Many of the earlier studies in Brazil used anti-HEV assays of poor sensitivity, which could result in significant underestimated rates compared to this study that is the first to use a validated, highly sensitive assay in the region. The same was observed in England where HEV seroprevalence rose from 3.6% to 16% when a more sensitive IgG assay, i.e., the Wantai test, was used.17 A recent study, also using the Wantai test, demonstrated a similar seroprevalence of anti-HEV IgG (10%) among blood donors in the metropolitan area of Itajai Valley, Southern Brazil.18
The eating habits of the population could also influence the higher seroprevalence of HEV antibodies, since the ingestion of raw or undercooked porcine meat has been associated with HEV infection19, 20 and probable zoonotic transmission of HEV has been demonstrated in Brazil, as very high homology was found between human and swine HEV strains.15, 21, 22 The consumption of pork varies according to multiple factors, including cultural and socioeconomical aspects.23 In the present study, the anti-HEV IgG prevalence varied significantly among demographic zones, with the highest rates in the Central (21.1%) and South-Central (19.4%) zones. Interestingly, these zones are both classified as having high guarantee of economic and social rights of all inhabitants in the region, which accounts for employment, income, housing, health and education. Moreover, these are the regions with the highest income per capita in the city, with less than five per cent low-income inhabitants.24 Thus, the higher prevalence of anti-HEV IgG antibodies in these regions could be a result of higher pork consumption, which is known to be related to better economic conditions.25 Accordingly, the South zone, which presented with one of the lowest HEV seroprevalence (5.8%), is classified as having poor guarantee of economic and social rights and is the zone with the highest percentual of low-income inhabitants (20% or more earning less than half the minimum wage).24
Only 1 of the 49 anti-HEV IgG positive subjects studied presented with anti-HEV IgM, a marker of acute infection. None of the subjects presented with HEV-RNA. Nonetheless, this study demonstrates a higher rate of past HEV infection than it was known to occur in the region, showing that this infection is probably underdiagnosed in the country.
The authors acknowledge some limitations to this study. It was not possible to enquiry the subjects on clinical history, possible risk factors and eating habits that could help understand the occurrence of hepatitis E in this population. Thus, further and larger epidemiological studies should be performed.
In conclusion, a high prevalence of anti-VHE IgG was observed in Sao Paulo, which is the highest prevalence ever reported in the region and the second highest in the country.
Financial support
FAPESP 2012/22925-3, 2013/03701-0.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors with to thank the COLSAN blood donor center and all employees for their support. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
References
- 1.Rein D.B., Stevens G.A., Theaker J., Wittenborn J.S., Wiersma S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Mirazo S., Ramos N., Mainardi V., Gerona S., Arbiza J. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med. 2014;6:45–59. doi: 10.2147/HMER.S63417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N., Dalton H.R., Abravanel F., Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierro N.A., Realpe M., Meraz-Medina T., Roman S., Panduro A. Hepatitis E virus: an ancient hidden enemy in Latin America. World J Gastroenterol. 2016;22:2271–2283. doi: 10.3748/wjg.v22.i7.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panduro A., Escobedo Meléndez G., Fierro N.A., Ruiz Madrigal B., Zepeda-Carrillo E.A., Román S. Epidemiology of viral hepatitis in Mexico. Salud Publica Mex. 2011;53(Suppl. 1):S37–S45. [PubMed] [Google Scholar]
- 6.Riveiro-Barciela M., Rodríguez-Frías F., Buti M. Hepatitis E virus: new faces of an old infection. Ann Hepatol. 2013;12:861–870. [PubMed] [Google Scholar]
- 7.Echevarría J.M., González J.E., Lewis-Ximenez L.L., et al. Hepatitis E virus infection in Latin America: a review. J Med Virol. 2013;85:1037–1045. doi: 10.1002/jmv.23526. [DOI] [PubMed] [Google Scholar]
- 8.Hering T., Passos A.M., Perez R.M., et al. Past and current hepatitis E virus infection in renal transplant patients. J Med Virol. 2014;86:948–953. doi: 10.1002/jmv.23915. [DOI] [PubMed] [Google Scholar]
- 9.Vitral C.L., da Silva-Nunes M., Pinto M.A., et al. Hepatitis A and E seroprevalence and associated risk factors: a community-based cross-sectional survey in rural Amazonia. BMC Infect Dis. 2014;14:458. doi: 10.1186/1471-2334-14-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passos-Castilho A.M., de Sena A., Reinaldo M.R., Granato C.F. Hepatitis E virus infection in Brazil: results of laboratory-based surveillance from 1998 to 2013. Rev Soc Bras Med Trop. 2015;48:468–470. doi: 10.1590/0037-8682-0036-2015. [DOI] [PubMed] [Google Scholar]
- 11.IBGE [Brazilian Institute of Geography and Statistics. Population estimates, 2016]. Available at: http://www.cidades.ibge.gov.br/xtras/perfil.php?lang=&codmun=355030&search=sao-paulo|sao-paulo. Accessed: 15.9.2016.
- 12.Vasickova P., Kralik P., Slana I., Pavlik I. Optimisation of a triplex real time RT-PCR for detection of hepatitis E virus RNA and validation on biological samples. J Virol Methods. 2012;180:38–42. doi: 10.1016/j.jviromet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Jothikumar N., Cromeans T.L., Robertson B.H., Meng X.J., Hill V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Gyarmati P., Mohammed N., Norder H., Blomberg J., Belák S., Widén F. Universal detection of hepatitis E virus by two real-time PCR assays: TaqMan and primer-probe energy transfer. J Virol Methods. 2007;146:226–235. doi: 10.1016/j.jviromet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Luo W., Yang H., Rathbun K., Pau C.P., Ou C.Y. Detection of human immunodeficiency virus type 1 DNA in dried blood spots by a duplex real-time PCR assay. J Clin Microbiol. 2005;43:1851–1857. doi: 10.1128/JCM.43.4.1851-1857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos-Castilho A.M., Porta G., Miura I.K., et al. Chronic hepatitis E virus infection in a pediatric female liver transplant recipient. J Clin Microbiol. 2014;52:4425–4427. doi: 10.1128/JCM.02286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos D.C., Souto F.J., Lopes dos Santos D.R., Vitral C.L., Gaspar A.M. Seroepidemiological markers of enterically transmitted viral hepatitis A and E in individuals living in a community located in the North Area of Rio de Janeiro, RJ, Brazil. Mem Inst Oswaldo Cruz. 2002;97:637–640. doi: 10.1590/s0074-02762002000500007. [DOI] [PubMed] [Google Scholar]
- 18.Bendall R., Ellis V., Ijaz S., Ali R., Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 19.Passos-Castilho A.M., de Sena A., Geraldo A., Spada C., Granato C.F. High prevalence of hepatitis E virus antibodies among blood donors in Southern Brazil. J Med Virol. 2016;88:361–364. doi: 10.1002/jmv.24336. [DOI] [PubMed] [Google Scholar]
- 20.Colson P., Borentain P., Queyriaux B., et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 21.Legrand-Abravanel F., Kamar N., Sandres-Saune K., et al. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835–844. doi: 10.1086/655899. [DOI] [PubMed] [Google Scholar]
- 22.Lopes dos Santos D.R., Lewis-Ximenez L.L., da Silva M.F., de Sousa P.S., Gaspar A.M., Pinto M.A. First report of a human autochthonous hepatitis E virus infection in Brazil. J Clin Virol. 2010;47:276–279. doi: 10.1016/j.jcv.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Passos-Castilho A.M., Granato C.F. High frequency of hepatitis E virus infection in swine from South Brazil and close similarity to human HEV isolates. Braz J Microbiol. 2017 doi: 10.1016/j.bjm.2016.10.022. pii: S1517-8382(16)30349-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlindwein M.M., Kassouf A.L. Análise da influência de alguns fatores socioeconômicos e demográficos no consumo domiciliar de carnes no Brasil [Analysis of the influence of some socio-economic and demographic factors in household consumption of meat in Brazil] Rev Econ Sociol Rural. 2006;44:549–572. [in Portuguese] [Google Scholar]
- 25.Prefeitura Municipal de Sao Paulo. [Intra-urban system of human rights monitoring, 2005]. Available at: http://www9.prefeitura.sp.gov.br/simdh/index.html. Accessed: 15.09.2016.